Abstract
Background
Vitamin D has immunomodulatory properties and seems to reduce the risk of infections. Whether low vitamin D concentrations are independent risk factors for nosocomial postoperative infections in surgical patients remains to be studied in detail.
Methods
In 3,340 consecutive cardiac surgical patients, we investigated the association of circulating 25-hydroxyvitamin D (25OHD; indicator of nutritional vitamin D status) and 1,25-dihydroxyvitamin D (1,25[OH]2D; active vitamin D hormone) with nosocomicial infections. The primary endpoint was a composite of thoracic wound infection, sepsis, and broncho-pulmonary infection. Vitamin D status was measured on the last preoperative day. Infections were assessed until discharge. Logistic regression analysis was used to examine the association between vitamin D metabolite concentrations and the composite endpoint.
Results
The primary endpoint was reached by 5.6% (n = 186). In patients who reached and did not reach the endpoint, in-hospital mortality was 13.4% and 1.5%, respectively (P<0.001). Median (IQR) 25OHD and 1,25(OH)2D concentrations were 43. 2 (29.7–61.9) nmol/l and 58.0 (38.5–77.5) pmol/l, respectively. Compared with the highest 1,25(OH)2D quintile (>81.0 pmol/l), the multivariable–adjusted odds ratio of infection was 2.57 (95%CI:1.47–4.49) for the lowest 1,25(OH)2D quintile (<31.5 pmol/l) and 1.85 (95%CI:1.05–3.25) for the second lowest quintile (31.5–49.0 pmol/l). There was no significant association between 25OHD concentrations and the primary endpoint.
Conclusions
Our data indicate an independent association of low 1,25(OH)2D levels with the risk of postoperative infections in cardiac surgical patients. Future studies should pay more attention on the clinical relevance of circulating 1,25(OH)2D and its regulation.
Introduction
Nosocomial infections occur worldwide and are a significant burden both for the patients and for public health [1]. Infections of surgical wounds, the urinary tract, and the lower respiratory tract are the most frequent infections [1]. Cardiac surgical patients are at an increased risk of developing nosocomial infections. Incidence rates of up to 20% and more have been reported [2]. Although serious postoperative complications are uncommon, they are potentially devastating. For example, in a large study in 331,429 cardiac surgical patients, patients who developed an infection were more likely to have a prolonged in-hospital stay compared with patients who did not develop a postoperative infection [3]. In addition, in-hospital mortality was significantly higher in patients with major postoperative infection (17.3% versus 3.0%).
Antibiotic prophylaxis has demonstrated a large benefit in the prevention of wound infection [4] and is therefore standard practice in cardiac surgery. Cardiac-surgery-specific guidelines advocate antibiotic prophylaxis for up to 48 h post-operatively [5,6]. Standard or double-dose cefazolin or cefuroxime are most commonly recommended [5,7–11]. However, antibiotic prophylaxis does not completely prevent nosocomial infections [12].
Besides its pivotal role in musculoskeletal health, vitamin D has a broad range of additional effects, which also cover the immune system [13]. There is likely evidence that vitamin D decreases the risk of airway infections [14,15]. Moreover, vitamin D supplements seem to decrease the need for antibiotics [16], especially in elderly patients [17]. In cardiac surgical patients, the prevalence of vitamin D deficiency (circulating 25-hydroxyvitamin D [25OHD] < 30 nmol/l) is between 15% and 38% [18–20].
The present study aimed to investigate whether low vitamin D status is associated with an increased risk of developing postoperative infections.
Methods
Patients
This investigation is based on data of the CALCITOP (Calcitriol and clinical outcome in cardiac surgical patients) study, a prospective cohort study of 3,852 cardiac surgical patients [21]. Study participants were recruited between February 2012 and December 2013 at a tertiary heart center in East-Westphalia, Germany (Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen). Eligible for inclusion were cardiac surgical patients aged 18 years and over. Patients with heart transplants and pacemaker/defibrillator implants were excluded. In 3,340 patients, preoperative serum concentrations of 25OHD and 1,25(OH)2D as well as postoperative data on infections were available. This dataset was used to perform the statistical analyses. The study was approved by the Ethics Committee of the Ruhr University Bochum at Bad Oeynhausen and was registered at clinicaltrials.gov as NCT02192528. All patients provided their written informed consent.
Data collection
We prospectively collected preoperative, perioperative, and postoperative data using the electronic patient databases of our institution. Besides 25OHD (indicator of nutritional vitamin D status) and 1,25(OH)2D (active, hormonal form of vitamin D), we assessed additional preoperative and surgical characteristics such as age, gender, body mass index (BMI), left ventricular ejection fraction (LVEF), current smoking, diabetes mellitus, concentrations of creatinine, glucose, C-reactive protein (CRP) and leukocytes, previous cardiac surgery, operation priority, and type of surgery. Moreover, we assessed four postoperative outcome parameters, namely infection, intensive care unit (ICU) stay, in-hospital stay, and in-hospital mortality.
Antibiotic Prophylaxis and Treatment
According to institutional standards, antibiotic prophylaxis was performed routinely with 2g cephalosporin (Cefazolin-Sandoz, Sandoz Pharmaceuticals, Basel Switzerland), preoperatively, intra-operatively and during postoperative drainage (up to 48 h after surgery). In case of an infection, antibiotic therapy was selected according to the sensitivity of the organism and the clinical response of the patient.
Biochemical Analyses
Circulating levels of 25OHD and 1,25(OH)2D, creatinine, CRP, leukocytes, and glucose levels were measured as previously described [21]. Estimated glomerular filtration rate (eGFR) was calculated using the creatinine-based modification of diet in renal disease formula. Routine microbiological tests were used to assess the type of pathogen in case of an infection.
Endpoints
The primary endpoint was a composite of clinically relevant infections such as thoracic wound infection, sepsis, and broncho-pulmonal infection. Infections were diagnosed according to standard procedures, such as the presence of positive results of microbial culture, pyrexia, tachycardia, and tachypnea. Events were assessed until discharge. Additional endpoints were urogenital and other infections, ICU stay, in-hospital stay, and in-hospital mortality.
Statistics
Categorical variables are summarized as percentages. Since several continuous variables were non-normally distributed (CRP, glucose), continuous variables are reported as median and interquartile range (IQR). We used the Kolmogorov-Smirnov test to check normal data distribution of continuous variables. Normal distribution was a consideration when probability values were >0.05. Differences in preoperative categorical variables and continuous variables between the groups with and without infection were assessed using Fisher’s exact test and the Mann-Whitney U-test, respectively.
We carried out multiple logistic regression analysis to assess the independent relationship of the preoperative 25OHD or 1,25(OH)2D3 categories with the risk of infection. According to previous classification [21], we used the following cut-off values for classifying 25OHD: risk of deficiency (<30 nmol/L), risk of inadequacy (30 nmol/L to 49.9 nmol/L), borderline status (50–74.9 nmol/L), adequacy (75–100 nmol/L), and potentially harmful (>100 nmol/L, to convert nanomolar to nanogram per milliliter divide by 2.496). The group with adequate vitamin D status was used as the reference group. Regarding 1,25(OH)2D, we divided the study cohort into quintiles, since no generally accepted classification exists. We performed age- and gender-adjusted analyses and used multivariable-adjusted models to examine the association between vitamin D metabolites and the incidence of the primary endpoint. Since the number of variables that can be included for multivariable testing is equal to the square root of the number of events [22], we restricted the covariates to important demographic parameters (age, gender, and BMI), cardiac surgical-related parameters (redo, operation priority, type of cardiac surgery), and additional parameters that differed significantly between patients who reached or did not reach the primary endpoint (see Table 1). We calculated absolute (incidence) rates and odds ratios (ORs) and corresponding 95% confidence intervals (CI). In subgroup analyses, we restricted the study cohort to non-diabetes patients and patients aged ≥ 70 years. In sensitivity analyses, we used 50 nmol/l as cutoff for the lowest 25OHD category and divided the study cohort into tertiles of 1,25(OH)2D levels. Moreover, we compared the status of vitamin D metabolites in patients with any infection (broncho-pulmonal infection, thoracic wound infection, sepsis, urogenital infection or other infection) with non-infected patients. To reduce the risk of study bias through already existing preoperative infections, we also restricted the analysis to a comparison between patients with thoracic wound infection and patients without infection. We considered P values <0.05 as statistically significant. All P values are reported two-sided. Analyses were performed using the statistical software package IBM® SPSS®, version 21.
Table 1. Baseline characteristics of patients with and without wound infection.
| Parameter | Without Infection n = 3154 | With Infection n = 186 | P value |
|---|---|---|---|
| Age (years) | 71 (62–77) | 73 (63–78) | 0.086 |
| Gender, Males (%) | 66.9 | 67.2 | >0.999 |
| Body Mass Index (kg/m2) | 27.1 (24.6;30.1) | 27.4 (24.7;31.4) | 0.492 |
| Left-Ventricular Ejection Fraction (%) | 60 (51–65) | 56 (48–62) | <0.001 |
| EuroSCORE (logistic) | 5.1 (2.4;10.9) | 8.4 (3.7;19.9) | <0.001 |
| Smoker (%) | 28.5 | 30.6 | 0.713 |
| Diabetes Mellitus (%) | 25.9 | 40.3 | <0.001 |
| Re-Do (%) | 9.7 | 23.7 | <0.001 |
| NYHA Class > II (%) | 41.0 | 55.9 | <0.001 |
| Operation Priority, Urgent/Emergent (%) | 3.9 | 14.0 | <0.001 |
| eGFR (ml/min/1.73m2) | 77.4 (61.5;90.2) | 68.9 (49.5;89.1) | 0.001 |
| C-Reactive Protein (mg/l) | 2.5 (1.1;6.6) | 7.5 (2.5;26.0) | <0.001 |
| Glucose (mg/dl) | 105 (94;129) | 111 (92;138) | 0.384 |
| Type of Surgery | |||
| CABG (%) | 35.7 | 28.5 | 0.048 |
| Valve Surgery (%) | 36.5 | 39.6 | 0.059 |
| Combined CABG and Valve Surgery (%) | 15.1 | 23.1 | 0.005 |
| Others (%) | 12.7 | 18.8 | 0.024 |
| On-Pump Surgery (%) | 66.9 | 68.3 | 0.749 |
CABG: coronary artery bypass graft
Results
The incidence of the primary endpoint was 5.6% (n = 186). In detail, broncho-pulmonary infection was most prevalent (n = 112), followed by thoracic wound infection (n = 76), including 13 patients with deep sternal wound infection, and sepsis (n = 19). In some patients (n = 21) more than one infection was diagnosed. The incidence of urinary tract and other infections requiring antibiotic treatment like veneous catheter infection, foot infection, and oto-laryngeal or anal smear-positive antibiotic-resistant pathogens was 1.6% (n = 52) and 11.1% (n = 372), respectively. No postoperative case of endocarditis appeared. Patients who reached the primary endpoint were infected with different types of bacteria, among them Staphylococcus epidermidis (n = 51) and other coagulase-negative Staphylococci (n = 44), Enterococci faecalis and faecium (n = 37), Serratia marcescens (n = 18), Escherichia coli (n = 12), Staphylococcus aureus (n = 11), Pseudomonas aeruginosa (n = 10), Enterobacter cloacae (n = 9), and others (n = 51). Moreover, some patients were infected with Candida albicans (n = 78), non-albicans Candida species (n = 48), and Aspergillus fumigatus (n = 4).
Patients who reached the primary endpoint differed significantly from other patients concerning various demographic parameters, several preoperative clinical variables, and type of surgery (Table 1).
However, gender distribution, BMI, smoking status, blood glucose levels, and percent on-pump surgery did not differ significantly between groups.
Median (IQR) 25OHD and 1,25(OH)2D concentrations were 43.2 (29.7–61.9) nmol/l and 58.0 (38.5–77.5) pmol/l, respectively. Circulating preoperative 1,25(OH)2D levels, but not circulating 25OHD levels, were significantly lower in patients who reached the primary endpoint than in other patients (Table 2).
Table 2. Vitamin D metabolites and postoperative outcomes in patients with and without wound infection.
| Parameter | Without Infection n = 3154 | With Infection n = 186 | P value |
|---|---|---|---|
| Preoperative Vitamin D Metabolites | |||
| 25-hydroxyvitamin D (nmol/l) | 43.2 (30.0;61.9) | 42.1 (30.0;60.4) | 0.235 |
| 1,25-dihydroxyvitamin D (pmol/l) | 58.5 (39.5;78.0) | 42.5 (24.5;65.8) | <0.001 |
| Mechanical Ventilator Support (h) | 9 (7;13) | 105 (12;414) | <0.001 |
| Intensive-Care Unit stay (h) | 24 (20;66) | 240 (44;668) | <0.001 |
| In-Hospital Stay (days) | 13 (11;15) | 21 (15;40) | <0.001 |
| In-Hospital Mortality (%) | 1.5 | 13.4 | <0.001 |
Continuous data are presented as median with interquartile range
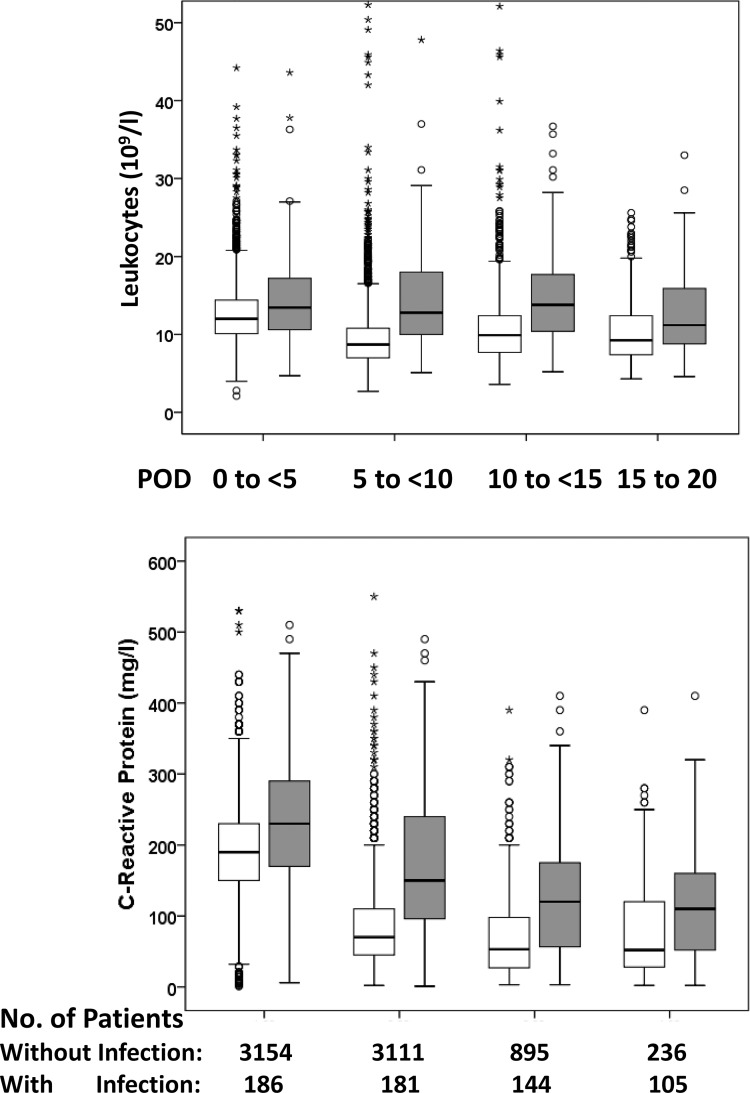
During postoperative hospitalization, infected patients had higher median leukocyte and CRP concentrations than patients who did not reach the primary endpoint (Fig 1).
Fig 1. Postoperative time course of leukocyte and C-reactive protein concentrations in cardiac surgical patients with and without infection.
The boxes express the upper and lower quartiles, and the central lines show the median. The whiskers represent the values below and above the interquartiles. The circles illustrate outliers, and the stars denote extremes. white boxes, patients without infection; grey boxes, patients with infection; POD, postoperative day
Moreover, ICU-stay and in–hospital stay were longer, and in-hospital mortality was significantly higher in infected patients than in other patients (13.4% vs. 1.5%, Table 2). Of the 25 patients who reached the primary endpoint and died, causes of death were sepsis (n = 8), multiorgan failure (n = 6), heart failure (n = 6), cardiogenic shock (n = 1), and others (n = 4).
Of the study cohort, 25.5% had deficient 25OHD levels (< 30 nmol/l). Patients in the lowest 1,25(OH)2D quintile had 1,25(OH)2D levels < 31.5 pmol/l. In Table 3, the ORs for the primary endpoint are given by categories of 25OHD and 1,25(OH)2D.
Table 3. Unadjusted and adjusted odds ratio (OR) for wound infection by cutoffs of 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D.
| Vitamin D | N | Primary Endpoint N (%) | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | Model 4 OR (95% CI) |
|---|---|---|---|---|---|---|
| 25OHD | ||||||
| <30 nmol/l | 838 | 58 (6.9) | 1.77 (0.96–3.28) | 1.94 (1.03–3.65) | 1.83 (0.97–3.47) | 1.62 (0.87–3.20) |
| 30–49.9 nmol/l | 1183 | 56 (4.7) | 1.15 (0.62–2.14) | 1.28 (0.68–4.41) | 1.30 (0.69–2.46) | 1.28 (0.67–2.43) |
| 50–74.9 nmol/l | 850 | 47 (5.5) | 1.36 (0.72–2.55) | 1.54 (0.81–2.93) | 1.60 (0.84–3.07) | 1.56 (0.81–3.02) |
| 75–100 nmol/l | 318 | 13 (4.1) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| >100 nmol/l | 151 | 12 (7.9) | 2.01 (0.72–4.51) | 1.90 (0.82–4.36) | 1.91 (0.83–4.43) | 1.78 (0.76–4.17) |
| 1,25(OH)2D | ||||||
| Lowest Quintile (<31.5 pmol/l) | 668 | 62 (9.3) | 3.97 (1.74–4.18) | 3.37 (1.98–5.71) | 3.11 (1.82–5.29) | 2.57 (1.47–4.49) |
| Second Lowest Quintile (31.5–49.0 pmol/l) | 668 | 43 (6.4) | 2.23 (0.93–2.43) | 2.07 (1.20–3.59) | 2.02 (1.16–3.51) | 1.85 (1.05–3.25) |
| Intermediate Quintile (49.1–63.0 pmol/l/l) | 668 | 30 (4.5) | 1.49 (0.84–2.66) | 1.42 (0.79–2.54) | 1.38 (0.77–2.48) | 1.36 (0.75–2.45) |
| Second Highest Quintile (63.1–81.0 pmol/l) | 668 | 31 (4.6) | 1.58 (0.89–2.80) | 1.55 (0.87–2.75) | 1.49 (0.84–2.65) | 1.48 (0.82–2.65) |
| Highest Quintile (>81.0 pmol/l) | 668 | 20 (3.0) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
Model 1: adjusted for age and gender
Model 2: adjusted as in model 1 and for body mass index, redo, operation priority, and type of surgery
Model 3: adjusted as in model 2 and for left ventricular ejection fraction, NYHA function class, and EuroSCORE
Model 4: adjusted as in model 3 and for kidney function (eGFR), inflammatory process (CRP), and diabetes mellitus
In the age- and gender-adjusted model, the OR for patients in the lowest 25OHD and 1,25(OH)2D category was non-significantly and significantly higher, namely 1.77 (95%CI: 0.96–3.28; P = 0.071) and 3.97 (95%CI: 1.74–4.18; P<0.001), compared with the respective reference group. In the fully adjusted model, the OR for patients in the lowest 25OHD category was attenuated and did not differ significantly from the reference category. The ORs remained however significantly higher for the patients in the two lowest 1,25(OH)2D categories, compared with the highest 1,25(OH)2D category (Table 3). Results did not differ substantially if only non-diabetes patients (n = 2484) or patients ≥ 70 years (n = 1887) were included in the data analysis (S1 and S2 Tables). Similar results were also obtained in sensitivity analyses, when 25OHD levels < 50 nmol/l were considered as lowest 25OHD category or 1,25(OH)2D levels were divided into tertiles. In detail, the fully adjusted OR for patients in the 25OHD category < 50 nmol/l was 1.44 (95%CI: 0.78–2.67; P = 0.244), compared with the reference category. Regarding 1,25(OH)2D, the OR for patients in the lowest tertile was 2.06 (95%CI: 1.36–3.10; P = 0.001), compared with the highest tertile. If all cases of infection (n = 430) were considered in the statistical analysis, the ORs for the lowest 25OHD category and lowest 1,25(OH)2D quintile was 1.32 (95%CI: 0.87–2.00; P = 0.197) and 2.09 (95%CI: 1.46–3.02; P<0.001), compared with the respective reference category. If only patients with thoracic wound infection were compared with non-infected patients, the ORs for the lowest 25OHD category and lowest 1,25(OH)2D quintile was 2.47 (95%CI: 0.76–8.10; P = 0.134) and 4.12 (95%CI: 1.73–9.80; P = 0.001), compared with the respective reference category.
Discussion
Our data support earlier findings [12] that despite antibiotics prophylaxis, postoperative infection remains a significant complication in cardiac surgical patients. To improve prophylaxis, the use of second- or third-generation cephalosporins as well as prophylaxis prolongation up to 48 h post-operatively has been recommended in cardiac surgical patients [12]. However, the broad spectrum of different pathogens and infections in our study cast doubt that this strategy would completely solve the problem of postoperative infection.
In our study, low 1,25(OH)2D levels were independently associated with an increased risk of a composite of clinically relevant infections. The risk was lowest in patients with circulating 1,25(OH)2D levels above 81.0 pmol/l. No such association was observed for circulating 25OHD levels. Thus, our data do not support a systematic review and meta-analysis of observational studies that found a significantly increased risk of infection and sepsis in critically ill patients with circulating 25OHD levels below 50 nmol/l [23]. However, it is noteworthy that the meta-analysis reported risk ratios for infection and sepsis of 1.49 (95%CI: 1.12–1.99) and 1.46 (95%CI: 1.27–1.68), respectively, which are on average very similar to the non-significantly higher OR we observed in the sensitivity analysis of our study, compared with adequate 25OHD levels. Thus, our data do not definitively rule out a higher risk of infection at insufficient or deficient 25OHD levels. With respect to 1,25(OH)2D, our results support data of a small earlier study in cardiac surgical patients [24]. In that investigation, lower circulating 1,25(OH)2D levels were significantly associated with higher risk of a composite of low cardiac output syndrome, infection, or in-hospital death. Notably, infection was the most prevalent complication (n = 4) among the 7 postoperative events that occurred.
Experimental studies provide insights into potential mechanisms of 1,25(OH)2D-mediated effects on the immune system: vitamin D receptors are expressed in monocytes and these cells differentiate into macrophages under the influence of 1,25(OH)2D [25]. Macrophages express their own 1α-hydroxylase isoenzyme which intracellularly synthesizes 1,25(OH)2D from its precursor 25OHD [26]. 1,25(OH)2D directly and indirectly regulates the expression of important antimicrobial proteins, such as cathelicidin and defensins [27,28], and of lysosomal enzymes and reactive oxygen species like nitric oxide [29]. The combination of antimicrobial peptides and oxygen species may destroy intracellular viruses, fungi, and bacteria in the autolysosomes. Notably, monocytic cathelicidin production is reduced in individuals with low 25OHD and 1,25(OH)2D levels [30].
Normalizing tissue 25OHD may be necessary for providing adequate amounts of substrate for local tissue production of 1,25(OH)2D [31]. Interestingly, in patients with low circulating 1,25(OH)2D levels administration of 1,25(OH)2D can increase 25OHD uptake in monocytes [32], suggesting that adequate circulating 1,25(OH)2D levels are necessary for sufficient 25OHD availability in vitamin D target cells.
Usually, 1,25(OH)2D synthesis is suppressed by low substrate availability, e.g. deficient 25OHD levels [15]: In children and young female adults, for instance, an increase in circulating 25OHD of 10 nmol/l was associated with an increase in circulating 1,25(OH)2D of approximately 5 pmol/l [33,34]. In patients of the CALCITOP study, however, the corresponding increase in circulating 1,25(OH)2D levels was only 0.7 pmol/l [21], whereas several clinical parameters such as kidney function, EuroSCORE (a surgical risk score), diabetes, CRP, and diuretic use were inversely correlated with circulating 1,25(OH)2D levels in these patients. Similarly, in heart transplant recipients kidney function and inflammatory parameters were much more predictive of circulating 1,25(OH)2D levels than circulating 25OHD levels [35]. These differences between healthy individuals and cardiac surgical patients may at least in part explain why vitamin D supplements have beneficial effects on upper respiratory tract infection in otherwise healthy individuals [14,15], whereas deficient 25OHD levels (as an indicator of nutritional vitamin D status) were not significantly associated with infection in the present study. Consequently, we should not be too enthusiastic to believe that in the clinical setting simple vitamin D supplementation would be able to restore all vitamin D-related derangements.
It may well be that in our fully adjusted statistical model the association between low circulating 1,25(OH)2D levels and postoperative infections was underestimated by the fact that adjustments were made for those parameters which are related to both clinical outcome and circulating 1,25(OH)2D levels. Circulating 1,25(OH)2D is related to eGFR, even in individuals without chronic kidney disease [36]. As mentioned before, in the patients of the CALCITOP study 1,25(OH)2D was also inversely interrelated with EuroScore, diabetes and CRP values [21]. Therefore, we cannot definitively rule out that our study results are biased by over-adjustment. Of note, our data indicate a stronger association of circulating 1,25(OH)2D with the risk of infection in models not adjusted for the aforementioned parameters.
Our study has several strengths, such as the large number of included patients, the availability of data on both 25OHD and 1,25(OH)2D levels, and the short follow-up period. However, some limitations also have to be addressed. First, we do not have exact data on postoperative antibiotics use in our study cohort. Second, no data on circulating postoperative 1,25(OH)2D levels were available in our study. Earlier investigations could demonstrate a significant transient cardiac surgery-related decline in circulating 1,25(OH)2D [24], which may have contributed to the increased risk of infections. Postoperative concentrations were, however, consistently lower in patients with relatively low preoperative 1,25(OH)2D levels than in patients with relatively high preoperative levels of this vitamin D metabolite [24], suggesting that the preoperative 1,25(OH)2D level is also indicative for the postoperative 1,25(OH)2D level of a patient. Third, no subgroup analyses regarding the association of vitamin D with gram positive bacteria, gram negative bacteria, or fungi could be performed, because many patients were simultaneously infected with two or three of these major groups of pathogens.
Finally, there is evidence for novel pathways of vitamin D3 metabolism initiated by CYP11A1 [37,38], indicating that vitamin D action is not only mediated by the sequence vitamin D3 → 25OHD3 → 1,25(OH)2D3, but also by other metabolites which were not analyzed in this study.
In conclusion, our data indicate an independent association of low circulating 1,25(OH)2D levels with the risk of postoperative infections in cardiac surgical patients, whereas 25OHD levels were not significantly related to the risk of infection. Future studies should therefore pay more attention on circulating 1,25(OH)2D, its regulation, and clinical relevance.
Supporting Information
(DOCX)
(DOCX)
Data Availability
This study analyzes quantitative data for which the participants did not consent to have their full data made publicly available. For requests contact Armin Zittermann at azittermann@hdz-nrw.de.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. Prevention of hospital-aquired infections, WHO/CDS/CSR, EPH, 2002.12. http://apps.who.int/medicinedocs/documents/s16355e/s16355e.pdf (assessed January 2016)
- 2.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296: 2460–6. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG Jr, O'Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112: I358–65. [DOI] [PubMed] [Google Scholar]
- 4.Kreter B, Woods M. Antibiotic prophylaxis for cardiothoracic operations. Meta-analysis of thirty years of clinical trials. J Thorac Cardiovasc Surg. 1992;104: 590–9. [PubMed] [Google Scholar]
- 5.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol. 2004;44: e213–e310. [DOI] [PubMed] [Google Scholar]
- 6.Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR; Society of Thoracic Surgeons. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part I: Duration. Ann Thorac Surg. 2006;81: 397–404. [DOI] [PubMed] [Google Scholar]
- 7.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20: 250–78. [DOI] [PubMed] [Google Scholar]
- 8.Bratzler DW, Houck PM, Surgical Infection Prevention Guidelines Writers Workgroup, American Academy of Orthopaedic Surgeons, American Association of Critical Care Nurses, American Association of Nurse Anesthetists, et al. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38: 1706–15. [DOI] [PubMed] [Google Scholar]
- 9.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, et al. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg. 2007;83: 1569–76. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. 2008. http://www.nice.org.uk/nicemedia/live/11743/42378/42378.pdf (assessed 5 January 2016)
- 11.Scottish Intercollegiate Guidelines Network. Antibiotic Prophylaxis in Surgery: A National Clinical Guideline. 2008. Updated 2014. http://www.sign.ac.uk/pdf/sign104.pdf (assessed January 2016)
- 12.Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, et al. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67: 541–50. 10.1093/jac/dkr470 [DOI] [PubMed] [Google Scholar]
- 13.Zittermann A, Gummert JF. Nonclassical vitamin D action. Nutrients. 2010;2: 408–25. 10.3390/nu2040408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman P, Lindh AU, Björkhem-Bergman L, Lindh JD. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 2013;8: e65835 10.1371/journal.pone.0065835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zittermann A, Pilz S, Hoffmann H, März W. Vitamin D and airway infections: a European perspective. Eur J Med Res. 2016;21: 14 10.1186/s40001-016-0208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2007;36: 574–7. [DOI] [PubMed] [Google Scholar]
- 17.Tran B, Armstrong BK, Ebeling PR, English DR, Kimlin MG, van der Pols JC, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. 2014;99: 156–61. 10.3945/ajcn.113.063271 [DOI] [PubMed] [Google Scholar]
- 18.Zittermann A, Kuhn J, Dreier J, Knabbe C, Gummert JF, Börgermann J. Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur Heart J. 2013;34: 1358–64. 10.1093/eurheartj/ehs468 [DOI] [PubMed] [Google Scholar]
- 19.Turan A, Grady M, You J, Mascha EJ, Keeyapaj W, Komatsu R, et al. Low vitamin D concentration is not associated with increased mortality and morbidity after cardiac surgery. PLoS One. 2013;8: e63831 10.1371/journal.pone.0063831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun LA, Spitzer O, Levkovich B, Bailey M, Stanguts C, Hose L, et al. Prevalence of vitamin D deficiency prior to cardiothoracic surgery. Heart Lung Circ. 2014;23: 978–80. 10.1016/j.hlc.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 21.Zittermann A, Kuhn J, Ernst JB, Becker T, Dreier J, Knabbe C, et al. 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and postoperative outcome in cardiac surgery. J Clin Endocrinol Metab. 2015;100: 72–80. 10.1210/jc.2014-3013 [DOI] [PubMed] [Google Scholar]
- 22.Roodnat JI, Mulder PG, Tielens ET, van Riemsdijk IC, van Gelder T, Weimar W. The Cox proportional hazards analysis in words: Examples in the renal transplantation field. Transplantation. 2004;77: 483–8. [DOI] [PubMed] [Google Scholar]
- 23.de Haan K, Groeneveld AB, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. 2014;18: 660 10.1186/s13054-014-0660-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Börgermann J, Lazouski K, Kuhn J, Dreier J, Schmidt M, Gilis-Januszewski T, et al. 1,25-Dihydroxyvitamin D fluctuations in cardiac surgery are related to age and clinical outcome. Crit Care Med. 2012;40: 2073–81. 10.1097/CCM.0b013e31824e8c42 [DOI] [PubMed] [Google Scholar]
- 25.Provvedini DM, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 promotes in vitro morphologic and enzymatic changes in normal human monocytes consistent with their differentiation into macrophages. Bone. 1986;7: 23–8. [DOI] [PubMed] [Google Scholar]
- 26.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89: 552–72. [DOI] [PubMed] [Google Scholar]
- 27.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12: 388–90. [DOI] [PubMed] [Google Scholar]
- 28.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311: 1770–3. [DOI] [PubMed] [Google Scholar]
- 29.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276: 35482–93. [DOI] [PubMed] [Google Scholar]
- 30.Dini C, Bianchi A. The potential role of vitamin D for prevention and treatment of tuberculosis and infectious diseases. Ann Ist Super Sanita. 2012;48: 319–27. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diab. 2002;9: 87–98. [Google Scholar]
- 32.Gallieni M, Kamimura S, Ahmed A, Bravo E, Delmez J, Slatopolsky E, et al. Kinetics of monocyte 1 alpha-hydroxylase in renal failure. Am J Physiol. 1995;268: F746–53. [DOI] [PubMed] [Google Scholar]
- 33.Zittermann A, Scheld K, Stehle P. Seasonal variations in vitamin D status and calcium absorption do not influence bone turnover in young women. Eur J Clin Nutr. 1998;52: 501–6. [DOI] [PubMed] [Google Scholar]
- 34.Docio S, Riancho JA, Pérez A, Olmos JM, Amado JA, González-Macías J. Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res. 1998;13: 544–8. [DOI] [PubMed] [Google Scholar]
- 35.Zittermann A, Schleithoff SS, Götting C, Fuchs U, Kuhn J, Kleesiek K, et al. Calcitriol deficiency and 1-year mortality in cardiac transplant recipients. Transplantation. 2009;87: 118–24. 10.1097/TP.0b013e31818c2708 [DOI] [PubMed] [Google Scholar]
- 36.Zittermann A, Schulz U, Lazouski K, Fuchs U, Gummert JF, Börgermann J. Association between glomerular filtration rate and 1,25-dihydroxyvitamin D in cardiac surgery. Scand Cardiovasc J. 2012;46: 359–65. 10.3109/14017431.2012.725478 [DOI] [PubMed] [Google Scholar]
- 37.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN et al. In vivo evidence for a novel pathway of vitamin D₃ metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012; 26: 3901–15. 10.1096/fj.12-208975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015; 5:14875 10.1038/srep14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
This study analyzes quantitative data for which the participants did not consent to have their full data made publicly available. For requests contact Armin Zittermann at azittermann@hdz-nrw.de.