ABSTRACT
Quorum sensing (QS) is a prevalently found intercellular signaling system in bacteria. QS system bestows behavioral coordination ability in bacteria at high population density. QS via acylated homoserine lactone (AHL) is extensively conserved in Gram-negative bacteria and plays crucial role in regulating many biological processes. The role of QS genes coding for AHL synthase enzyme (lasI and rhlI) was established in bioremediation of polycyclic aromatic hydrocarbons (PAHs) viz. phenanthrene and pyrene. AHL producing biofilm forming marine bacterium Pseudomonas aeruginosa N6P6 was isolated by selective enrichment on PAHs. AHL production was confirmed using AHL bioreporters and GC-MS analysis. Biofilm development and its architecture was significantly (P < 0.05) affected by alterations in lasI/rhlI expression. The lasI/rhlI gene expression pattern significantly influences biofilm formation and subsequent degradation of PAHs. The integrated density of Pseudomonas aeruginosa N6P6 biofilm was highest for 48 h old biofilm and the PAHs (phenanthrene and pyrene) degradation was also found maximum (85.6 % and 47.56 %) with this biofilm. A significant positive correlation (P < 0.05) was observed between lasI expression and PAHs degradation. The role of QS genes in biofilm formation and degradation of PAHs was validated by blocking the transcription of lasI/rhlI by a QS inhibitor (QSI) tannic acid. Further, application of such QS positive isolates in PAHs contaminated sites could be a promising strategy to improve the PAHs bioremediation.
KEYWORDS: AHL, biofilm, PAHs, quorum sensing, tannic acid
Introduction
Density sensing mechanism mediated by N- acyl- homoserine lactone (AHL) based quorum sensing (QS) endowed in gram-negative bacteria empowers them with enhanced bioremediation potential. Various key features regulated by QS genes alleviating the biodegradation of polycyclic aromatic hydrocarbons (PAHs) are biofilm formation, biosurfactant production, horizontal gene transfer and catabolic gene expression.1 Bacterial biofilm has been recognized as an impeccable agent in bioremediation technology. QS via AHLs regulates biofilm architecture, development and maturation.2 Diffusible autoinducers (AIs) such as AHLs aid in biofilm formation. Marine bacteria under the genera Pseudoalteromonas, Thalassomonas, Vibrio and Pseudomonas often form dense biofilm with the help of these AIs. Among the known QS positive isolates, Pseudomonas aeruginosa has been explored for its QS associated properties. P. aeruginosa is found in diverse environments such as clinical, terrestrial, and aquatic set- ups and show tremendous potential to degrade various xenobiotics.3 The QS system of P. aeruginosa is very well conserved consisting of lasI/R and rhlI/R genes coding for the Lux family transcriptional activators.4
The bacterial communication system mediated via AHL involves binding of AHL to transcriptional factor which further initiates the signaling cascades to regulate appropriate gene(s). In P. aeruginosa, the synthesis of AHL signal molecule i.e. N-(3-oxododecanoyl)-l-homoserine lactone (3O-C12-HSL) is regulated by lasI/R. In order to become an active transcription factor, lasR requires the released AHL molecule (3O-C12-HSL). The transcription of various genes involved in the synthesis of biosurfactant, EPS and biofilm development relies on this signaling molecule 3O-C12-HSL. Only multimeric form of LasR has the potential to bind DNA, whereas the formation of multimeric LasR is dependent on 3O-C12-HSL.5,6
RhlI and RhlR proteins constitute the second QS system in P. aeruginosa. Both the QS system work synchronously and constitute a regulatory cascade. The rhlI/R mediated QS system always works under the control of the lasI/R QS system. RhlI synthase facilitates the synthesis of short chain AHL such as N-butyryl-l-homoserine lactone (C4-HSL) via the transcriptional regulator, RhlR.7 RhlR often forms complex with C4-HSL and promotes the expression of several other genes (e.g rhamnosyltransferase, aminopeptidase, endoproteinase etc.). Both the 3O-C12-HSL and C4-HSL signaling molecule diffuse freely from the bacterial cells and initiates the communication cascade.8 However, 3O-C12-HSL has high molecular weight and it diffuses at a significantly slower rate in comparison to C4-HSL.
Among the phenotypes regulated by QS system, biofilm formation holds the premier position. On the basis of comparison made between the biofilm structure of wild type P. aeruginosa strain and isogenic rhlI, lasI and lasIrhlI mutant, an initial relationship between biofilm formation and QS was established.9 Structured biofilm was formed in wild-type and rhlI mutant, whereas, undifferentiated and flat biofilm was observed in lasI and lasIrhlI mutant. The flat biofilm formed in the lasIrhlI mutant strains were prone to various stress conditions and were susceptible to the surfactants such as sodium dodecyl sulfate.9 On the other hand, the structured biofilms formed by the wild type P. aeruginosa was found to be resistant. Bacterial motility (Swimming, swarming and twitching motility) in case of P. aeruginosa enhances biofilm mediated bioremediation by many folds as actively growing cells sense the pollutant and colonises. However, QS mutants lack swarming motility and exhibits reduced biofilm formation and bioremediation.10
PAHs comprising of 2 or more fused aromatic rings are of great concern because of their persistent nature.11 PAHs are hydrophobic in nature which is a major limiting factor for their degradation in the aquatic environment.12 However, biofilm forming marine bacteria are effective xenobiotic degraders. They have potential to strive in harsh and fluctuating environment and the biofilms provide an appropriate aid for enhancing the degradation of hydrophobic PAH in both aqueous and terrestrial environment.13,14 The extracellular polymeric substances (EPS) secreted by bacterial biofilm composed of many hydrophobic cores, which can increase the solubility of PAHs in the aqueous environment. Besides, bacteria from marine environment with QS system which regulate biofilm formation can further enhance degradation of PAH by increasing the cell density around the vicinity of the PAH compounds.15
The focus of our study was to establish a relationship between QS, biofilm formation and bioremediation of PAH. QS positive marine bacterium P. aeruginosa N6P6 was used in the present study. The bacterium can form dense biofilm after 48 h and it harbours both rhl and las QS system. The expression profiling of rhlI and lasI, encoding AHL synthase, was studied under different phase of biofilm growth and PAH stress such as phenanthrene and pyrene. AHLs produced by this bacterium were characterized by various chromatographic techniques. The effect of QS gene(s) expression on biofilm growth and its consequent impact on PAHs (phenanthrene and pyrene) degradation was monitored. We applied a potent QS inhibitor (QSI), tannic acid, for further validating the role of QS in biofilm formation and bioremediation. Tannic acid is a phenolic compound obtained from plants. It has multiple phenol groups linked together and hence often called as polyphenol. The presence of many phenols together constituting characteristically different structures makes them physically, chemically and biologically inimitable and endows them with many unique properties (metabolic, toxic, therapeutic, etc.).16 In the present study, the transcription of lasI and rhlI was directly inhibited by tannic acid by some unknown mechanism, which potentially affects the expression and function of Lux protein.17
Results and discussion
AHL characterization and genetic basis of AHL production
QS has been reported to regulate various phenotypes in P. aeruginosa N6P6. Biofilm formation is one such QS regulated phenotype having crucial role in degradation of PAHs. P. aeruginosa N6P6 synthesizes 2 different types of AHL molecules, which was confirmed through TLC and GC-MS analysis. Both TLC (via overlaying the QS bioreporters) and GC-MS analysis revealed the presence of C4HSL and 3OC12-HSL [compared with standard AHLs mass spectra (1 mg/ml stock was prepared in methanol)]. Chromobacterium violaceum CV026 (CECT 5999), a violacein-negative, mini-Tn5 mutant was used as a bioreporter for detecting short chain AHLs.18 Pigment production can be restored in the strain by incubating it with AHLs positive isolates. Similarly, for detecting long chain AHL, Agrobacterium tumefaciens NTLR4 (ATCC-BAA2240) was used. A. tumefaciens NTLR4 harbours a β-galactosidase gene driven by a promoter traI. Long chain AHL signaling molecules induce the expression of β-galactosidase. In the presence AHLs, β-galactosidase is induced and it cleaves X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) resulting in the formation a blue precipitate.19 The AHL production was further confirmed by comparing P. aeruginosa N6P6 crude AHL extract with standard AHLs retention time through HPLC analysis. The genetic basis of AHL production in P. aeruginosa N6P6 was confirmed by amplifying the quorum sensing (QS) genes (lasI and rhlI) coding for AHL synthase with the aid of gene-specific primers.
To understand the expression pattern of AHL synthase during planktonic and biofilm mode of growth, semi-quantitative PCR was performed. During biofilm mode of growth, the expression of lasI gene increased with time. The expression of rhlI remained constant throughout the observed time frame. QS genes play crucial role in the synthesis of EPS and biofilm maturation. For validating the role of QS genes in biofilm formation and development, RNA was extracted from biofilm cultures at different time interval (15–72 h) and relative expression of QS genes was studied with qRT-PCR. lasI expression increased with increase in biofilm growth and declines slightly for 72 h old biofilm. Development of mature biofilm follows a cascade of events i.e., attachment to substratum, colonization, EPS production, primary and secondary maturation and ultimately dispersion. EPS are the most crucial component of the biofilm. lasI regulates the expression of several gene(s) involved in the production of glucose rich EPS-matrix. The slight decrease in lasI expression at this point indicates that lasI might not have any essential role once a biofilm community has been established. The other reason for decreased expression of 72 h old biofilm of P. aeruginosa N6P6 might be the initiation of dispersion phase of biofilm.
The trend observed in rhlI expression was completely different from lasI expression pattern. rhlI gene remained positively upregulated for 15 h, 48 h and 72 h, but down regulation was observed for 24 h old biofilm. To correlate the QS genes expression with biofilm development, the biofilm surface architecture of 15 h, 24 h and 48 h old P. aeruginosa N6P6 biofilm was studied with the help of fluorescence microscopy. Biofilm growth and lasI expression follows similar trend i.e. biofilm growth and lasI expression increases from 15 to 48 h followed by reduction in biofilm growth and lasI expression at 72 h. P. aeruginosa N6P6 biofilm surface architecture turned more rough and heterogeneous with time. However, a significant positive correlation (P > 0.05) was not established between P. aeruginosa N6P6 biofilm growth and expression of rhlI gene. The fold difference between lasI and rhlI gene expression level was least for 15 h old biofilm and was maximum for 24 h old biofilm specifying the significance of both these genes in initial biofilm development and maturation. Large difference between lasI and rhlI gene expression level resulted in topologically smooth surface biofilm at this point of time. A very structured biofilm was observed at 48 h and 72 h old biofilm. The upregulation of rhlI gene at 48 h and 72 h. might be a reason behind the metamorphosis of smooth surface biofilm to distinct 3-dimensional architecture.
Role of QS genes in biofilm growth and PAHs degradation
After establishing the relationship between biofilm development and QS genes expression, our next aim was to establish relationship between QS genes expression, biofilm growth and PAHs degradation. Two representative members of PAH family, phenanthrene and pyrene were selected for the degradation study. The efficiency of 48 h old and 72 h old biofilm in degrading phenanthrene was almost same. 21.5%, 54.2%, 85.6% and 85.7% phenanthrene degradation was achieved in 7 d by 15 h, 24 h, 48 h and 72 h old biofilm respectively. Day wise analysis of phenanthrene degradation indicated that biofilm growth and QS gene (lasI and rhlI) expression had significant effect on phenanthrene degradation. Similar trend was also observed during pyrene degradation. 48 h old biofilm was more effective in degrading pyrene as compared to other. A significant positive correlation (P < 0.05) was observed between biofilm growth, lasI expression and pyrene degradation.
Further, to understand P. aeruginosa N6P6 QS gene expression pattern under PAH stress, the bacterium was cultured in PAH substituted media. Under phenanthrene stress, the expression of lasI was 3 fold higher than rhlI in P. aeruginosa N6P6 biofilm culture. In contrast, 9 fold higher expression of rhlI gene than lasI was observed for biofilm culture grown under pyrene stress. Cells embedded in the biofilm are often released if PAH compounds are present in the growth medium. The availability of hydrophobic compounds in the growth medium might play crucial role in the production of emulsifiers. Production of emulsifying agents enhances the solubility of a PAH molecule and makes it available to the bacterial cells encased in biofilm matrix.
Tannic acid as potent QS inhibitor
To confirm whether biofilm formation is only time depended factor or it actually rely on QS genes (i.e., with time the cells aggregate and form biofilm or QS based genetic mechanism governs the phenomenon), a QS inhibitor tannic acid was used for validating the role of QS in biofilm formation and PAHs degradation. Tannic acid blocks the transcription of QS genes, hence the production of AHL synthase is also inhibited.16 Different concentrations of tannic acid were tested to find the threshold concentration for complete inhibition of AHL production in P. aeruginosa N6P6. AHLs were extracted from P. aeruginosa N6P6 culture after treatment with different concentration of tannic acid. The extracted AHLs were incubated with bioreporter Chromobacterium violaceum CV026 (CECT 5999) for 24 h. In the presence of AHL, CV026 produces deep violet pigment. In the present experimental study, 0.3 mg/ml of tannic acid completely inhibited AHL production in P. aeruginosa N6P6 without interfering with its growth.
To ascertain the non-toxic effect of tannic acid on P. aeruginosa N6P6 growth, the bacterium was grown in the presence of tannic acid at a threshold concentration. Tannic acid showed no toxic effect on the growth of the bacterium. However, other phenotypes regulated by QS genes viz. biofilm formation, pyocyanin production etc. were observed to be inhibited totally in the presence of tannic acid. Tannic acid treated biofilm was stained with aqueous solution of acridine orange (0.02 %) were analyzed with fluorescence microscope (Olympus, 1X71, Japan) under 20X magnification. Fluorescence micrograms were processed to construct biofilm 3D surface plot using IMAGE J interactive 3D surface plot tool. Tannic acid treatment caused a decrease in biofilm density by 83.2%. Flat biofilm without any structural heterogeneity was observed in the presence of 0.3 mg/ml tannic acid, whereas highly structured biofilm was formed by P. aeruginosa N6P6 in absence of tannic acid. The biofilm growth declined in the growth medium supplemented with tannic acid. The biofilm growth retardation becomes more prominent with increasing concentration of tannic acid and ultimately biofilm appears as straight line with no surface coverage (Fig. 1). Biofilm growth was quantified as raw integrated density calculated using IMAGE J. The raw integrated density reduces by around 94% on addition of 0.1mg/ml tannic acid. Further increase in tannic acid concentration completely inhibited the biofilm growth resulting in significant reduction in integrated density (P < 0.0001; One way ANOVA) (Fig. 2). In the similar manner, pyocyanin production was also reduced by 64.2% compared to the untreated P. aeruginosa N6P6 culture.
Figure 1.
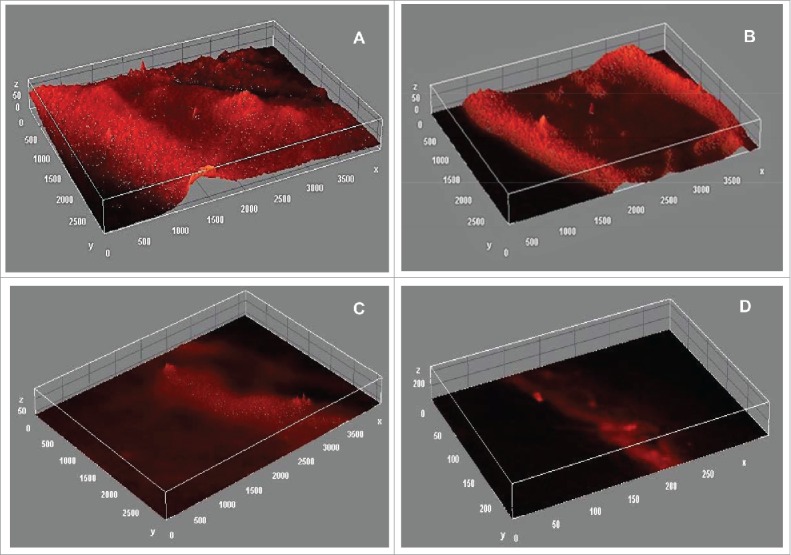
Effect of tannic acid on P. aeruginosa N6P6 biofilm growth and structure (A) Control (no tannic acid) (B) 0.1 mg/ml tannic acid (C) 0.2 mg/ml tannic acid (D) 0.3 mg/ml tannic acid.
Figure 2.
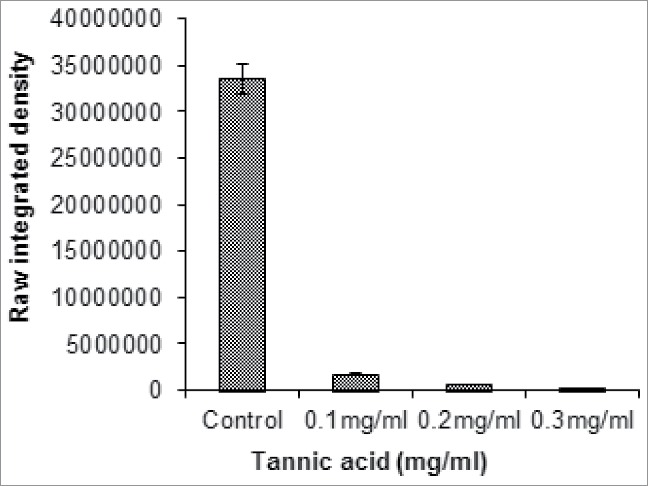
P. aeruginosa N6P6 biofilm growth in the presence of tannic acid (calculated as raw integrated density using IMAGE J).
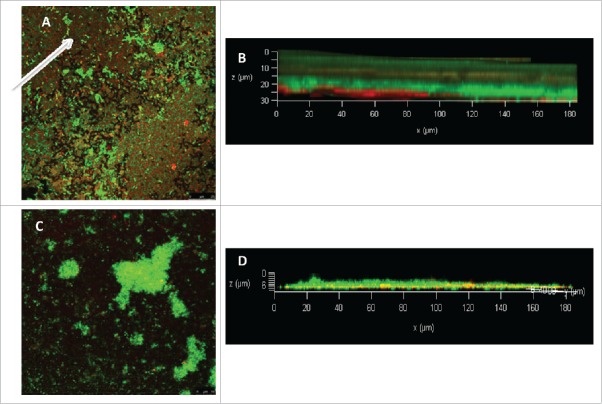
The centered aim of this study was to understand the involvement of the QS genes in PAHs bioremediation. It was observed that treatment with 0.3 mg/ml tannic acid resulted 90% reduction in lasI and 56.6 % reduction in rhlI gene expression. Such reduction in QS genes expression caused a decrease in PAHs degradation. 18.6% and 15.38 % reduction in phenanthrene and pyrene degradation was observed respectively in presence of tannic acid. PAHs degradation by planktonic culture of P. aeruginosa N6P6 was also decreased by 23% when tannic acid was added in the degradation medium. EPS production plays crucial role in degradation of PAHs by acting as biosurfactant to enhance its solubility. rhlI gene expression controls the EPS production resulting in partial solubilisation of PAHs in aqueous environment. Solubilisation of PAHs makes it easily available to bacterial cells for ensuing degradation. For analyzing the effect of QS inhibitor tannic acid on EPS production, confocal laser scanning microscopic (CLSM) studies were performed for tannic treated and untreated biofilm after staining with 5 μmol l−1 Syto9 and 100 μg ml−1 of ConA-TRITC (concanavalin A-tetramethylrhodamine isothiocyanate).14 Syto9 binds to the cells staining them green whereas, ConA-TRITC binds to the EPS portion of biofilms staining them red. Tannic acid (0.3 mg/ml) treatment reduces the EPS production considerably as the red stained portions diminishes completely in the treated biofilm (Fig. 3). The z stack images (Fig. 3 D) clearly reveal the reduction in EPS production and overall reduction in biofilm thickness on treatment with QS inhibitor tannic acid.
Figure 3.
CLSM images of P. aeruginosa N6P6 biofilm growth. (A) Untreated, without tannic acid (arrow indicating EPS portions); (B) Z stack image of untreated biofilm; (C) Biofilm treated with 0.3 mg/ml tannic acid; (D) Z stack image of tannic acid (0.3 mg/ml) treated biofilm. Red stained area was significantly reduced in tannic acid treated biofilm indicating no or very less EPS. The bacterial cells were not affected as their number did not reduce after tannic acid treatment (green stained).
Yong and Zhang21 reported similar trend of decreased aromatic xenobiotic degradation by a QS mutant isolate. A significant decrease in the degradation of an aromatic xenobiotic was observed when rhl QS system of Pseudomonas aeruginosa CGMCC1.860 was deleted. Catechol 2,3-dioxygenase a key enzyme responsible for degradation of various xenobiotics was also repressed on deletion of rhl QS system of Pseudomonas aeruginosa CGMCC1.860. Sarabhai et al.22 also reported similar effects of ellagic acid (a type of tannin) in inhibiting QS genes expression. Ellagic acid treatment resulted in 89 and 90% reduction in lasI and rhlI expression in P. aeruginosa PAO1. Biofilms are natural emulsifiers that disperse the oil and provide greater surface area for growth. Such decrease in PAHs degradation indicates that QS genes play crucial role in the regulation of catabolic genes responsible for PAHs degradation. To comprehend the role of QS genes in catabolic gene expression and regulation, further experiments with directed real time PCR expression analysis is needed. To expedite the bioremediation of PAHs, a combinatorial approach comprising of QS and PAHs catabolic genes will be studied in future in this potential marine bacterium.
Conclusion
The findings from the present study suggest that lasI gene encoding long chain-AHL and rhlI gene encoding short chain-AHL regulates biofilm formation and development and EPS production in P. aeruginosa N6P6. These factors along with intracellular signaling molecules significantly enhance the bioremediation performance. The QS mediated intracellular signaling process operates at society level; hence the activities regulated via QS process might play crucial role in enhancing bioremediation of natural contaminated sites. Bacterial biofilm is one such structured community, envisioned in nature till date. Unique chemical composition and communication capabilities of biofilm provide resistance to various stresses and make it the best aspirant for restoring the contaminated environment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Huang Y, Zeng Y, Yu Z, Zhang J, Feng H, Lin X. In silico and experimental methods revealed highly diverse bacteria with quorum sensing and aromatics biodegradation systems–a potential broad application on bioremediation. Bioresour Technol 2005; 148:311-316; http://dx.doi.org/ 10.1016/j.biortech.2013.08.155 [DOI] [PubMed] [Google Scholar]
- [2].Das S. Microbial Biodegradation and Bioremediation 2014. 1st Edition: Elsevier, Germany. [Google Scholar]
- [3].Chakraborty J, Das S. Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11. Environ Sci Pollut Res 2014; 21:14188-141201; http://dx.doi.org/ 10.1007/s11356-014-3308-7 [DOI] [PubMed] [Google Scholar]
- [4].Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 1997; 17918:5756-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci 1994; 91:197-201. 4; http://dx.doi.org/ 10.1073/pnas.91.1.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kiratisin P, Tucker KD, Passador L. LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J Bacteriol 2002; 184:4912-4919; PMID:12169617; http://dx.doi.org/ 10.1128/JB.184.17.4912-4919.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ochsner UA, Koch AK, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 1994; 176:2044-2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acyl homoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci 1995; 92:1490-1494; http://dx.doi.org/ 10.1073/pnas.92.5.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998; 280(5361):295-8; PMID:9535661; http://dx.doi.org/ 10.1126/science.280.5361.295 [DOI] [PubMed] [Google Scholar]
- [10].Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol Microbiol 2006; 62(5):1264-77; PMID:17059568; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05421.x [DOI] [PubMed] [Google Scholar]
- [11].IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Polynuclear aromatic compounds, Part 1, Chemical, environmental and experimental data 1983; 32:1-453 [PubMed] [Google Scholar]
- [12].Mangwani N, Shukla SK, Rao TS, Das S. Calcium-mediated modulation of Pseudomonas mendocina NR802 biofilm influences the phenanthrene degradation. Colloids Surf B 2014; 114:301-309; http://dx.doi.org/ 10.1016/j.colsurfb.2013.10.003 [DOI] [PubMed] [Google Scholar]
- [13].Shimada K, Itoh Y, Washio K, Morikawa M. Efficacy of forming biofilms by naphthalene degrading Pseudomonas stutzeri T102 toward bioremediation technology and its molecular mechanisms. Chemosphere 2012; 87:226-233; PMID:22285037; http://dx.doi.org/ 10.1016/j.chemosphere.2011.12.078 [DOI] [PubMed] [Google Scholar]
- [14].Mangwani N, Shukla SK, Kumari S, Rao TS, Das S. Characterization of Stenotrophomonas acidaminiphila NCW-702 biofilm for implication in the degradation of polycyclic aromatic hydrocarbons. J Appl Microbiol 2014; 18:12602. [DOI] [PubMed] [Google Scholar]
- [15].Dash HR, Mangwani N, Chakraborty J, Kumari S, Das S. Marine bacteria: potential candidates for enhanced bioremediation. Appl Microbiol Biotechnol 2013; 97(2):561-571; PMID:23212672; http://dx.doi.org/ 10.1007/s00253-012-4584-0 [DOI] [PubMed] [Google Scholar]
- [16].Salminen JP, Karonen M, Sinkkonen J. Chemical ecology of tannins: recent developments in tannin chemistry reveal new structures and structure–activity patterns. Chem Eur J 2011; 17(10):2806-16; http://dx.doi.org/ 10.1002/chem.201002662 [DOI] [PubMed] [Google Scholar]
- [17].Chang CY, Krishnan T, Wang H, Chen Y, Yin WF, Chong YM, Chan KG. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci Rep 2014; 4: 7245; http://dx.doi.org/ 10.1038/srep07245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997; 143(12):3703-11; PMID:9421896; http://dx.doi.org/ 10.1099/00221287-143-12-3703 [DOI] [PubMed] [Google Scholar]
- [19].Kawaguchi T, Chen YP, Norman RS, Decho AW. Rapid screening of quorum-sensing signal N-acyl homoserine lactones by an in vitro cell-free assay. Appl Environ Microbiol 2008; 74(12): 3667-71; PMID:18424536; http://dx.doi.org/ 10.1128/AEM.02869-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wei Q, Ma LZ. Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int J Mol Sci 2013; 14(10): 20983-1005; PMID:24145749; http://dx.doi.org/ 10.3390/ijms141020983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yong YC, Zhong JJ. Regulation of aromatics biodegradation by rhl quorum sensing system through induction of catechol meta-cleavage pathway. Bioresour Technol 2013; 136: 761-765; PMID:23582222; http://dx.doi.org/ 10.1016/j.biortech.2013.03.134 [DOI] [PubMed] [Google Scholar]
- [22].Sarabhai S, Sharma P, Capalash N. Ellagic acid derivatives from Terminalia chebula Retz. downregulate the expression of quorum sensing genes to attenuate Pseudomonas aeruginosa PAO1 virulence. PLoS One 2013; 8(1):e53441; PMID:23320085; http://dx.doi.org/ 10.1371/journal.pone.0053441 [DOI] [PMC free article] [PubMed] [Google Scholar]