ABSTRACT
Signal peptides play an important role in directing and efficiently transporting secretory proteins to their proper locations in the endoplasmic reticulum of mammalian cells. The aim of this study was to enhance the expression of recombinant coagulation factor VII (rFVII) in CHO cells by optimizing the signal peptides and type of fed-batch culture medium used. Five sub-clones (O2, I3, H3, G2 and M3) with different signal peptide were selected by western blot (WB) analysis and used for suspension culture. We compared rFVII expression levels of 5 sub-clones and found that the highest rFVII expression level was obtained with the IgK signal peptide instead of Ori, the native signal peptide of rFVII. The high protein expression of rFVII with signal peptide IgK was mirrored by a high transcription level during suspension culture. After analyzing culture and feed media, the combination of M4 and F4 media yielded the highest rFVII expression of 20 mg/L during a 10-day suspension culture. After analyzing cell density and cell cycle, CHO cells feeding by F4 had a similar percentage of cells in G0/G1 and a higher cell density compared to F2 and F3. This may be the reason for high rFVII expression in M4+F4. In summary, rFVII expression was successfully enhanced by optimizing the signal peptide and fed-batch medium used in CHO suspension culture. Our data may be used to improve the production of other therapeutic proteins in fed-batch culture.
KEYWORDS: cell cycle, CHO, rFVII, SFM, signal peptide
Introduction
Coagulation factor VII (FVII) is a vitamin K-dependent serine protease that circulates in the blood. Recombinant FVII (rFVII) is used clinically to stop bleeding in patients with bleeding disorders including hemophilia A and B patients with inhibitors against FVIII and FIX. Recombinant proteins produced in prokaryotic cells fold improperly due to a lack of post-translational modification. Therefore mammalian cells are widely used to express rFVII. The first report of rFVII expression in baby hamster kidney (BHK) cells was in 19861 and rFVII is now commercially available as “NovoSevenRT” for clinical use. Currently, several recombinant protein expression systems, such as Chinese hamster ovary (CHO), HEK 293 and insects cells, have been developed to express rFVII.2-5 Moreover, Hwang et al. reported using transgenic fish as bioreactors to express rFVII.6 However, the main problem with rFVII production is its low expression level during culture.
Proper localization is important for the structure, post-translational modification and function of secreted proteins in eukaryotic cells. Most secretory proteins are synthesized with a 5-30-amino-acid signal peptide at the N-terminal end which serves to transport proteins to the correct subcellular compartment.7,8 As a signal peptide is synthesized by a ribosome, it is recognized by a signal recognition particle (SRP) and forms a complex of SRP-ribosome-nascent chain (SRP-RNC), which is delivered to the target ER membrane by interacting with the SRP receptor (SR) for sequential post-translational modification.7 Therefore, the affinity of a signal peptide for an SRP determines the efficiency of the translocation of the synthesized polypeptide chain. Most signal peptides have an N-terminal polar region, a hydrophobic core region and a C-terminal polar region.8 However, the effect of signal peptides on protein secretion has been varied, as reported by previous reports, suggesting that the native signal peptide is not always the most efficient.9 Therefore, it is important to optimize the signal peptide for enhancing protein expression in mammalian cells.
CHO cells are the most popular mammalian expression system for recombinant therapeutic protein production. Recombinant therapeutic proteins expressed by prokaryotes and yeast often cause immunogenicity or the shorten half-life because of the incorrect post-translational modification especially non-human glycosylation.10,11 Therefore, CHO cells have been the predominant host for the production of recombinant therapeutic proteins due to advantages of similarly post-translational modification to human and easily genetic regulation.12 Most therapeutic proteins expressed by CHO cells were non-cell growth associated and the final product concentration varied depending on cell density, culture time and specific production rate. Fed-batch is an effective method for increasing the duration of viable cell density by avoiding the depletion of nutrients and the accumulation of by-products. Some nutrient feeding and culture strategies were developed to enhance the expression and improve the quality of antibodies and other therapeutic proteins.13-15 In addition, SFM formulae have been optimized to better support the physiology and metabolism of the cell and enhance target protein expression.16-18
The aim of this study was to enhance the expression of rFVII by screening signal peptides and optimizing fed-batch media for efficient and long-term production. Five signal peptides from different sources were fused to the N-terminus of rFVII and its expression level during suspension culture was analyzed. The effects of 5 culture media and 3 feed media on rFVII expression were compared and cell cycle analysis was carried out during suspension culture. The results of this study showed effective enhancement of rFVII expression and will be beneficial to the production of other recombinant therapeutic proteins in CHO cells.
Results
Establishment and screening of stable high rFVII expression CHO clones
In this study, the rFVII encoding gene with different signal peptides, including Ori, IgK, Ht, Gl and Mutant, were inserted into plasmid pMH3 and transfected into CHO cells. After G418 selection, the surviving cells were cultured in 96-well and 6-well microplates and protein expression in the supernatants of the cell culture media in 6-well microplates after culturing 2 d were analyzed by WB (Fig. 1). The relatively high expression sub-clone in each CHO/rFVII expression cell lines with different signal peptides was selected by comparing bands in WB analysis. The selected sub-clones for different signal peptides were O2, I3, H3, G2 and M3, and rFVII expression levels were further compared in suspension culture.
Figure 1.
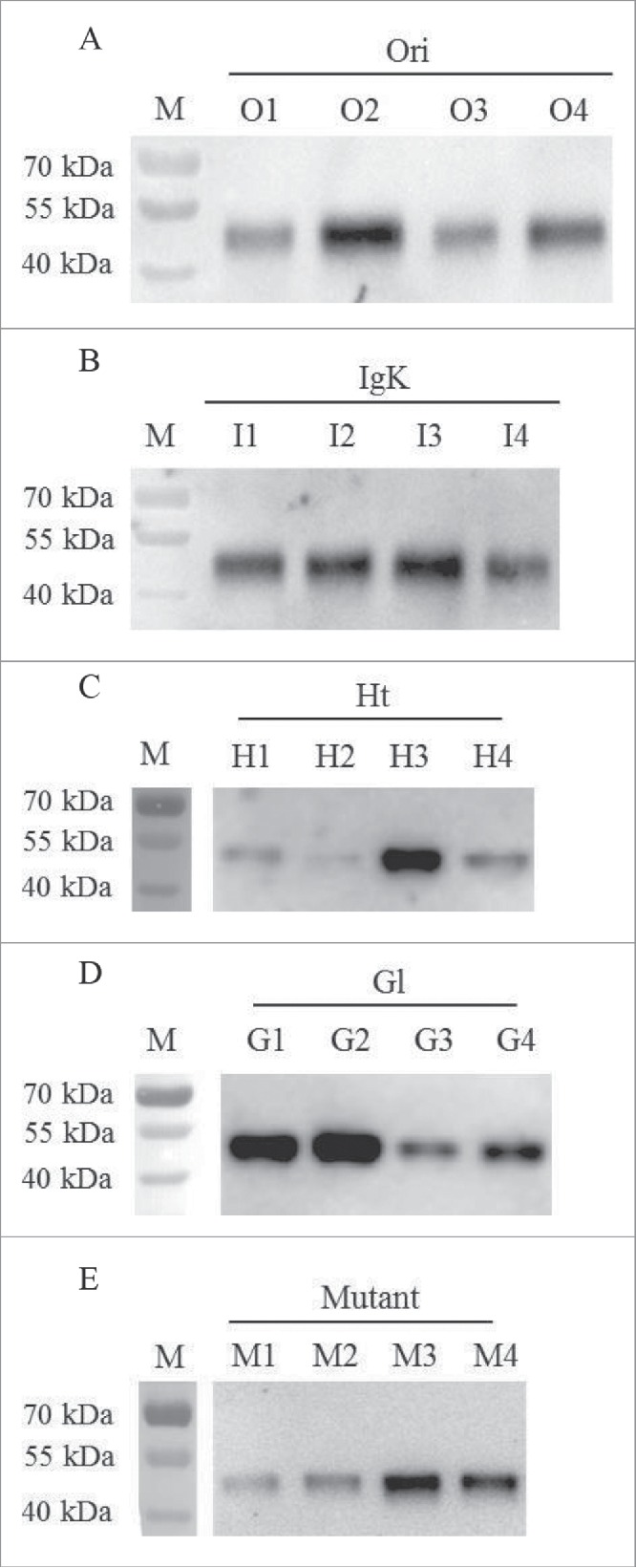
Screening of the relatively high rFVII expression sub-clone in each CHO/rFVII expression cell line with different signal peptides by WB analysis. (A) Ori (O1-O4); (B) IgK (I1-I4); (C) Ht (H1-H4); (D) Gl (G1-G4); (E) Mutant (M1-M4).
Analysis of the expression and transcription levels of rFVII with different signal peptides in CHO suspension culture
To select the suitable signal peptide, rFVII expression levels of various sub-clones with different signal peptide were compared under suspension culture with M2 SFM for 6 d. The sub-clone with IgK signal peptide had the highest rFVII concentration at 5.42 mg/L compared with the 4 other sub-clones (Fig. 2A). In contrast, rFVII expression with Ori, the native signal peptide of rFVII, was 3.58 mg/L. Analysis by qRT-PCR showed that fvii transcription level in the IgK signal peptide clone was higher than that of the other 4 signal peptides, which agreed with the corresponding rFVII protein expression (Fig. 2B). The transcription of fvii transcription in the clone with the Ori signal peptide was lower than the 4 other tested clones.
Figure 2.
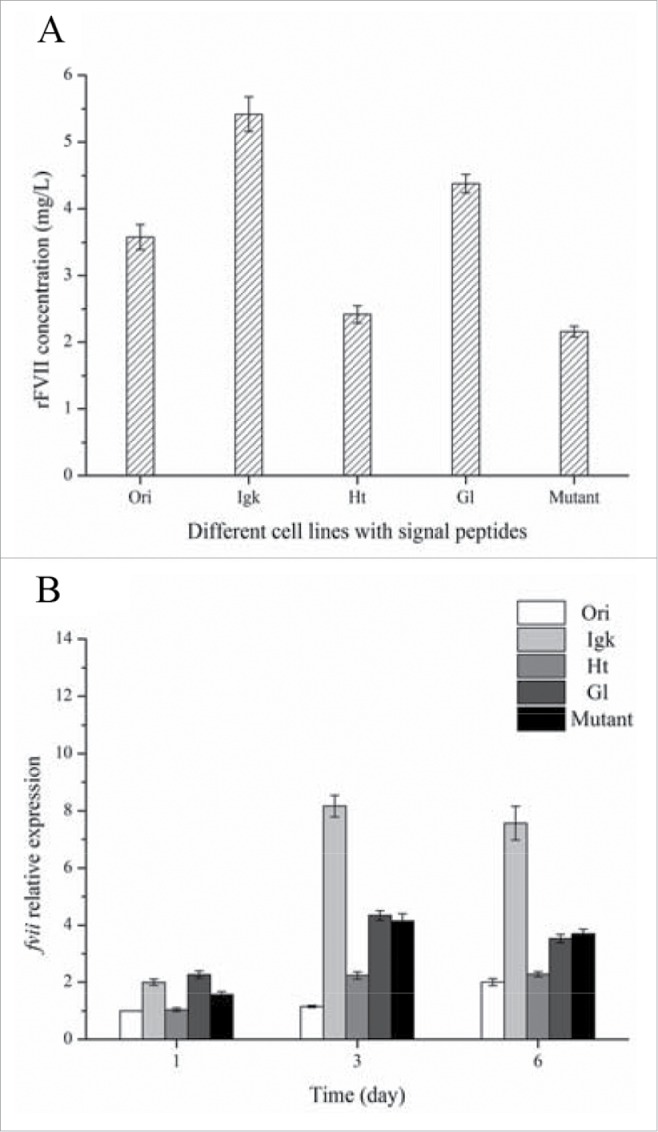
Protein expression (A) and transcription levels (B) of rFVII in CHO cell lines with Ori, IgK, Ht, Gl and Mutant signal peptides.
Effects of culture and feed medium on rFVII expression in CHO cells
To enhance rFVII expression, 5 different SFMs were applied to the CHO/IgK suspension culture (Fig. 3A). Among these SFMs, M4 medium produced the highest (6.76 mg/L) concentration of rFVII. Therefore, M4 was selected as the medium for the suspension culture of the CHO/IgK cell line. The main nutrient and carbon source in SFM is glucose. The initial glucose concentration in M4 was 5 g/L and it dropped to less than 1 g/L on day 4, which was an insufficient concentration of glucose for long term rFVII production. Therefore, it was necessary to supply additional medium in order to sustain the culture. In this study, 3 concentrated feed media (F2, F3, and F4) were added to the culture broth of CHO/IgK cell lines on day 3. The resulting rFVII expression levels after the addition of the feed media are shown in Fig. 3B. The highest expression level of rFVII, 20 mg/L, was obtained using feed medium F4.
Figure 3.
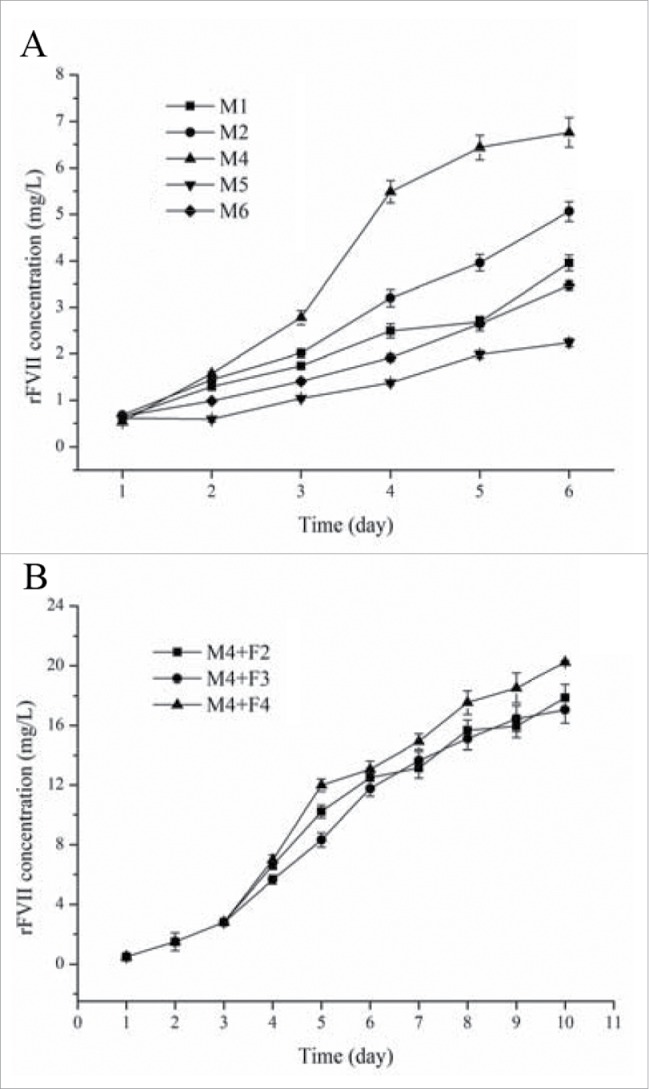
rFVII expression in suspension culture with different SFM (A) and in fed-batch culture, M4 media supplemented with 3 types of feed media (B).
Cell cycle analysis during feed-batch suspension culture
During feed-batch culture, CHO cell lines were cultured in M4 medium and fed with F2, F3 and F4 feed media. After feed media supplementation at day 3, the abundant nutrients caused the constantly increase of viable cell density, which peaked on day 5 (Fig. 4A). The whole CHO cells were analyzed and divided into the percentages of cells in different phases of the cell cycle (G0/G1, G2 and S phases) (Fig. 4B-4D). In all tested feed medium, cells in G0/G1 phase maintained a high level of exceeding 80%, which may be caused by some cell cycle arresting components. The percentages of cells in G0/G1 phase were similar under different feed media. Some cell cycle arresting chemicals had toxic effect on cell growth and thus cell density reduced after adding this chemicals.19-21 In this study, cell density also reduced after day 6 and this may be caused by cell cycle arresting component in feed media. However, the cell density decline in M4+F4 was slower than other 2 fed-batch culture (M4+F2 and M4+F3) (Fig. 4A). Therefore, high cell density under M4+F4 and simultaneously high percentage of cells in G0/G1 phase led to the high rFVII expression during suspension culture.
Figure 4.
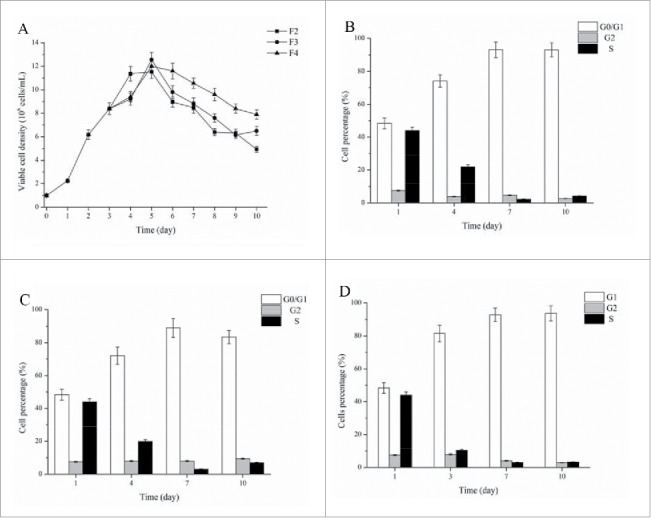
Viable cell density (A) and cell cycle percentage (B-D) during fed-batch culture. (B) M4 + F2 media cell cycle percentages; (C) M4 + F3 media cell cycle percentages; (D) M4 + F4 media cell cycle percentages.
Discussion
As proteins are synthesized by ribosomes, signal peptides at the N-terminal of nascent polypeptides emerge from the translating ribosome and are recognized by SRP.7,8 Therefore, the affinity of the SRP for the signal peptide determines the efficiency of post-translation modification of the proteins in the endoplasmic reticulum (ER) and their subsequent secretion. Previous studies have shown that optimization of signal peptides effectively enhances the production of recombinant therapeutic proteins.20-22 It has been shown that the native signal peptide is not always the most effect signal peptide for recombinant protein expression.9
In this study, 5 signal peptides from different sources, including the native signal peptide, were fused to the N-terminal of rFVII and expressed in CHO cells. The results showed that the IgK signal peptide was more suitable for rFVII expression than other signal peptides. Ori, the active signal peptide for rFVII, had a lower rFVII expression level than the optimized signal peptide IgK. This result agrees with previous reports that the native signal peptide is not always the most effective in recombinant protein expression.9 Our data showed the varied effects of different signal peptides on rFVII expression and thus demonstrated the importance of signal peptide selection when optimizing recombinant protein production in a mammalian cell culture system. In addition, the CHO/IgK cell line had high rFVII transcription and expression during culture. Commonly, the function of signal peptide is directing synthesis proteins to proper location. It seems that signal peptides also increase transcription and enhance recombinant protein expression during suspension culture. The reason for this result will be investigated in further research.
Previous studies showed that rFVII production in CHO, HEK 293 and insect cell expression systems was low.2,4,5 Xiao et al. expressed rFVII in CHO cells and the expression level was approximately 300 ng/mL.23 Low expression of rFVII in these studies may be caused by the low cell density and short culture time of adhered cells. Therefore, suspension culture already the main method used to produce therapeutic proteins should be developed and applied to rFVII expression. The optimal combination of culture and feed media resulted in 20 mg/L of rFVII expression in CHO cells, a result that is higher than previously reported studies.2,4,5,23 Moreover, it has been reported that the cell cycles phase, specifically the percentage of cells in the G0/G1 phase, significantly influences the specific productivity and production of recombinant proteins in CHO cells.16-19 Many researchers have focused on screening for chemicals that pause the cells in the G0/G1 phase to enhance protein specific productivity in CHO cells.16,24 Several tested chemicals had toxic effect on the cell growth and led to the decline of viable cell density.19-21 In this study, high percentage of G0/G1 phase cells was accompanied by the reduced cell density in all tested feed media. However, the decreased of cell density after adding 3 feed media was different and F4 had a slower cell density decline. This result indicated that CHO cells feeding by F4 had a similar percentage of G0/G1 phase cells and meanwhile had a higher cell density compared to F2 and F3. Therefore, the high cell density and simultaneously high specific productivity caused by cells in G0/G1 phase was contribute to the high rFVII expression by M4+F4.
Materials and methods
Cell line and culture medium
The serum-free adapted CHO K1 cells (Amprotein Co.) were used for cell line establishment. Adherent cells were cultured in DMEM/F12 (Gibco) medium supplemented with 10% fetal bovine serum (Gibco), 100 μg/ml of streptomycin, and 100 U/ml of penicillin. In suspension culture, SFM M1, M2, M4, M5 and M6 were supplemented with 6 mM of glutamine, as were feed media F2, F3 and F4. Serum-free media and feed media were purchased from Kangju Co.
Plasmid construction and transfection
To amplify rFVII with different signal peptide genes, 5 primers containing different signal peptides were designed. The tested signal peptides were from human FVII (Ori), murine Immunoglobulin Kappa (IgK), Gaussia princeps luciferase (Gl), human trypsinogen-2 (Ht) and mutant 1 signal peptide of Oikosin 1 protein (Mutant) (see Table 1 for amino acid sequences). The PCR products obtained from the designed primers were digested by both NotI and EcoRI and ligated into the pMH3 plasmid (Amprotein Co.). The constructed plasmids were transfected to CHO K1 cells by electroporation using 400V for 40 μs. The electroporation reaction mixture contained 3×106 cells, 20 μg of plasmid, and 5 μg of salmon sperm DNA.
Table 1.
Signal peptide sequences.
| Signal peptide | Amino acid sequences |
|---|---|
| Ori | MVSQALRLLCLLLGLQGCLA |
| IgK | METDTLLLWVLLLWVPGSTG |
| Ht | MNLLLILTFVAAAVA |
| Gl | MGVKVLFALICIAVAEA |
| Mutant | MLLLSALLLGLAFGYS |
Clonal isolation
The transfected cells were cultured in DMEM/F 12 with 10% fetal bovine serum (FBS) and selected with 2.0 mg/ml G418 at 37°C, 5% CO2 for 48 h. The surviving clones were picked and cultured in a 96-well microplate with DMEM/F 12 supplemented with 10% FBS. After culturing the sub-clones for 6 d, the cells were cultured in M1 SFM for 2 d. The supernatant from each well was analyzed by dot blot and high expression clones were subsequently cultured in a 6-well microplate. High expression clones in 6-well microplates were screened by protein gel blot (WB) and the subsequent high expression sub-clones were selected for further suspension culture.
Cell line suspension culture
To compare rFVII expression levels, sub-clones with various signal peptides were cultured in M2 medium for 6 d under 37°C, 100 rpm. High expression cell lines were cultured in M1, M2, M4, M5 and M6 to further investigate the rFVII expression level. To select a suitable feed medium, F2, F3 and F4 were added to culture broth at day 3 to attain a 3 g/L glucose concentration in the culture broth. In all suspension fermentations, a cell density of 1×106 cells/ml was inoculated in 100 ml of culture medium in a 250 ml Erlenmeyer flask and incubated at 37°C, 100 rpm. Culture broth was collected for the analysis of cell density and rFVII protein concentration.
Western blot (WB) analysis
We collected the culture supernatant of CHO/rFVII expression cells in 6-well microplates after 2-day culture, extracted the protein and separated it using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins in the gel were transferred to PVDF membranes and blocked using 5% skim milk, and visualized using sheep polyclonal anti-human factor VII (Cedarlane Labs).
rFVII concentration and cell density
The concentration of rFVII in the cell culture supernatant was analyzed by ELISA kit (Assaypro) according to the manufacturer's instructions. Viable cell density was analyzed by the trypan blue dye method.
RNA and cDNA preparation and quantitative reverse transcriptase PCR (qRT-PCR)
Cells expressing rFVII using different signal peptides were collected and total RNA was extracted using a Trizol Total RNA Purification Kit (Sangon). We used a RevertAid First Strand cDNA Synthesis Kit (Fermentas) to reverse transcribe total RNA to cDNA. Expression levels of fvii were analyzed by qRT-PCR using SYBR green (TaKaRa) according to the manufacturer's instructions. Product specificity was confirmed by melt curve analysis. In all cases, gene expression levels were normalized to β-actin expression.
Cell cycle analysis
CHO cells during fed-batch culture were collected on different days and fixed with 70% ethanol in PBS. The obtained cells were suspended in 0.5 mL PBS with 50 mg/mL of propidium iodide and 100 mg/mL of DNase-free RNase and then immediately analyzed by FACSCalibur (Becton Dickinson).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Chinese New Medicine Research Fund (No. 2013ZX09102033), Chinese National High-Tech Programs of China (No. 2014AA021003 and 2015AA020802), Chinese Natural Science Fund (No. 81273437) and Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- [1].Berkner K, Busby S, Davie E, Hart C, Insley M, Kisiel W, Kumar A, Murray M, O'Hara P, Woodbury R, et al.. Isolation and expression of cDNAs encoding human factor VII. Cold Spring Harbor symposia on quantitative biology 1986; 51: 531-41; PMID:3034487; http://dx.doi.org/ 10.1101/SQB.1986.051.01.065 [DOI] [PubMed] [Google Scholar]
- [2].Halabian R, Roudkenar MH, Esmaeili NS, Masroori N, Roushandeh AM, Najafabadi AJ. Establishment of a cell line expressing recombinant factor VII and its subsequent conversion to active form FVIIa through hepsin by genetic engineering method. Vox Sang 2009; 96: 309-15; PMID:19175565; http://dx.doi.org/ 10.1111/j.1423-0410.2008.01158.x [DOI] [PubMed] [Google Scholar]
- [3].Mirzaahmadi S, Asaadi-Tehrani G, Bandehpour M, et al. Expression of recombinant human coagulation factor VII by the Lizard Leishmania expression system. J Biomed Biotechnol 2011:873874-81. http://dx.doi.org/ 10.1155/2011/873874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Masroori N, Halabian R, Mohammadipour M, Roushandeh AM, Rouhbakhsh M, Najafabadi AJ, Fathabad ME, Salimi M, Shokrgozar MA, Roudkenar MH. High-level expression of functional recombinant human coagulation factor VII in insect cells. Biotechnol Lett 2010; 32: 803-9; PMID:20213530; http://dx.doi.org/ 10.1007/s10529-010-0227-7 [DOI] [PubMed] [Google Scholar]
- [5].Wajih N, Owen J, Wallin R. Enhanced functional recombinant factor VII production by HEK 293 cells stably transfected with VKORC1 where the gamma-carboxylase inhibitor calumenin is stably suppressed by shRNA transfection. Thromb Res 2008; 122: 405-10; PMID:18177690; http://dx.doi.org/ 10.1016/j.thromres.2007.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hwang GL, Muller F, Rahman MA, Williams DW, Murdock PJ, Pasi KJ, Goldspink G, Farahmand H, Maclean N. Fish as bioreactors: Transgene expression of human coagulation factor VII in fish embryos. Mar Biotechnol 2004; 6: 485-92; PMID:15129328; http://dx.doi.org/ 10.1007/s10126-004-3121-2 [DOI] [PubMed] [Google Scholar]
- [7].Saraogi I, Shan S. Molecular Mechanism of Co-translational Protein Targeting by the Signal Recognition Particle. Traffic 2011; 12: 535-42; PMID:21291501; http://dx.doi.org/ 10.1111/j.1600-0854.2011.01171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hegde RS, Bernstein HD. The surprising complexity of signal sequences. Trends Biochem Sci 2006; 31: 563-71; PMID:16919958; http://dx.doi.org/ 10.1016/j.tibs.2006.08.004 [DOI] [PubMed] [Google Scholar]
- [9].Young R, Rance J. Mammalian expression vector with a highly effient secretory signal sequence. 2008. Patent No: WO 2008/148519 A2.
- [10].Chemmanur AT, Wu GY. Drug evaluation: Albuferon-alpha - an antiviral interferon-alpha/albumin fusion protein. Curr Opin Investig Drugs 2006; 7: 750-8; PMID:16955687 [PubMed] [Google Scholar]
- [11].Fliedl L, Grillari J, Grillari-Voglauer R. Human cell lines for the production of recombinant proteins: on the horizon. N Biotechnol 2015; 6: 673-9; http://dx.doi.org/ 10.1016/j.nbt.2014.11.005 [DOI] [PubMed] [Google Scholar]
- [12].Jayapal KR, Wlaschin KF, Hu WS, Yap MGS. Recombinant protein therapeutics from CHO cells - 20 years and counting. Chem Eng Prog 2007; 10: 40-7. [Google Scholar]
- [13].Seo JS, Min BS, Kim YJ, Cho JM, Baek E, Cho MS, Lee GM. () Effect of glucose feeding on the glycosylation quality of antibody produced by a human cell line, F2N78, in fed-batch culture. Appl Microbiol Biotechnol 2014; 98: 3509-15; PMID:24384750; http://dx.doi.org/ 10.1007/s00253-013-5462-0 [DOI] [PubMed] [Google Scholar]
- [14].Chen F, Ye Z, Zhao L, Liu X, Fan L, Tan WS. Biphasic addition strategy of hypoxanthine and thymidine for improving monoclonal antibody production. J Biosci Bioeng 2012; 114: 347-52; PMID:22652083; http://dx.doi.org/ 10.1016/j.jbiosc.2012.04.015 [DOI] [PubMed] [Google Scholar]
- [15].Selvarasu S, Ho YS, Chong WPK, Wong NS, Yusufi FN, Lee YY, Yap MG, Lee DY. Combined in silico modeling and metabolomics analysis to characterize fed-batch CHO cell culture. Biotechnol Bioeng 2012; 109: 1415-29; PMID:22252269; http://dx.doi.org/ 10.1002/bit.24445 [DOI] [PubMed] [Google Scholar]
- [16].Du Z, Treiber D, McCarter JD, Fomina-Yadlin D, Saleem RA, McCoy RE, Zhang Y, Tharmalingam T, Leith M, Follstad BD, et al.. Use of a Small Molecule Cell Cycle Inhibitor to Control Cell Growth and Improve Specific Productivity and Product Quality of Recombinant Proteins in CHO Cell Cultures. Biotechnol Bioeng 2015; 112: 141-55; PMID:25042542; http://dx.doi.org/ 10.1002/bit.25332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carvalhal AV, Santos SS, Calado J, Haury M, Carrondo MJ. Cell growth arrest by nucleotides, nucleosides and bases as a tool for improved production of recombinant proteins. Biotechnol Prog 2003; 19: 69-83; PMID:12573009; http://dx.doi.org/ 10.1021/bp0255917 [DOI] [PubMed] [Google Scholar]
- [18].Dutton RL, Scharer J, Moo-Young M. Cell cycle phase dependent productivity of a recombinant Chinese hamster ovary cell line. Cytotechnology 2006; 52: 55-69; PMID:19002865; http://dx.doi.org/ 10.1007/s10616-006-9041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen F, Kou TC, Fan L, et al.. The combined effect of sodium butyrate and low culture temperature on the production, sialylation, and biological activity of an antibody produced in CHO cells. Biotechnol Bioprocess Eng 2011; 16: 1157-65; http://dx.doi.org/ 10.1007/s12257-011-0069-8 [DOI] [Google Scholar]
- [20].Haryadi R, Ho S, Kok YJ, Pu HX, Zheng L, Pereira NA, Li B, Bi X, Goh LT, Yang Y, et al.. Optimization of Heavy Chain and Light Chain Signal Peptides for High Level Expression of Therapeutic Antibodies in CHO Cells. Plos One 2015; 10: e0116878; PMID:25706993; http://dx.doi.org/ 10.1371/journal.pone.0116878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nagano R, Masuda K. Establishment of a signal peptide with cross-species compatibility for functional antibody expression in both Escherichia coli and Chinese hamster ovary cells. Biochem Biophys Res Commun 2014; 447: 655-9; PMID:24755069; http://dx.doi.org/ 10.1016/j.bbrc.2014.04.060 [DOI] [PubMed] [Google Scholar]
- [22].Barash S, Wang W, Shi YG. Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem Biophys Res Commun 2002; 294: 835-42; PMID:12061783; http://dx.doi.org/ 10.1016/S0006-291X(02)00566-1 [DOI] [PubMed] [Google Scholar]
- [23].Xiao W, Li CQ, Xiao XP, Lin FZ. Expression and fast preparation of biologically active recombinant human coagulation factor VII in CHO-K1 cells. Genet Mol Res 2013; 12: 6813-24; PMID:24391029; http://dx.doi.org/ 10.4238/2013.December.16.7 [DOI] [PubMed] [Google Scholar]
- [24].Bi JX, Shuttleworth J, Ai-Rubeai M. Uncoupling of cell growth and proliferation results in enhancement of productivity in p21(C1P1)-arrested CHO cells. Biotechnol Bioeng 2004; 85: 741-9; PMID:14991652; http://dx.doi.org/ 10.1002/bit.20025 [DOI] [PubMed] [Google Scholar]


