Abstract
In experiments conducted over 60 years ago, the lateral hypothalamic area (LHA) was identified as a critical neuroanatomical substrate for motivated behavior. Electrical stimulation of the LHA induces voracious feeding even in non-restricted animals. In the absence of food, animals will work tirelessly, often lever-pressing 1000’s of times per hour, for electrical stimulation at the same site that provokes feeding, drinking, and other species-typical motivated behaviors. Here we review the classic findings from electrical stimulation studies and integrate them with more recent work that has utilized contemporary circuit-based approaches to study the LHA. We identify specific anatomically and molecularly defined LHA elements that integrate diverse information arising from cortical, extended amygdala, and basal forebrain networks to ultimately generate a highly specified and invigorated behavioral state conveyed via LHA projections to downstream reward and feeding specific circuits.
The hypothalamus, while accounting for only ~3% of brain tissue, has direct control over essential homeostatic functions and primitive behavioral states. The hypothalamus can readily be divided based on gene expression1–4, function5, or classical anatomical boundaries6–8, but a large portion of the hypothalamus consists of an extended field of neurons and fibers with substantially less anatomical definition9–11 referred to as the lateral hypothalamic area (LHA). As studies continue to uncover the precise circuitry and cellular phenotypes within the LHA that encode and orchestrate behavior, it is important to revisit many of the well-described findings using classical anatomical and electrical stimulation methods previously used to elucidate LHA function. In this article, we review some of these seminal findings from the 1950’s–80’s, and integrate them with more recent discoveries utilizing optogenetic neurocircuit approaches. A holistic synthesis of these findings paints an emerging picture of multiple, well-defined neurocircuit elements, embedded within the LHA, that interface with downstream systems to ultimately generate specific motivational and actionable states.
Classic experiments on LHA function
The LHA is a richly heterogeneous structure residing posterior to the preoptic area and anterior to the ventral tegmental area. The LHA contains a number of genetically distinct cell populations (for review see12) and forms a bed nucleus through which the fibers of the medial forebrain13 bundle pass. Lesion studies conducted in the 1940’s – 1980’s described the effects of electrolytic or chemical ablation of the LHA and subsequent effects on feeding and drinking. This work collectively demonstrated the importance of the LHA for homeostatic physiology and behavior. Electrolytic lesions of the LHA suppresses feeding14, and drinking15 while lesioning of the nearby VMH promotes feeding and body weight gain16. Later studies that utilized chemical lesions to destroy catecholaminergic fibers containing either norepinephrine17 or dopamine18 demonstrated that these fibers of passage contained within the median forebrain bundle are important components controlling feeding and drinking. Anand and Brobeck14 suggested that fibers of passage, but also fibers of origin from somata distributed throughout the LHA are also important for controlling feeding. Chemical lesions that ablate LHA somata but spare passing fibers also suppressed feeding and drinking19–21. The pioneering early studies that utilized electrical stimulation of the LHA in rodents showed that gross electrical activation of this region produces voracious feeding behavior22, as well as reinforced lever-pressing behavior to gain additional stimulation23, (Fig. 1). This suggests that the LHA and associated brain regions are not only critical for feeding and other drive-like effects, but also reinforcement processes24,25. Intra-LHA injection of neurotransmitter agonists or antagonists further demonstrated that glutamate receptor activation can also induce feeding26, while GABA agonist can suppress it27. These studies show that modulation of neurotransmission within the LHA can generate feeding responses similar to those observed following electrical stimulation or lesions. However, it is worth noting that electrical stimulation of the LHA can become aversive if its intensity is too strong or its duration too long28,29. Additionally, electrical stimulation at sites in this and adjacent levels of the medial forebrain bundle motivate a variety of species-typical behaviors–e.g., feeding, drinking, copulation, gnawing and nest building30–32, in addition to reinforcement.
Figure 1. Electrical stimulation of the LHA produces reinforcement.
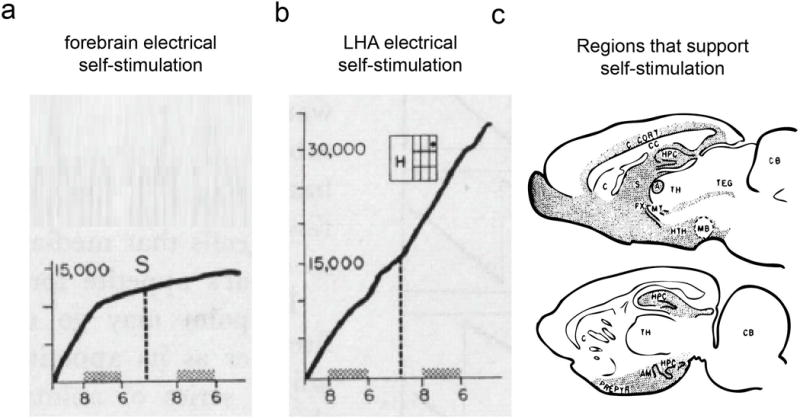
a. Animals will self-stimulate in many regions of the ventral forebrain, but only the LHA electrical self-stimulation is largely insatiable (b). c. Illustration showing that the forebrain and hypothalamus sites (shaded) that supports electrical self-stimulation. Adapted from150.
Lateral hypothalamic electrical stimulation
Electrical stimulation of the LHA and other portions of the medial forebrain bundle can motivate a variety of species-typical, biologically primitive behavior patterns including eating, drinking, and gnawing in sated animals6,32,33. With respect to feeding and copulation, there seems to be clear anatomical separation between systems24,30. For feeding, gnawing, and predatory attack, the systems appear to overlap32,34. Because the LHA had been implicated in feeding and drinking by lesion studies as described above14, a good deal of attention was paid to the motivational effects of lateral hypothalamic stimulation. Such stimulation does not elicit specific motor responses, but rather establishes a state of heightened responsiveness to a variety of environmental stimuli. Stimulation in this region might produce different reactions in animals such as feeding in one animal, drinking in another, gnawing of wood in another, or predatory attack in yet another31,32,35. These differences are not due to differences in stimulation region within the LHA36, but rather are the result of response patterns that develop during the stimulation trials37,38. That is, responsiveness to a given goal object increases with repeated stimulation trials38 and the dominant response of a given animal can change as a function of what goal objects are offered32,37. In the case of feeding and drinking, the animal behaves in much the same way it would under food and water deprivation. First, electrical LHA stimulation motivates the learning of food-reinforced instrumental responses35,39,40 as well as the performance of such responses as were previously learned under conditions of deprivation41. The LHA evoked feeding is also influenced by unconditioned42 and conditioned taste aversions43. In cats, LHA stimulation appears to motivate goal-directed behavior involving response sequences such as, in the case of predatory attack, visual stalking, approach, pouncing and bringing the prey to the mouth, mouth opening, and, finally, snapping shut of the mouth44. Each act in the sequence has its own environmental triggering stimulus, and the effect of stimulation is to make the animal more responsive to the triggering stimulus45,46. The behavior observed in a given experiment depends to a great extent on what stimuli are available for interaction in the testing chamber37. This suggests that the activated substrate is more a general arousal system than a set of specific motivational pathways. Against this view are the findings that stimulation at different points along the medial forebrain bundle are differentially sensitive to modulation by food restriction and leptin (LHA at the A-P level of the ventromedial nucleus)47,48 on the one hand and testosterone (posterior hypothalamic MFB)47,48 on the other, that stimulation-induced eating and drinking are preferentially responsive to low and high (respectively) stimulation frequencies49. Morgane suggested that even the feeding response results from activation of two LHA “hunger-motivational” subsystems, one slightly lateral to the other50. Because electrical stimulation activates neurons near the electrode tip rather indiscriminately51, and because 50 or more fiber systems share this region, the question of one or multiple systems has not been resolved by electrical stimulations studies.
Whereas the effects of lesions and stimulation led to the labeling of the LHA as a “hunger system”50 a “feeding center”14 and a “drinking center”52, the discovery that stimulation of this region was rewarding led also to the label of a “pleasure center”53. The fact that rats would work for stimulation of a brain region where stimulation appeared to make them hungry24,25,54 was termed the “drive-reward” paradox and raised the issue of whether a single arousal system or two independent systems mediated the drive-like effects and the rewarding effects of the stimulation55.
Pharmacological studies of stimulation-induced feeding56 and LHA brain stimulation reward57–61 suggested an important role for the forebrain-projecting midbrain dopamine systems. However, parametric studies of brain stimulation reward soon falsified the hypothesis that the rewarding effects of stimulation were primarily due to the depolarization, at the electrode tip, of dopaminergic fibers of passage. First, the dopamine system was insensitive to changes of stimulation frequency over the range that altered the rewarding impact of stimulation62. Paired pulse studies showed that the refractory periods for the directly stimulated “first stage” fibers (the fibers depolarized at the electrode tip) were too fast to reflect direct activation of the ascending dopamine fiber system63,64. At least two sub-populations were implicated, one of which was undefined and one was an ultra-fast sub-population that was sensitive to cholinergic receptor blockade65; each, however, appeared to contribute to both the feeding effect and the rewarding effect of LHA electrical stimulation.
Dual electrode paired-pulse studies followed and showed that despite the fact that the LHA and VTA were connected by reward-relevant fibers, their conduction velocities were, like the refractory periods, too fast to reflect a significant contribution of the small unmyelinated dopaminergic fiber system66. Finally, by challenging the effects of cathodal stimulation at one level with anodal stimulation at another, Bielajew and Shizgal showed that the bulk of the reward-relevant fibers of the LHA project caudally, toward, not away from, the ventral tegmental area (VTA)67. Subsequent studies showed axonal connectivity between the lateral preoptic area and the VTA, suggesting that the reinforcing effects of lateral hypothalamic stimulation were likely due to activation of descending fibers of passage originating in or rostral to the anterior hypothalamus68. Taken together, these studies suggested that brain stimulation reward resulted from activation of descending medial forebrain fibers of passage that activated, directly69 or indirectly70, the VTA dopaminergic system that had been implicated not only in brain stimulation reward but also in the rewarding effects of food71 and psychomotor stimulants72,73.
The paired-pulse parametric techniques that were developed to characterize the substrate of brain stimulation reward were also used in studies to characterize the substrate of stimulation-induced feeding and to explore whether a common substrate might mediate stimulation-induced feeding and reward. As in the case of LHA brain stimulation reward65, in the case of stimulation-induced feeding there again appeared to be two non-overlapping sub-populations of contributing first-stage fibers: an ultrafast subpopulation with refractory periods between 0.4 and 0.6 msec, and a non-overlapping slower subpopulation with refractory periods between 0.7 and approximately 2.0 msec74. Stimulation-induced feeding was induced by stimulation not only of the LHA but also by stimulation of the VTA and intervening levels of the medial forebrain bundle; and evidence for the same non-overlapping sub-populations of first-stage fibers was seen at each of these levels. Single electrode refractory period findings suggested that both effects were mediated by activation of two sub-populations of ultrafast and fast fibers of the medial forebrain bundle that extended at least from the lateral hypothalamus to the region of origin of the mesolimbic dopamine system.
Dual electrode studies suggested further evidence for a common substrate or substrates. Here, one electrode was aimed at the LHA and another was aimed more posterior at the VTA. The findings concluded that stimulation induced feeding and reward were each found with both placements The two electrodes were thus inferred to be aligned along the path of the same axons whenever the effects of stimulation at one electrode cancelled the effects of stimulation of the other at short inter-pulse intervals. In those cases where alignment was found for stimulation-induced feeding, it was also found for reward. As with the refractory periods for the fibers mediating the two behaviors, the conduction velocities were very similar75. These findings do not rule out the possibility that different subsets of fibers are involved in the two responses to stimulation, but the common sites, trajectories, refractory period distributions and conduction velocities continue to point to the possibility of a common neural substrate.
Molecular phenotypes and functions of LHA neurons
The LHA encompasses a plethora of genetically and functionally distinct cell types that utilize various signaling modalities, including various neurotransmitters and neuropeptides76–83. Vesicular glutamate transporter type 2 (Vglut2; a marker for glutamate neurons) mRNA expression is abundant in the LHA78,84,85 (Fig. 2), suggesting that numerous LHA neuronal subpopulations synthesize the excitatory neurotransmitter, glutamate. In addition to glutamate, the LHA is enriched with GABAergic neuronal markers82,84,86,87 (Fig. 2), which are largely segregated from Vglut2-expressing LHA cells85. Some LHA neurons also produce several important neuropeptides, including orexin/hypocretin (Orx), melanin-concentrating hormone (MCH), neurotensin (Nts), and galanin (GAL). While these neuropeptide expressing cell populations likely play an important role in regulating feeding and reward (see below), it is worth noting that some of these cells groups not only regulate feeding, but also metabolism, likely through different circuits88.
Figure 2. The LHA contains a mixture of inhibitory and excitatory neurons.
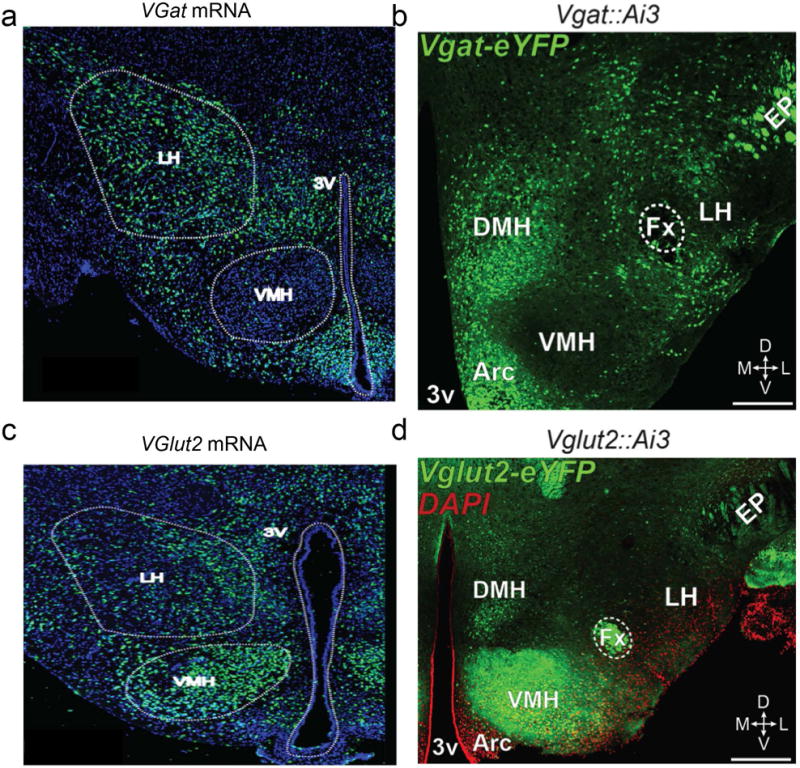
a. In situ hybridization image of LHA Vgat expression. b. Vgat targeted neurons in the Vgat-ires-Cre mouse line. c. In situ hybridization image of LHA Vglut2 expression. d. Vglut2-targeted neurons in the Vglut2-ires-Cre mouse line.
Neurons that synthesize and release the neuropeptide orexin/hypocretin89 (~3,500 – 5,000 total in rodents)90 are restricted to the LHA and also have been reported to express Vglut284. Orx neurons are thought to primarily regulate arousal, but also feeding and reward-related behaviors. Consistent with this, injections of the peptide into the lateral ventricle increases food intake91, while Orx receptor antagonists and genetic removal of Orx decrease consumption92. Furthermore, chemical activation of Orx cells as well as infusions of the peptide into the VTA, an anatomical target of LHA Orx neurons, reinstates drug-and food-seeking behaviors93. However, these neurons are also heavily involved with arousal, as optogenetic stimulations of Orx neuron increases wakefulness94, while genetic ablation of the cells causes narcolepsy95. Therefore, they are likely a contributor, but not primary determinant of motivated behavioral output mediated by the LHA. For further review of Orx neuronal function see96.
Melanin-concentrating hormone (MCH) producing neurons are also predominantly found in the LHA, project widely throughout the brain, and are distinct from Orx neurons1,97,98. Some MCH neurons express markers for GABA (glutamatic acid decarboxylase; GAD67) while others express markers for glutamate (Vglut2)98–100, suggesting that MCH neurons are composed of subsets of inhibitory and excitatory cells. MCH neurons have also been implicated in the regulation of feeding and sleep-wakefulness balance. Intracerebroventricular injections of the peptide increases feeding and body weight in rodents101. Further, recent genetic studies revealed that overexpression of MCH results in hyperphagia and obesity102, while mice lacking MCH neurons or MCH are hypophagic and lean103,104. In contrast to Orx neurons, activation of MCH neurons promotes REM sleep100, consistent with an opposing role of these cells to Orx neurons in controlling arousal states. For additional reviews of the neurocircuitry of Orx and MCH LHA neurons see5,105. Collectively, these studies demonstrate a role for LHA ORX and MCH neurons in regulating arousal and sleep in addition to feeding and body weight. Thus, and interesting possibility is that the feeding phenotypes associated with these cell types are more related to an animals natural behavioral patterns that would normally occur in particular states of arousal.
A separate neuropeptide-containing cell population concentrated in the preoptic and anterior hypothalamic region but overlapping with the LHA106, Neurotensin (Nts) producing neurons, have been hypothesized to be involved with negative energy balance. Peripheral and central administration of Nts suppresses feeding107, and both the genetic ablation of a subset of Nts neurons, as well as the removal of the Nts receptor (NTR1), result in hyperphagia and obesity108,109. Nts neurons highly co-localize with galanin expressing neurons (~95% overlap), but not with MCH and Orx cells82. Interestingly, LHA neurons that express vesicular GABA transporters (Vgat-ires-Cre)110 show little to no co-localization with neurons that are immunopositive for either MCH or Orx111 (Fig. 3). This suggests at least some of the LHA neurons that have been previously targeted for manipulation in the Vgat-ires-Cre line may also be Nts expressing neurons, although this will need to be fully investigated in future studies.
Figure 3. Vgat-targeted neurons are distinct from MCH and Orexin producing LHA neurons.
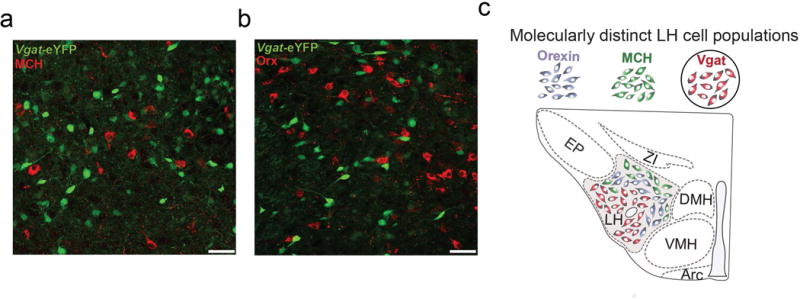
a. YFP expressing Vgat neurons (green) and MCH immunopositive neurons (red) in the LHA. b. YFP expressing Vgat neurons (green) and Orexin immunopositive neurons (red) in the LHA. c. VGat target LHA neurons thus represent a distinct population of LHA cells that mediate feeding. Data adapted from111.
Optogenetic studies to delineate LHA function
The introduction of optogenetic stimulation methods has provided a powerful new tool for identifying the substrates of motivation and reward. Electrical stimulation preferentially activates fibers of passage and does not differentiate between fibers from arising at the stimulation site and fibers of passage with distal origins51. Electrical stimulation allows only crude differentiation of different fiber types and provides little information as to the type of fibers activated. Optogenetic techniques make it possible to activate only fibers of origin from a confirmed cell group of interest and to trace and selectively activate only the fibers that arise from that cell group and project to or through a given target area. Whereas electrical stimulation activates a set of fibers by causing the opening of cation channels that are voltage-sensitive and expressed in the membranes of all neuronal elements, optogenetic stimulation activates fibers by causing the opening of cation channels that are light sensitive and that are expressed only by neurons originating in a particular brain regions and expressing particular gene used to at least partially delineate a cellular phenotype. Thus, optogenetic studies have already begun to unravel which of the numerous molecularly defined neuronal fibers that originate in or pass through the LHA13 contribute to motivational and reward function. Cell-type specific optogenetic targeting approaches have begun to ascribe functional roles for distinct LHA populations for orchestrating feeding and reward.
Direct optogenetic activation of VGat expressing LHA neurons produces voracious feeding and optical self-stimulation behavior111, a phenotype that is strikingly reminiscent of that seen with electrical stimulation of the LHA24,25. Interestingly, optogenetic stimulation of VGlut2 expressing LHA neurons has the opposite effect; it reduces feeding in hungry mice, as well as producing an aversion to locations where stimulation of these cells occurs112. Consistent with the idea that VGat and VGlut2 expressing LHA neurons exert opposing behavioral effects, selective genetic ablation of VGat expressing LHA neurons reduces feeding, body weight gain, and motivation to obtain palatable caloric rewards111, while ablation of VGlut2 expressing LHA neurons enhances both feeding and body weight gain113. Thus, perhaps Vgat and Vglut2 expressing LHA neurons produce a bidirectional output signal, which is then directly and indirectly conveyed to VTA dopamine neurons to homeostatically invigorate behavioral output (Fig. 4). Second, complex, but reoccurring environmental representations are likely encoded in upstream cellular networks in the cortex and hippocampus, which in turn convey representational information to LHA neuronal circuits. However, in order to mechanistically understand how LHA signals are processed; first consider the neural circuit input architecture.
Figure 4. Proposed neurocircuit-wiring diagram based on optogenetic studies.
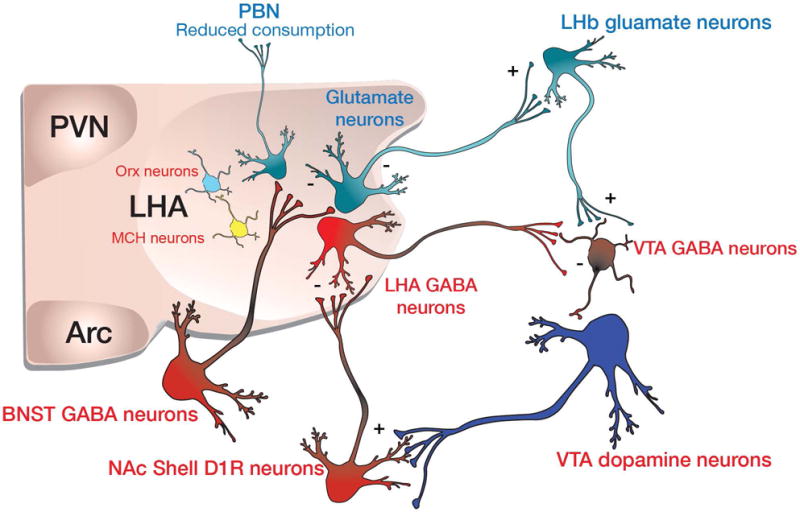
LHA GABAergic neurons inhibit VTA GABAergic neurons to disinhibit VTA dopamine neurons. Dopamine is release within the NAc where it excites D1R expressing MSNs and induces plasticity. These inhibitory signals then feedbacks to inhibit LHA GABAergic neurons to terminate feeding bouts. BNST GABAergic neurons preferentially inhibit LHA Glutamate neurons, some of which may project to the lateral habenula.
Input circuitry to the LHA
The LHA receives multiple excitatory and inhibitory inputs from both cortical and subcortical structures. Direct, electrical stimulation of the medial prefrontal cortex produces many distinct mono and polysynaptic activity patterns in LHA neurons114. Monosynaptic excitatory fibers arriving via the fornix, also likely provide important hippocampal information related to the ongoing processing of space and context115. Inhibitory GABAergic subcortical fibers innervate the LHA from the lateral septum116 and much of the basal forebrain and extended amygdala including the nucleus accumbens shell117,118, the BNST/preoptic area112, the ventral pallidum119 and nucleus basalis/substantia innominata120. Midbrain and brainstem inputs to the LHA are more sparse but arise from classical processing centers of autonomic function including the parabrachial nucleus and periaqueductal grey121. Neuromodulators including dopamine, norepinephrine122, and serotonin123, are also released within the LHA where they can act to further sculpt circuit dynamics. Furthermore, intra-hypothalamic connectivity providing input to the LHA from regions such as the arcuate nucleus1,124, periventricular hypothalamus125, and ventral medial hypothalamus126 have also been described. Importantly, optogenetic stimulation of ArcuateAGRP-LHA or PVHGABA-LHA pathways are capable of evoking feeding behavior124,125. Collectively, these findings suggest that arcuate nucleus circuitry directly controls homeostatic feeding in response to energetic demands while LHA circuits drive compulsive and/or hedonic feeding, due to the tight linkage to the VTA reward circuitry.
The functional input architecture from regions of the extended amygdala that interface with definable LHA neurons is beginning to emerge. GABAergic neurons from the ventral BNST and related structures send monosynaptic inputs that preferentially inhibit postsynaptic LHA glutamate neurons (Fig. 4). Direct optogenetic stimulation of the vBNSTGABA-LHAGlutamate circuit produces robust feeding behavior that is initiated rapidly, correlated with stimulation frequency112, and directed towards the most palatable, calorically dense foods available. Furthermore, mice readily engage in optical self-stimulation of this circuit, and self-stimulation output is strongly modulated by food deprivation or satiety states112, consistent with a dual role of the LHA to orchestrate both motivation and feeding behaviors. Inhibitory input to the LHA from the nucleus accumbens shell arises from both D1 and D2 expressing medium spiny projection neurons (O’Connor et al., in press,117,118) (Fig. 4). Functionally, the importance of this pathway was first described by Ann Kelley and colleagues who reported that AMPA receptor antagonism or GABA-mediated inhibition in the NAc shell elicits feeding that is dependent on the LHA127–129. Circuit input from the NAc shell to the LHA was recently investigated in more detail (O’Connor et al., in press). The majority of NAc shell MSNs that project to the LHA are D1 expressing MSNs, with only a minority arising from the D2R expressing MSN population. Fibers from D1R expressing MSNs innervate the more ventral lateral aspects of the LHA where they functionally target LHA GABAergic neurons, and not Orexin or MCH producing neurons. Optogenetic stimulation of the NAcshellD1R-LHAGABA pathway suppresses licking for a palatable reward, while optogenetic inhibition of postsynaptic LHAGABA neurons suppresses consumption of food130. Collectivity, it appears likely that distinct subcircuits arising from various extended amygdala and neighboring structures provide inhibitory input that preferentially targets molecularly distinct LHA postsynaptic neurons to regulate feeding and reward (Fig. 4).
Output circuitry of the LHA
Given that multiple populations of functionally and genetically classifiable neurons exist in the LHA, it is also of importance to consider the projection target structures of these cells. Some of the most well described outputs from classical anatomy studies demonstrated the existence of multiple projection specific outputs to brain regions such as the VTA, periventricular thalamus, lateral habenula, and many others131,132. A recent study by Nieh et al., demonstrated that both glutamatergic and GABAergic LHA fibers functionally innervate both VTA GABA neurons and VTA dopamine neurons133. It seems likely that these inhibitory fibers may preferentially innervate VTA GABA neurons, as does the pathway from the BNST to the VTA,134 as optogenetic stimulation of LHGABA-VTA pathway also produces feeding behavior133, and as mice will readily engage in optical self-stimulation of this pathway135. This could occur by transiently increasing VTA dopaminergic neuronal activity via a dis-inhibitory mechanism to thus control motivation (Fig. 4). Consistent with this, brief optogenetic stimulation of VTA GABAergic neurons suppresses cue-evoked licking for a caloric reward136 and is aversive137.
In addition to the glutamatergic projection to the VTA, Vglut2-expressing neurons in the LHA also project to the lateral habenula to excite LHb neurons that likely project to VTA/RMTg GABAergic neurons, which in turn can inhibit VTA dopamine neurons113,138,139 (Fig. 4). Consistent with this, optogenetic inhibition of the LHAVglut2-LHb pathway enhances licking for a caloric reward and produces aversion when optogenetically activated113. Intriguingly, optogenetic stimulation of glutamatergic projections from the neighboring endopeducular nucleus (EP) is also aversive140, suggesting that glutamatergic neuronal populations in the LHA, the zona inserta, and EP may share a common behavioral/circuit function. In our studies, we have also observed substantially weaker LHb innervation from LHA GABAergic neurons113. However, LHAVGat neurons appear to innervate midline thalamic structures just ventral to the LHb such as the periventricular thalamus (PVT), a brain region shown to produce GABA-mediated feeding141. Both LHA GABAergic and glutamatergic neurons project heavily to the parabrachial nucleus (PBN). While it is still functionally untested, it seems feasible that LHA excitatory and inhibitory signals can also tune PBN circuits shown to play an important role in regulating feeding and taste aversion142,143. In addition, LHA projections to the arcuate nucleus have also been documented144, which could likely play an important role in generating feeding. Clearly, additional studies are required to more fully elucidate the functional wiring output of the LHA (Fig. 4). It is also worth noting that a dearth of Cre-driver mouse lines exist to selectively parcel out LHA cellular function. Furthermore, as this circuit architecture is deduced, we can begin to consider the dynamic nature of these systems during ongoing innate and learned behavior.
Neuronal encoding dynamics of LHA neurons
Much of the previously described work has focused on contemporary circuit mapping and optogenetic approaches to identify the functions of LHA components. However, it is important to note that approaches that have recently revolutionized systems neuroscience cannot yet detail circuit dynamics, and thus are largely a way to imply circuit function based on imprecise and artificial activity patterns. For example, while LHA neurons are capable of firing up to or even beyond 20 Hz, the collection of LHA cellular subtypes likely do not fire in highly synchronous patterns generated by bulk optogenetic methods. Even with inhibitory optogenetic and chemogenetic approaches, neuronal activity is suppressed over extended time epochs, which is also likely inconsistent with neurotypical signaling dynamics, and may also increase activity if sufficient light is delivered to heat the tissue. Thus, an ongoing area of intense research is to examine the endogenous neural encoding properties of distinct LHA neuronal networks and populations. These rich datasets, collected from 100s of LHA neurons, can in turn be used to accurately test which aspects of LHA spatiotemporal signaling are critical for motivated behavioral states.
Early in vivo electrophysiological studies in rodents, rabbits, and primates demonstrated that individual LHA neurons are responsive to rewarding, aversive, and associated conditioned stimuli145–148. Ono et al146 identified populations of LHA neurons that were responsive to rewarding or aversive stimuli presentation. LHA neurons that are responsive to primary rewards, tended to not respond to aversive stimuli, but if they did, they tended to respond in the opposite direction (i.e. excited by rewarding, inhibited by aversive stimuli). Additionally, LHA neurons that displayed activity changes in response to a caloric reward displayed similar response patterns to electrical stimulation that could evoked ICSS146, suggesting that distinct types of rewards can engage the same LHA neurons. Moreover, a subset of LHA neurons also respond to conditioned stimuli presentation, but these cells are largely distinct from the LHA neurons that responded to primary rewards133,146,148. While these early studies demonstrated the existence of distinct LHA neuronal populations that respond to aspects of feeding and reward, it is difficult to designate the functional properties of these neuronal subtypes due to the inability at the time to record from identified LHA neurons.
Two recent studies have begun to further unravel the signaling properties of defined subsets of reward-relevant LHA neurons based on their projection targets or genetic specificity111,133. Nieh, Mathews, et al.133 studied LHA neurons based on their connectivity with the VTA. Specially, they introduced channelrhodopsin-2 into LHA neurons that projected to the VTA using retrograde Cre-encoding virus strategy (this strategy did not differentiate whether these targeted cells were glutamatergic and GABAergic). Neurons could then be identified as directly projecting or as polysynaptically connected with the VTA based on the electrophysiological response to intra-LHA blue light pulses. Some LHA neurons that were classified as directly-projecting to the VTA tended to respond with excitations while others responded with inhibitions when mice entered a reward retrieval port. In contrast, LHA neurons that were classified as polysynaptically connected to the VTA responded to nosepokes and cues that predicted rewards as well as to reward port entry. Using microendoscopic calcium imaging Jennings, Ung et al.111 selectively imaged neuronal activity from 100’s of LHA Vgat-expressing (putative GABAergic) neurons in behaving mice. Individual LHA GABAergic neurons displayed changes in their activity timelocked to either nosepokes required to produce the delivery of a caloric reward or to the first lick following reward delivery, but very few LHA GABAergic neurons responded to both nosepokes and lick events. These data suggest that LHA neurons that respond to primary rewards, aversive stimuli, or conditioned stimuli may be dissociable based on their circuit connectivity or molecular phenotype.
Future outlook
While a detailed description of LHA circuitry and cellular encoding properties is far from complete, emerging evidence generated with contemporary techniques coupled with historic findings are beginning to reveal reoccurring themes and principles of these circuits. For example, both classical electrical stimulation and recordings studies as well cell type specific optogenetic studies have suggested that discrete LHA circuits not only play an important role in reward and feeding, but can also produce aversive states24,25,111–113,146. While there is limited (but some) information detailing how a few defined LHA neural populations encode rewards and predictive cues, there is currently no published data on whether subsets (or all) of these same cell types also respond to aversive stimuli, or whether these are encoded by different cell populations. Recent studies have begun to define LHA neuronal subtypes based on the small molecular neurotransmitters they are capable of utilizing or by the production of a few select neuropeptides100,109,111–113, that are involved in motivation and reward. While this is an ideal first pass approach to study LHA circuits, a good deal more must be done to accurately define circuit architecture and function. An even more fundamental problem is that it remains to be determined precisely how many neuronal phenotypes are present within the LHA. High throughput single-cell transcriptional profiling strategies appear to be rapidly emerging, and have already begun to elucidate the number of cell subclasses in other parts of the central nervous system149. If these methodologies are applied to 100,000 or more LHA neurons, it may be possible to accurately identify the number of neuronal phenotypes based on quantitative genetic data. Thus, coupled with state-of-the-art circuit targeting strategies, delineation of LHA circuits will continue to evolve.
While a clearer picture of LHA neuronal classes and connectivity will continue to emerge, it is not certain whether ‘rewarding’ and ‘feeding’ phenotypes that are readily engaged by neuromodulation of the LHA are actually dissociable from each other, and thus drive reward paradox, with respect to the LHA, still remains unresolved. Current evidence suggests that bulk optogenetic modulation can activate or inhibit up to 1 mm of brain tissue, or up to 10,000 LHA neurons and their associated circuit components, while the spatiotemporal activity dynamics of LHA neurons are highly complex even at the cellular level. Thus, accurately recording and modulating LHA network dynamics to living neurocircuits will require single-cell optogenetic modulation capabilities. Another perhaps more immediately testable scenario is that LHA circuit dynamics are largely based upon their input and output circuitry. Stimulus evoked activity patterns can be conveyed from one individual cortical neuron to another, and thus aggregate afferent input from the extended amygdala, basal forebrain, cortex, and other hypothalamic nuclei may ultimately provide more specified ensemble information to LHA circuits which in turn generates appropriate behavioral drive.
Acknowledgments
We thank Joshua Jennings for input on the manuscript and members of the Stuber Lab for helpful discussion. This work was supported by the Klarman Family Foundation, the Brain and Behavior Research Foundation, the Foundation for Prader-Willi Research, the Foundation of Hope, the National Institute on Drug Abuse (DA032750 and DA038168), and the Department of Psychiatry at UNC Chapel Hill (G.D.S.). R.A.W. was supported by the Intramural Research Program at the National Institute on Drug Abuse.
References
- 1.Broberger C, De Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/Orexin-and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: Relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 2.Lee H, et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 4.Puelles L, Rubenstein JLR. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 5.Saper CB, Chou TC, Elmquist JK. The Need to Feed: Homeostatic and Hedonic Control of Eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 6.Hess WR. The functional organization of the diencephalon. Grune & Stratton; New York: 1957. [Google Scholar]
- 7.Martini L, Ganong WF. Neuroendocrinology. Elsevier; 2013. [Google Scholar]
- 8.THE HUMAN HYPOTHALAMUS IN HEALTH AND DISEASE. Elsevier; 1992. [Google Scholar]
- 9.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: Neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci Biobehav Rev. 1993;17:141–193. doi: 10.1016/s0149-7634(05)80149-6. [DOI] [PubMed] [Google Scholar]
- 10.Millhouse OE. A Golgi study of the descending medial forebrain bundle. Brain Res. 1969;15:341–363. doi: 10.1016/0006-8993(69)90161-9. [DOI] [PubMed] [Google Scholar]
- 11.Palkovits M, Van Cuc H. Quantitative light and electron microscopic studies on the lateral hypothalamus in rat. Cell and synaptic densities. Brain Res Bull. 1980;5:643–647. doi: 10.1016/0361-9230(80)90199-9. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- 14.Anand BK, Brobeck JR. Localization of a ‘feeding center’ in the hypothalamus of the rat. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 15.Montemurro DG, Stevenson JA. Adipsia produced by hypothalamic lesions in the rat. Can J Biochem Physiol. 1957;35:31–37. [PubMed] [Google Scholar]
- 16.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec. 1940;78:465–466. [Google Scholar]
- 17.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 18.Kapatos G, Gold RM. Evidence for ascending noradrenergic mediation of hypothalamic hyperphagia. Pharmacol Biochem Behav. 1973;1:81–87. doi: 10.1016/0091-3057(73)90060-9. [DOI] [PubMed] [Google Scholar]
- 19.Grossman SP, Dacey D, Halaris AE, Collier T, Routtenberg A. Aphagia and adipsia after preferential destruction of nerve cell bodies in hypothalamus. Science. 1978;202:537–539. doi: 10.1126/science.705344. [DOI] [PubMed] [Google Scholar]
- 20.Grossman SP, Grossman L. Iontophoretic injections of kainic acid into the rat lateral hypothalamus: effects on ingestive behavior. Physiol Behav. 1982;29:553–559. doi: 10.1016/0031-9384(82)90281-5. [DOI] [PubMed] [Google Scholar]
- 21.Stricker EM, Swerdloff AF, Zigmond MJ. Intrahypothalamic injections of kainic acid produce feeding and drinking deficits in rats. Brain Res. 1978;158:470–473. doi: 10.1016/0006-8993(78)90692-3. [DOI] [PubMed] [Google Scholar]
- 22.DELGADO JMR, ANAND BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172:162–168. doi: 10.1152/ajplegacy.1952.172.1.162. [DOI] [PubMed] [Google Scholar]
- 23.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 24.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 25.Margules DL, Olds J. Identical ‘feeding’ and ‘rewarding’ systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 26.Stanley BG, Ha LH, Spears LC, Dee MG., 2nd Lateral hypothalamic injections of glutamate, kainic acid, D,L-alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993;613:88–95. doi: 10.1016/0006-8993(93)90458-y. [DOI] [PubMed] [Google Scholar]
- 27.Kelly J, Rothstein J, Grossman SP. GABA and hypothalamic feeding systems. I. Topographic analysis of the effects of microinjections of muscimol. Physiol Behav. 1979;23:1123–1134. doi: 10.1016/0031-9384(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 28.Bower GH, Miller NE. Rewarding and punishing effects from stimulating the same place in the rat’s brain. J Comp Physiol Psychol. 1958;51:669–674. doi: 10.1037/h0038925. [DOI] [PubMed] [Google Scholar]
- 29.Mendelson J, Freed WJ. Do rats terminate hypothalamic stimulation only in order to turn it on again? Behav Biol. 1973;8:619–628. doi: 10.1016/s0091-6773(73)80147-6. [DOI] [PubMed] [Google Scholar]
- 30.Caggiula AR, Hoebel BG. ‘Copulation-reward site’ in the posterior hypothalamus. Science. 1966;153:1284–1285. doi: 10.1126/science.153.3741.1284. [DOI] [PubMed] [Google Scholar]
- 31.Mogenson GJ, Stevenson JA. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp Neurol. 1967;17:119–127. doi: 10.1016/0014-4886(67)90139-2. [DOI] [PubMed] [Google Scholar]
- 32.Roberts WW, Carey RJ. REWARDING EFFECT OF PERFORMANCE OF GNAWING AROUSED BY HYPOTHALAMIC STIMULATION IN THE RAT. J Comp Physiol Psychol. 1965;59:317–324. doi: 10.1037/h0022030. [DOI] [PubMed] [Google Scholar]
- 33.Glickman SE, Schiff BB. A biological theory of reinforcement. Psychol Rev. 1967;74:81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson RR, Renfrew JW. Stalking attack and eating behaviors elicited from the same sites in the hypothalamus. J Comp Physiol Psychol. 1966;61:360–367. doi: 10.1037/h0023250. [DOI] [PubMed] [Google Scholar]
- 35.Coons EE, Levak M, Miller NE. Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science. 1965;150:1320–1321. doi: 10.1126/science.150.3701.1320. [DOI] [PubMed] [Google Scholar]
- 36.Wise RA. Individual differences in effects of hypothalamic stimulation: the role of stimulation locus. Physiol Behav. 1971;6:569–572. doi: 10.1016/0031-9384(71)90207-1. [DOI] [PubMed] [Google Scholar]
- 37.Valenstein ES, Cox VC, Kakolewski JW. Modification of Motivated Behavior Elicited by Electrical Stimulation of the Hypothalamus. Science. 1968;159:1119–1121. doi: 10.1126/science.159.3819.1119. [DOI] [PubMed] [Google Scholar]
- 38.Wise RA. Hypothalamic motivational systems: fixed or plastic neural circuits? Science. 1968;162:377–379. doi: 10.1126/science.162.3851.377. [DOI] [PubMed] [Google Scholar]
- 39.Mendleson J. The role of hunger in the T-maze learning for food by rats. J Comp Physiol Psychol. 1966;62:341–349. [Google Scholar]
- 40.Mendelson J, Chorover SL. LATERAL HYPOTHALAMIC STIMULATION IN SATIATED RATS: T-MAZE LEARNING FOR FOOD. Science. 1965;149:559–561. doi: 10.1126/science.149.3683.559. [DOI] [PubMed] [Google Scholar]
- 41.Andersson B, Wyrwicka W. The elicitation of a drinking motor conditioned reaction by electrical stimulation of the hypothalamic drinking area in the goat. Acta Physiol Scand. 1957;41:194–198. doi: 10.1111/j.1748-1716.1957.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 42.Tenen SS, Miller NE. STRENGTH OF ELECTRICAL STIMULATION OF LATERAL HYPOTHALAMUS, FOOD DEPRIVATION, AND TOLERANCE FOR QUININE IN FOOD. J Comp Physiol Psychol. 1964;58:55–62. doi: 10.1037/h0043359. [DOI] [PubMed] [Google Scholar]
- 43.Wise RA, Albin J. Stimulation-induced eating disrupted by a conditioned taste aversion. Behav Biol. 1973;9:289–297. doi: 10.1016/s0091-6773(73)80179-8. [DOI] [PubMed] [Google Scholar]
- 44.Flynn JP. Neural aspects of attack behavior in cats. Ann N Y Acad Sci. 1969;159:1008–1012. doi: 10.1111/j.1749-6632.1969.tb12993.x. [DOI] [PubMed] [Google Scholar]
- 45.MacDonnell MF, Flynn JP. Sensory control of hypothalamic attack. Anim Behav. 1966;14:399–405. doi: 10.1016/s0003-3472(66)80036-2. [DOI] [PubMed] [Google Scholar]
- 46.MacDonnell MF, Flynn JP. Control of sensory fields by stimulation of hypothalamus. Science. 1966;152:1406–1408. doi: 10.1126/science.152.3727.1406. [DOI] [PubMed] [Google Scholar]
- 47.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 48.Fulton S, Woodside B, Shizgal P. Potentiation of brain stimulation reward by weight loss: evidence for functional heterogeneity in brain reward circuitry. Behav Brain Res. 2006;174:56–63. doi: 10.1016/j.bbr.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Mogenson GJ, Gentil CG, Stevenson JA. Feeding and drinking elicited by low and high frequencies of hypothalamic stimulation. Brain Res. 1971;33:127–137. doi: 10.1016/0006-8993(71)90311-8. [DOI] [PubMed] [Google Scholar]
- 50.Morgane PJ. Evidence of a ‘hunger motivational’ system in the lateral hypothalamus of the rat. Nature. 1961;191:672–674. doi: 10.1038/191672a0. [DOI] [PubMed] [Google Scholar]
- 51.Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 52.Greer MA. Suggestive evidence of a primary drinking center in hypothalamus of the rat. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1955;89:59–62. doi: 10.3181/00379727-89-21716. [DOI] [PubMed] [Google Scholar]
- 53.Olds J. Pleasure centers in the brain. Sci Am. 1956;195:105–116. [Google Scholar]
- 54.Wise RA. Lateral hypothalamic electrical stimulation: does it make animals ‘hungry’? Brain Res. 1974;67:187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]
- 55.Wise RA. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biol Psychiatry. 2013;73:819–826. doi: 10.1016/j.biopsych.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips AG, Nikaido RS. Disruption of brain stimulation-induced feeding by dopamine receptor blockade. Nature. 1975;258:750–751. doi: 10.1038/258750a0. [DOI] [PubMed] [Google Scholar]
- 57.Fouriezos G, Hansson P, Wise RA. Neuroleptic-induced attenuation of brain stimulation reward in rats. J Comp Physiol Psychol. 1978;92:661–671. doi: 10.1037/h0077500. [DOI] [PubMed] [Google Scholar]
- 58.Fouriezos G, Wise RA. Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 1976;103:377–380. doi: 10.1016/0006-8993(76)90809-x. [DOI] [PubMed] [Google Scholar]
- 59.Franklin KB. Catecholamines and self-stimulation: reward and performances effects dissociated. Pharmacol Biochem Behav. 1978;9:813–820. doi: 10.1016/0091-3057(78)90361-1. [DOI] [PubMed] [Google Scholar]
- 60.Franklin KB, McCoy SN. Pimozide-induced extinction in rats: stimulus control of responding rules out motor deficit. Pharmacol Biochem Behav. 1979;11:71–75. doi: 10.1016/0091-3057(79)90299-5. [DOI] [PubMed] [Google Scholar]
- 61.Gallistel CR, Boytim M, Gomita Y, Klebanoff L. Does pimozide block the reinforcing effect of brain stimulation? Pharmacol Biochem Behav. 1982;17:769–781. doi: 10.1016/0091-3057(82)90360-4. [DOI] [PubMed] [Google Scholar]
- 62.Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- 63.Gallistel CR, Shizgal P, Yeomans JS. A portrait of the substrate for self-stimulation. Psychol Rev. 1981;88:228–273. [PubMed] [Google Scholar]
- 64.Yeomans JS. The absolute refractory periods of self-stimulation neurons. Physiol Behav. 1979;22:911–919. doi: 10.1016/0031-9384(79)90336-6. [DOI] [PubMed] [Google Scholar]
- 65.Gratton A, Wise RA. Hypothalamic reward mechanism: two first-stage fiber populations with a cholinergic component. Science. 1985;227:545–548. doi: 10.1126/science.2981439. [DOI] [PubMed] [Google Scholar]
- 66.Shizgal P, Bielajew C, Corbett D, Skelton R, Yeomans J. Behavioral methods for inferring anatomical linkage between rewarding brain stimulation sites. J Comp Physiol Psychol. 1980;94:227–237. doi: 10.1037/h0077668. [DOI] [PubMed] [Google Scholar]
- 67.Bielajew C, Shizgal P. Evidence implicating descending fibers in self-stimulation of the medial forebrain bundle. J Neurosci Off J Soc Neurosci. 1986;6:919–929. doi: 10.1523/JNEUROSCI.06-04-00919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bielajew C, Bushnik T, Konkle AT, Schindler D. The substrate for brain-stimulation reward in the lateral preoptic area. II. Connections to the ventral tegmental area. Brain Res. 2000;881:112–120. doi: 10.1016/s0006-8993(00)02565-8. [DOI] [PubMed] [Google Scholar]
- 69.Wise RA, Bozarth MA. Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol. 1981;5:467–474. doi: 10.1016/0364-7722(81)90028-x. [DOI] [PubMed] [Google Scholar]
- 70.Yeomans JS. The Neural Basis of Feeding and Reward. Haer Institute; 1982. pp. 405–417. [Google Scholar]
- 71.Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced ‘anhedonia’ in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 72.De Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- 73.Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- 74.Gratton A, Wise RA. Comparisons of refractory periods for medial forebrain bundle fibers subserving stimulation-induced feeding and brain stimulation reward: a psychophysical study. Brain Res. 1988;438:256–263. doi: 10.1016/0006-8993(88)91344-3. [DOI] [PubMed] [Google Scholar]
- 75.Gratton A, Wise RA. Comparisons of connectivity and conduction velocities for medial forebrain bundle fibers subserving stimulation-induced feeding and brain stimulation reward. Brain Res. 1988;438:264–270. doi: 10.1016/0006-8993(88)91345-5. [DOI] [PubMed] [Google Scholar]
- 76.Allen GV, Cechetto DF. Neurotensin in the lateral hypothalamic area: Origin and function. Neuroscience. 1995;69:533–544. doi: 10.1016/0306-4522(95)00261-g. [DOI] [PubMed] [Google Scholar]
- 77.Burdakov D, Alexopoulos H. Metabolic state signalling through central hypocretin/orexin neurons. J Cell Mol Med. 2005;9:795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collin M, et al. Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci. 2003;18:1265–1278. doi: 10.1046/j.1460-9568.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- 79.Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J Neurosci Off J Soc Neurosci. 2014;34:11405–11415. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Griffond B, Risold PY. MCH and feeding behavior-interaction with peptidic network. Peptides. 2009;30:2045–2051. doi: 10.1016/j.peptides.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Knight ZA, et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151:1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laque A, et al. Leptin receptor neurons in the mouse hypothalamus are co-localized with the neuropeptide galanin and mediate anorexigenic leptin action. Am J Physiol Endocrinol Metab. 2013 doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leinninger GM, et al. Leptin Acts via Leptin Receptor-Expressing Lateral Hypothalamic Neurons to Modulate the Mesolimbic Dopamine System and Suppress Feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 85.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- 86.Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of Neuropeptide Y, Peptide YY, and Pancreatic Polypeptide Inhibition of Identified Green Fluorescent Protein-Expressing GABA Neurons in the Hypothalamic Neuroendocrine Arcuate Nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karnani MM, Szabó G, Erdélyi F, Burdakov D. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin-and melanin-concentrating hormone neurons. J Physiol. 2013;591:933–953. doi: 10.1113/jphysiol.2012.243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luiten PGM, ter Horst GJ, Steffens AB. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 89.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harrison TA, Chen CT, Dun NJ, Chang JK. Hypothalamic orexin A-immunoreactive neurons project to the rat dorsal medulla. Neurosci Lett. 1999;273:17–20. doi: 10.1016/s0304-3940(99)00611-4. [DOI] [PubMed] [Google Scholar]
- 91.Sakurai T. Orexins and orexin receptors: implication in feeding behavior. Regul Pept. 1999;85:25–30. doi: 10.1016/s0167-0115(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 92.Haynes AC, et al. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. 2002;104:153–159. doi: 10.1016/s0167-0115(01)00358-5. [DOI] [PubMed] [Google Scholar]
- 93.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 94.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 96.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 97.Bittencourt JC, et al. The melanin-concentrating hormone system of the rat brain: An immuno-and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 98.Elias CF, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 99.Harthoorn LF, Sañé A, Nethe M, Heerikhuize JJ. Multi-Transcriptional Profiling of Melanin-Concentrating Hormone and Orexin-Containing Neurons. Cell Mol Neurobiol. 2005;25:1209–1223. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jego S, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 102.Ludwig DS, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci Off J Soc Neurosci. 2006;26:389–397. doi: 10.1523/JNEUROSCI.1203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 105.Brown JA, Woodworth HL, Leinninger GM. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front Syst Neurosci. 2015;9:9. doi: 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kahn D, Abrams GM, Zimmerman EA, Carraway R, Leeman SE. Neurotensin neurons in the rat hypothalamus: an immunocytochemical study. Endocrinology. 1980;107:47–54. doi: 10.1210/endo-107-1-47. [DOI] [PubMed] [Google Scholar]
- 107.Cooke JH, et al. Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obes Silver Spring Md. 2009;17:1135–1143. doi: 10.1038/oby.2008.652. [DOI] [PubMed] [Google Scholar]
- 108.Kim ER, Leckstrom A, Mizuno TM. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behav Brain Res. 2008;194:66–71. doi: 10.1016/j.bbr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 109.Leinninger GM, et al. Leptin Action via Neurotensin Neurons Controls Orexin, the Mesolimbic Dopamine System and Energy Balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vong L, et al. Leptin Action on GABAergic Neurons Prevents Obesity and Reduces Inhibitory Tone to POMC Neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jennings JH, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stamatakis AM, et al. Lateral hypothalamic glutamatergicneurons regulate feeding and rewardvia projections to the lateral habenula. Journal of Neuroscience. 2015 [Google Scholar]
- 114.Kita H, Oomura Y. Reciprocal connections between the lateral hypothalamus and the frontal cortex in the rat: Electrophysiological and anatomical observations. Brain Res. 1981;213:1–16. doi: 10.1016/0006-8993(81)91244-0. [DOI] [PubMed] [Google Scholar]
- 115.Nauta WJH. Hippocampal Projections and Related Neural Pathways to the Mid-Brain in the Cat. Brain. 1958;81:319–340. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- 116.Anthony TE, et al. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014;156:522–536. doi: 10.1016/j.cell.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 118.Zahm DS, Brog JS. On the significance of subterritories in the ‘accumbens’ part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 119.Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:23–53. [PubMed] [Google Scholar]
- 123.Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: Ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 124.Betley JN, Cao ZFH, Ritola KD, Sternson SM. Parallel, Redundant Circuit Organization for Homeostatic Control of Feeding Behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu Z, et al. GABAergic Projections from Lateral Hypothalamus to Paraventricular Hypothalamic Nucleus Promote Feeding. J Neurosci. 2015;35:3312–3318. doi: 10.1523/JNEUROSCI.3720-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 127.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stratford TR, Kelley AE. GABA in the Nucleus Accumbens Shell Participates in the Central Regulation of Feeding Behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stratford TR, Kelley AE. Evidence of a Functional Relationship between the Nucleus Accumbens Shell and Lateral Hypothalamus Subserving the Control of Feeding Behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O’Connor EC, et al. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron. 2015;88:553–564. doi: 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 131.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 132.Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: An autoradiographic investigation. Brain Res Bull. 1982;8:511–526. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- 133.Nieh EH, et al. Decoding Neural Circuits that Control Compulsive Sucrose Seeking. Cell. 2015;160:528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jennings JH, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kempadoo KA, et al. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci Off J Soc Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tan KR, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Poller WC, Madai VI, Bernard R, Laube G, Veh RW. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013 doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 139.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stratford TR, Wirtshafter D. Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res. 2013;1490:128–133. doi: 10.1016/j.brainres.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci Off J Soc Neurosci. 2015;35:4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Horvath TL, Diano S, van den Pol AN. Synaptic Interaction between Hypocretin (Orexin) and Neuropeptide Y Cells in the Rodent and Primate Hypothalamus: A Novel Circuit Implicated in Metabolic and Endocrine Regulations. J Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fukuda M, Ono T, Nishino H, Nakamura K. Neuronal responses in monkey lateral hypothalamus during operant feeding behavior. Brain Res Bull. 1986;17:879–883. doi: 10.1016/0361-9230(86)90102-4. [DOI] [PubMed] [Google Scholar]
- 146.Ono T, Nakamura K, Nishijo H, Fukuda M. Hypothalamic neuron involvement in integration of reward, aversion, and cue signals. J Neurophysiol. 1986;56:63–79. doi: 10.1152/jn.1986.56.1.63. [DOI] [PubMed] [Google Scholar]
- 147.Ono T, Nakamura K, Fukuda M, Kobayashi T. Catecholamine and acetylcholine sensitivity of rat lateral hypothalamic neurons related to learning. J Neurophysiol. 1992;67:265–279. doi: 10.1152/jn.1992.67.2.265. [DOI] [PubMed] [Google Scholar]
- 148.Schwartzbaum JS. Electrophysiology of taste, feeding and reward in lateral hypothalamus of rabbit. Physiol Behav. 1988;44:507–526. doi: 10.1016/0031-9384(88)90313-7. [DOI] [PubMed] [Google Scholar]
- 149.Macosko EZ, et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]


