Supplemental Digital Content is available in the text.
Keywords: epidemiology, hormones, stroke, women
Abstract
Background and Purpose—
The benefit/risk analysis of hormone therapy in postmenopausal women is not straightforward and depends on cardiovascular disease. Evidence supports the safety of transdermal estrogens and the importance of progestogens for thrombotic risk. However, the differential association of oral and transdermal estrogens with stroke remains poorly investigated. Furthermore, there are no data regarding the impact of progestogens.
Methods—
We set up a nested case–control study of ischemic stroke (IS) within all French women aged 51 to 62 years between 2009 and 2011 without personal history of cardiovascular disease or contraindication to hormone therapy. Participants were identified using the French National Health Insurance database, which includes complete drug claims for the past 3 years and French National hospital data. We identified 3144 hospitalized IS cases who were matched for age and zip code to 12 158 controls. Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI).
Results—
Compared with nonusers, the adjusted ORs of IS were1.58 (95% CI, 1.01–2.49) in oral estrogen users and 0.83 (0.56–1.24) in transdermal estrogens users (P<0.01). There was no association of IS with use of progesterone (OR, 0.78; 95% CI, 0.49–1.26), pregnanes (OR, 1.00; 95% CI, 0.60–1.67), and nortestosterones (OR, 1.26; 95% CI, 0.62–2.58), whereas norpregnanes increased IS risk (OR, 2.25; 95% CI, 1.05–4.81).
Conclusions—
Both route of estrogen administration and progestogens were important determinants of IS. Our findings suggest that transdermal estrogens might be the safest option for short-term hormone therapy use.
Despite a decline in the use of postmenopausal hormone therapy (HT) in the last decade, it continues to be prescribed to many women worldwide because of its effectiveness in correcting climacteric symptoms.1 Following the results of the Women’s Health Initiative HT clinical trials showing risks of HT to outweigh benefits,2 medical guidelines have been modified, and the current recommendation is that women should be prescribed the lowest effective dose for the shortest possible duration.3 In this context, venous thromboembolism (VTE) and stroke are the main harmful effects of HT use.4 Better understanding the cardiovascular risk attributable to HT would allow the benefit–risk ratio associated with its use to be improved. Epidemiological and biological data show that oral but not transdermal estrogens activate blood coagulation and increase risk of VTE.4,5 In addition, the type of progestogens associated with estrogens used by non-hysterectomized women has emerged as another important determinant of thrombotic risk.6,7 Taken together, these findings suggest that transdermal estrogens alone, or in combination with micronized progesterone, may be the safest option to reduce thrombotic risk. However, the extent to which the same is true for the risk of stroke in HT users is unknown. Data on the association of transdermal estrogens with risk of stroke are scarce. The only study to have examined this issue suggested that low doses of transdermal estrogens were likely to be safe in relation to ischemic cerebrovascular disease.8 However, this association has to be further investigated. In addition, the impact of different pharmacological classes of progestogens on stroke risk is yet to be examined. Accordingly, we used the French National Health Insurance database to construct a nested case/control study with the aim of investigating the role of estrogens by route of administration, as well as progestogens in ischemic stroke (IS).
Methods
Data Source and Study Design
Data are drawn from the French national health insurance system (Système National d’Information Inter-Régimes d’Assurance Maladie), a database that includes over 97% of the French population. It covers 3 consecutive years and contains comprehensive records of all outpatient drug reimbursements, demographic data (sex, age, zip code of residence), and information on the patient’s health status, including all severe long-term chronic diseases with the corresponding diagnoses codes. This database is linked to the French Hospital Discharge Database (Program de Médicalisation des Systèmes d’Information), which provides information on all patients admitted to French hospitals, including discharge diagnoses coded according to the International Classification of Diseases 10th edition.
We started out with all women aged 51 to 62 years between January 1, 2009, and December 31, 2011 (n=5 532 341), and we set up a nested case–control study of IS in which each case was matched to ≤4 controls using 1-year age band and exact zip code. The detailed selection process is presented as the flow chart (Figure 1) and described later.
Figure 1.
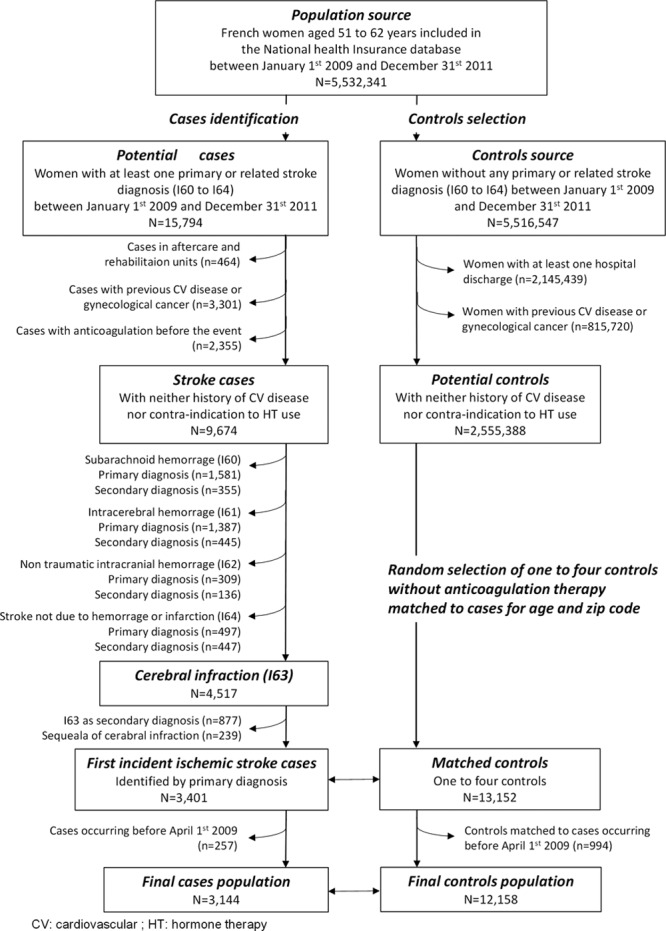
Flow-chart of the selection of cases and controls. CV indicates cardiovascular; and HT, hormone therapy.
This study was approved by the French ethical committee and the data protection agency (Commission Nationale Informatique et Liberté, no 911247).
Cases Identification
The starting point was all women aged 51 to 62 years (n=15 794) with a first hospitalization between January 1, 2009, and December 31, 2011, for a stroke (International Classification of Diseases 10th edition codes I60 to I64). We subsequently excluded cases identified in aftercare and rehabilitation units or home hospitalization (n=464) because these patients were more likely to have had a stroke before 2009. We also excluded cases who presented a contraindication to HT use defined as a personal history of cardiovascular disease or gynecological cancers (breast, uterine, ovary) before the stroke occurrence (n=3301). Then, we excluded cases using antithrombotic (including platelet aggregation inhibitors) therapy during the 3 months before the event (n=2355).
Of the 9674 remaining cases, we excluded non-IS cases who presented International Classification of Diseases 10th edition diagnoses codes I60 (n=1936), I61 (n=1832), I62 (n=445), and I64 (n=944), IS cases with the I63 code as a secondary diagnosis (n=877), and cases with a related diagnosis corresponding to sequela of cerebral infarction I69.3 (n=239), leading to 3401 first IS. Details of these exclusions and characteristics of patients by stroke subtypes are given in the online-only Data Supplement (please see Table I in the online-only Data Supplement). There were large differences in cardiovascular risk factors between ischemic and nonischemic cases, justifying not pooling stroke subtypes in the analyses.
Selection of Controls
Controls were randomly selected among healthy women who had never been hospitalized during the follow-up and who had no contraindication to HT use (same definition as for cases). Of the 5 516 547 women who were not potential stroke cases, we excluded those who had at least one hospitalization (n=2 145 439) and those with long-term chronic disease codes for cardiovascular disease or gynecological cancers (n=815 720). Among the 2 555 388 remaining women, ≤4 controls without antithrombotic therapy were selected randomly and matched to each case for 1-year age band and exact zip code (n=13 154). If 4 controls with adequate criteria were not found, ≤3 were selected. There were 2995 cases matched to 4 controls, 371 to 3, 26 to 2, and 9 to 1 control.
Assessment of Hormone Therapy
Each woman was classified according to her HT exposure history at the index date, defined as the time of event for cases and the corresponding date for matched controls. HT exposure was assessed using data on drugs reimbursement which occurred before the index data, and women were classified as current HT users if they had at least one reimbursement of HT at any time during the 3 last months before this date. HT users were then classified according to route of estrogen administration (oral or transdermal) and type of concomitant progestogen (progesterone, pregnane derivatives, norpregnane derivatives, and nortestosterone derivatives). Estrogen therapy was further subdivided according to dose (low: ≤1 mg/d of oral estrogens or <50 μg/d of transdermal estrogens; intermediate: 1.5 mg/d of oral estrogens or 50 μg/d of transdermal estrogens; high: ≥2 mg/d of oral estrogens or >50 μg/d of transdermal estrogens).
Final Study Population
As HT exposure was defined as lasting 3 months before the index date, we excluded cases occurring between January 1, 2009, and March 31, 2009, and their corresponding controls. The final study population consisted of 3144 IS cases and 12 158 controls.
Statistical Analysis
Continuous measures were expressed as means (SD) and categorical variables as absolute numbers and percentages. Characteristics of cases and controls were compared using univariate conditional logistic regressions.
The association of HT use with IS was assessed by odds ratios (OR) and their 95% confidence intervals (CI) estimated by conditional logistic regression. After having verified the lack of interaction between estrogens and progestogens, the main impact of estrogen use by route of administration and concomitant progestogen was estimated concurrently with nonuse of HT as the reference group. Analyses were adjusted for hypertension, diabetes mellitus, and dyslipidemia, each identified as at least one reimbursement for the relevant medication during the 3 months before the index date. The analyses were also adjusted for any long-term chronic diseases at the index date. We tested the homogeneity of ORs for oral and transdermal estrogens and for the different progestogens. The impact of estrogen dose was tested separately for oral and transdermal estrogens through tests of linear trend. Stratified analyses were conducted to assess the impact of age and cardiovascular risk factors on these associations, and potential multiplicative interactions were tested.
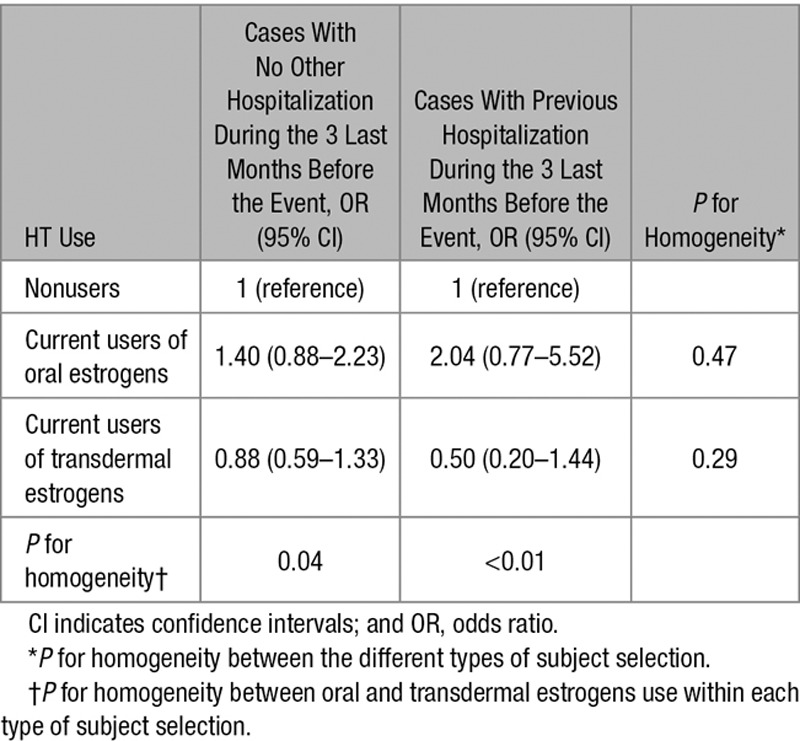
We also undertook a series of sensitivity analysis to assess the robustness of our findings. The first one consisted in exclusion of women treated by raloxifene, an osteoporosis prevention medication (n=177), or by oral contraceptives (n=115) who were initially included as nonusers in the main analysis. Second, we used polytomous logistic regression with a 3-level outcome variable (controls [reference], IS cases with no other hospitalization during the 3 last months before the event, and those with previous hospitalization over this period) to ensure similarity in these results with this classification.9 This approach allowed us to perform a test of homogeneity of the association of HT with the general health status of cases.
Finally, the number of IS and VTE cases that could be avoided by change from oral to transdermal estrogens alone or combined with progesterone was estimated using the excess incidence of stroke and VTE attributable to HT use in the Women’s Health Initiative HT trials.2
All statistical analyses were performed with SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC).
Results
Characteristics of cases and controls are shown in Table 1. By design, mean age of cases and controls was similar. Cases were more likely than controls to have reimbursement for antidiabetic (12.0% versus 4.1%; P<0.01), antihypertensive (34.8% versus 21.0%; P<0.01), and antidyslipidemia (16.9% versus 12.8%; P<0.01) medications. They were also more likely to present long-term chronic diseases (15.6% versus 1.1%; P<0.01). Overall, HT use was similar among cases and controls (6.2% versus 6.8%; P=0.19). Comparisons of the general characteristics of controls between users and nonusers and according to the route of estrogen administration and to the type of progestogens are detailed in the online-only Data Supplement (please see Tables II–IV in the online-only Data Supplement). General characteristics of controls did not differ according to the route of estrogen administration, whereas controls who used norpregnanes were more likely to be prescribed antihypertensive medication than those who used other type of progestogen.
Table 1.
General Characteristics of Cases and Controls
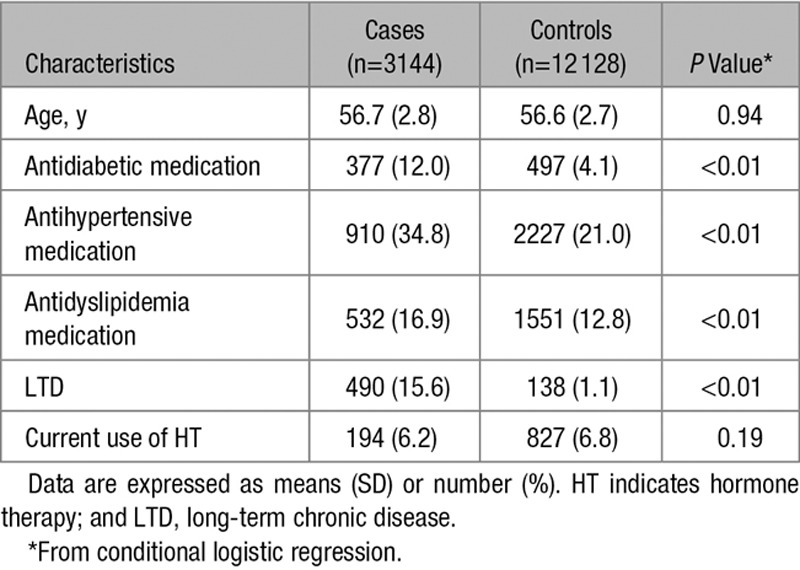
Table 2 shows the IS risk associated with HT use by route of estrogen administration and classes of progestogens. In this population, 17β-estradiol was the only prescribed estrogen, and there was no statistical interaction between route of estrogen administration and progestogens on IS risk (P=0.66). In adjusted models, the route of estrogen administration was differentially associated with IS risk (P<0.01), which was increased for oral (OR, 1.58; 95% CI, 1.01–2.49) but not transdermal (OR, 0.83; 95% CI, 0.56–1.24) estrogens. Among oral estrogens users, the association increased with the dose of HT (P for linear trend <0.01). The risk was borderline significant for low-dose estrogen users and the greatest in those on high doses (OR, 1.39; 95% CI, 1.00–1.99; OR, 1.84; 95% CI, 1.02–3.30; and OR, 2.41; 95% CI, 1.43–4.07 for users of low, intermediate, and high doses, respectively). However, there was no evidence for a dose–effect relation with transdermal estrogens use (OR, 0.69; 95% CI, 0.37–1.28; OR, 0.79; 95% CI, 0.40–1.58; and OR, 0.88; 95% CI, 0.57–1.37 for low, intermediate, and high doses, respectively; Figure 2).
Table 2.
Odds Ratios of Ischemic Stroke in Relation to Current HT Use by Route of Estrogen Administration and Pharmacological Classes of Progestogens
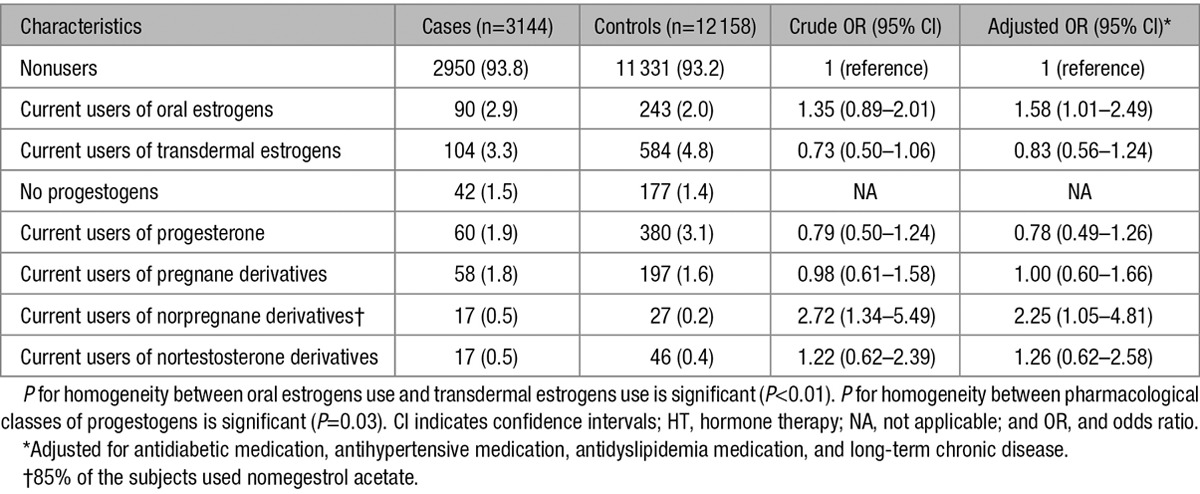
Figure 2.
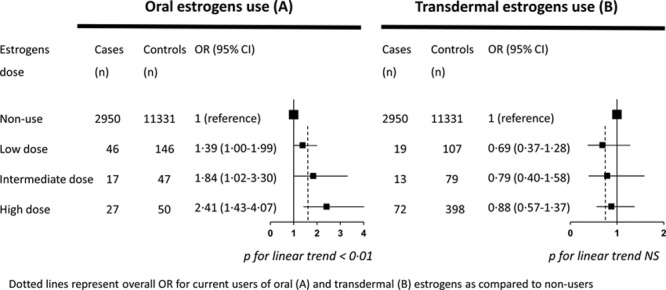
Odds ratios of ischemic stroke according to estrogen dose by route of administration. Dotted lines represent overall OR for current users of oral (A) and transdermal (B) estrogens compared with nonusers. CI indicates confidence interval; and OR, odds ratio.
The IS risk differed as a function of progestogens type (P homogeneity=0.03). Although progesterone, pregnane derivatives, and nortestosterone derivatives were not associated with IS (OR, 0.78; 95% CI, 0.49–1.26; OR, 1.00; 95% CI, 0.60–1.66; and OR, 1.26; 95% CI, 0.62–2.58, respectively), users of norpregnane derivatives had higher IS risk (OR, 2.25; 95% CI, 1.05–4.81). In this group, 85% of the subjects used nomegestrol acetate, and restricting analysis to this molecule led to similar results (OR, 2.85; 95% CI, 1.15–7.06).
Further analysis provided no evidence that age modified the association of oral and transdermal estrogens with IS risk. Using median age as a cutoff (57 years), the IS risk among users of oral and transdermal estrogens was 1.41 (95% CI, 0.98–2.03) and 0.88 (95% CI, 0.52–1.49), respectively, for younger women (1974 cases and 7678 controls) and 2.04 (95% CI, 1.37–3.03) and 0.75 (95% CI, 0.41–1.37), respectively, for older women (1427 cases and 5476 controls; P for interaction=0.62). On the contrary, presence of cardiovascular risk factors did not affect the association of oral and transdermal estrogens with IS risk (Figure 3). Finally, associations of IS with oral and transdermal estrogens were similar for cases with and without a hospitalization during the 3 last months before the event (P for homogeneity=0.47 for oral estrogens use and P for homogeneity=0.29 for transdermal estrogens use; Table 3). Excluding women using raloxifene or oral contraceptives (<2% in our sample) did not change the results (OR of IS associated with oral and transdermal estrogens: 1.68; 95% CI, 1.05–2.62; and 0.83; 95% CI, 0.56–1.24, respectively).
Figure 3.
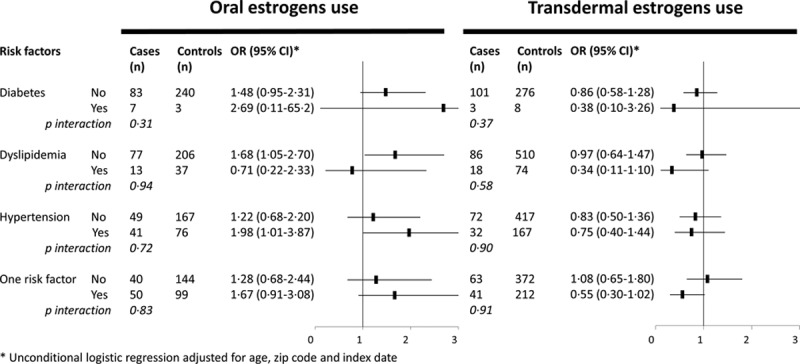
Odds ratios of ischemic stroke according to route of estrogen administration by cardiovascular risk factors. *Unconditional logistic regression adjusted for age, zip code, and index date. CI indicates confidence interval; and OR, odds ratio.
Table 3.
Odds Ratios of Ischemic Stroke in Relation to Oral and Transdermal Estrogens Use According to Whether Cases Were Hospitalized or Not During the 3 Last Months Before the Event
In the Women’s Health Initiative HT clinical trials, excess annual incidence of stroke and VTE was 9/10 000 and 21/10 000, respectively, for estrogens plus progestins users and 11/10 000 and 11/10 000, respectively, for estrogens alone. Based on these data, there were between 22 and 30 cases of stroke and VTE per 10 000 HT users that could have been avoided every year if women used transdermal rather than oral estrogens.
Discussion
Using a large French medical database, we found differences in the association of oral and transdermal estrogens with IS risk in postmenopausal women. Oral estrogens significantly increased this risk with a dose-dependent relationship, whereas transdermal estrogens displayed no association. In addition, we showed for the first time that type of concomitant progestogens was also an important determinant of this outcome. Although there was no significant association of IS with progesterone, pregnane derivatives, and nortestosterone derivatives, norpregnane derivatives were found to increase IS risk.
Our finding regarding the association of oral estrogens with IS are consistent with the overall evidence from both observational studies and randomized controlled trials that showed a significant increased risk in HT users.10 Studies that focused only on IS found an excess risk among oral estrogen users to be somewhat higher and similar to our estimates.11–17
The dose-dependent association of oral estrogens with stroke risk has been less often investigated. One early study found no association of estrogens with stroke, with dose not having any effect. However, these data cannot be extrapolated to ours because the authors compared ever to never HT users rather than current users and nonusers.11 Fifteen years ago and in an updated analysis, a clear relationship between dose of oral estrogens and IS risk was found in the Nurses’s Health Study.13,18 More recently, a large UK nested case/control study showed a more elevated risk of stroke among users of the highest estrogen doses.8 Thus, our data showing a dose-dependent association of estrogens dose with IS is consistent with these recent findings.
Although data on the association of transdermal estrogens with stroke risk are scarce, our results are consistent with previous findings. One study showed no relationship between transdermal estrogens and ischemic transitory attack, suggesting a potential neutral effect of nonoral components. In addition, Renoux et al found transdermal estrogens not to be associated with risk of stroke.8 However, no previous study has been able to show the lack of adverse effects of transdermal estrogens on IS, irrespective of the dose.
To the best of our knowledge, our study is the first to investigate the differential association of stroke risk with pharmacological classes of progestogens. Some observational data on IS provided estimates among users of unopposed estrogens and estrogens plus progestins separately, but the findings were inconclusive and appeared not to be interpretable.12,13,15 Indeed, results mostly concerned MPA and norethisterone acetate, 2 molecules belonging to pregnane and nortestosterone derivatives, respectively, that are not used in France. Other data on MPA are available from randomized controlled trials and suggest no clear effect on stroke risk.2,16,17,19 Our findings are nevertheless consistent with results concerning VTE risk. We have previously shown similar findings to the present study on thrombotic risk as a function of pharmacological classes of progestogens.6,7 Even though venous and arterial thromboembolism disorders cannot be compared directly, they share several physiopathological mechanisms, including a thrombotic component that allows us to draw parallels between these outcomes, making these new findings consistent with previous results.20
The potential mechanisms underlying the differential association of oral and transdermal estrogens with risk of IS may include imbalance in blood coagulation. There is increasing evidence for an important role of hemostatic component in the pathogenesis of IS.20–23 In particular, thrombin generation, an integrative maker of hypercoagulability state, has emerged as a new risk factor for cerebral infarction in postmenopausal women.20 In parallel with this physiological mechanism involved in stroke occurrence, it has been consistently demonstrated that oral but not transdermal estrogens activate blood coagulation and induce deleterious changes in thrombin generation among HT users.5,24 Further research is necessary to fully understand the biological mechanisms underlying the differential association of oral and transdermal estrogens with IS risk among HT users.
Physiological changes in hemostasis might also explain the increased risk of IS with use of norpregnane derivatives but not with other pharmacological classes of progestogens. There is little evidence in this domain but results from one recent cross-sectional study suggest that this pharmacological class of progestogens increases markers of blood coagulation activation and induce activated protein C resistance.25 Although the validity of this plasma phenotype as a surrogate biomarker of IS is debated,22,26 we cannot exclude its potential implication in IS because it is a derived measure of thrombin generation that has been associated with IS among postmenopausal women.20
Other biological mechanisms could involve atherogenesis, inflammation, and vascular hemodynamic changes in cerebral arteries, but the etiologic role of different HT components on these biological parameters is not yet clear.27,28
Our study presents several strengths. First, the French National medical databases offered a unique opportunity to set up a study with a large number of cases and controls to estimate associations with adequate statistical power, especially for subgroup analysis. Moreover, previous studies have shown hospital diagnosis for stroke to have good validity, especially in women who are middle-aged or older.29 In addition, we were able to verify the internal validity of our study because medications to control hypertension, the highest stroke risk factor, were more likely to be used by cases than controls. Finally, because HT exposure was not self-reported but assessed by drugs claims, participants were not subject to recall bias, and we were able to examine prospective associations.30
Our findings need to be interpreted in light to some limitations. One, we did not have data on compliance, an important issue because purchase of medication does not necessarily imply their consumption. However, it is conceivable that treatment is bought for 1 month and not used, but recurrent purchases are unlikely to remain unused. In our study, sensitivity analysis restricted to women who had >1 reimbursement during the 3 months before the index date led to similar results (OR, 1.69; 95% CI, 1.23–2.33 and OR, 0.76; 95% CI, 0.57–1.03 for oral and transdermal, respectively). In addition, lack of compliance would reduce estimates toward the null. As compliance is unlikely to be dependent on the route of administration31 and the effect size associated with use of oral estrogens was comparable to previous findings, it is unlikely that poor compliance explains, even partially, the lack of association between transdermal estrogens and stroke risk.
Another important limitation is incomplete data on risk factors and comorbidities. Some risk factors, such as hypertension, dyslipidemia, and diabetes mellitus, were correctly approximated using data on drug reimbursements that were included in the fully adjusted analysis. For example, in our study, the IS risk associated with antidiabetic medications used as a proxy of diabetes mellitus was 2.6 (95% CI, 2.2–3.0). This risk estimate is close to that reported by a study on women where diabetes mellitus was directly assessed by glycemia and antidiabetic medication use (relative risk, 3.0; 95% CI, 1.6–5.7).32 Unfortunately, other risk factors, such as body mass index or smoking status, could not be ascertained.
Another potential limitation could be the retrospective analysis of medical insurance databases. However, these data are collected prospectively. In particular, exposure to HT was systematically collected at the time of drug delivery before occurrence of clinical outcomes, independently of any information provided by the participants, therefore, precluding any recall bias.
Finally, we cannot exclude an indication bias in relation to the route of estrogen administration and type of progestogen. In theory, women with cardiovascular risk factors are more likely to be prescribed transdermal than oral estrogens. However, in our study, the prevalence of hypertension, dyslipidemia, and diabetes mellitus was similar among oral and transdermal users. Furthermore, if such bias existed, the effect would be to increase the estimated risk of IS associated with transdermal estrogens use. Differences in clinical characteristics of women could also explain a part of the excess risk associated with norpregnane derivatives. In our study, women using this class of progestogens presented a higher proportion of treated cardiovascular risk factors, but adjustment for these parameters explained <20% of the risk estimate. In addition, norpregnane derivatives are preferentially used by women with hyperestrogenic symptoms, including breast tenderness, mastodynia, and endometrial hypertrophy. Because there is some evidence that endogenous exposure to estrogens is related to an increased risk of arterial disease among postmenopausal women,33 we cannot exclude the possibility that such a bias could explain, at least in part, the high IS risk among women using norpregnane derivatives. This indication bias has also been discussed in the context of VTE.6,7
Our study provides new findings on the potential safety of transdermal estrogens in relation to risk of IS. It also suggests for the first time the importance of concomitant progestogens in determining the risk of IS. Taken together with findings for thrombotic risk, transdermal estrogens alone or combined with micronized progesterone may be the best option to improve the benefit/risk ratio of HT use and may represent the safest option with respect to both VTE and stroke risk. Based on these observations, ≤3000 cases of these pathologies per year per million HT users could be avoided by encouraging them to change from oral to transdermal estrogens and from synthetic progestins to micronized progesterone. Nevertheless, data from randomized controlled trials are needed to confirm these results.
Sources of Funding
The study was supported by National Institute of Health and Medical Research (INSERM) and Institute of Research in Public Health (IReSP) in France. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosures
Dr Carcaillon receives personal fees from Newron pharmaceuticals as she is a member of the independent safety monitoring board for phase III clinical studies on Parkinson disease treatment. The other authors report no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.116.013052/-/DC1.
References
- 1.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utian WH, Archer DF, Bachmann GA, Gallagher C, Grodstein Fn, Heiman JR, et al. North American Menopause Society. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American Menopause Society. Menopause. 2008;15(4 pt 1):584–602. doi: 10.1097/gme.0b013e31817b076a. doi: 10.1097/gme.0b013e31817b076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olié V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr Opin Hematol. 2010;17:457–463. doi: 10.1097/MOH.0b013e32833c07bc. doi: 10.1097/MOH.0b013e32833c07bc. [DOI] [PubMed] [Google Scholar]
- 5.Canonico M. Hormone therapy and hemostasis among postmenopausal women: a review. Menopause. 2014;21:753–762. doi: 10.1097/GME.0000000000000296. doi: 10.1097/GME.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 6.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Lévesque H, et al. Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 7.Canonico M, Fournier A, Carcaillon L, Olié V, Plu-Bureau G, Oger E, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30:340–345. doi: 10.1161/ATVBAHA.109.196022. doi: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- 8.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- 9.Moisan F, Spinosi J, Delabre L, Gourlet V, Mazurie JL, Bénatru I, et al. Association of Parkinson’s disease and its subtypes with agricultural pesticide exposures in men: a case-control study in France. Environ Health Perspect. 2015;123:1123–1129. doi: 10.1289/ehp.1307970. doi: 10.1289/ehp.1307970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paganini-Hill A, Ross RK, Henderson BE. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988;297:519–522. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitti DB, Sidney S, Quesenberry CP, Jr, Bernstein A. Ischemic stroke and use of estrogen and estrogen/progestogen as hormone replacement therapy. Stroke. 1998;29:23–28. doi: 10.1161/01.str.29.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 14.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 15.Løkkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Hundrup YA, et al. Increased risk of stroke in hypertensive women using hormone therapy: analyses based on the Danish Nurse Study. Arch Neurol. 2003;60:1379–1384. doi: 10.1001/archneur.60.10.1379. doi: 10.1001/archneur.60.10.1379. [DOI] [PubMed] [Google Scholar]
- 16.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 17.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. WHI Investigators. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 18.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–866. doi: 10.1001/archinte.168.8.861. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, et al. WISDOM group. Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007;335:239. doi: 10.1136/bmj.39266.425069.AD. doi: 10.1136/bmj.39266.425069.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carcaillon L, Alhenc-Gelas M, Bejot Y, Spaft C, Ducimetière P, Ritchie K, et al. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly: the Three-City cohort study. Arterioscler Thromb Vasc Biol. 2011;31:1445–1451. doi: 10.1161/ATVBAHA.111.223453. doi: 10.1161/ATVBAHA.111.223453. [DOI] [PubMed] [Google Scholar]
- 21.Undas A, Ariëns RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31:e88–e99. doi: 10.1161/ATVBAHA.111.230631. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 22.Rossouw JE, Johnson KC, Pettinger M, Cushman M, Sandset PM, Kuller L, et al. Tissue factor pathway inhibitor, activated protein C resistance, and risk of ischemic stroke due to postmenopausal hormone therapy. Stroke. 2012;43:952–957. doi: 10.1161/STROKEAHA.111.643072. doi: 10.1161/STROKEAHA.111.643072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sexton T, Smyth SS. Novel mediators and biomarkers of thrombosis. J Thromb Thrombolysis. 2014;37:1–3. doi: 10.1007/s11239-013-1034-5. doi: 10.1007/s11239-013-1034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarabin PY, Hemker HC, Clément C, Soisson V, Alhenc-Gelas M. Increased thrombin generation among postmenopausal women using hormone therapy: importance of the route of estrogen administration and progestogens. Menopause. 2011;18:873–879. doi: 10.1097/gme.0b013e31820eee88. doi: 10.1097/gme.0b013e31820eee88. [DOI] [PubMed] [Google Scholar]
- 25.Canonico M, Alhenc-Gelas M, Plu-Bureau G, Olié V, Scarabin PY. Activated protein C resistance among postmenopausal women using transdermal estrogens: importance of progestogen. Menopause. 2010;17:1122–1127. doi: 10.1097/gme.0b013e3181e102eb. doi: 10.1097/gme.0b013e3181e102eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey RC, de Larrañaga G, Lepera S, Cohen M, Saposnik G, Alonso B, et al. Activated protein C resistance in patients with arterial ischemic stroke. J Stroke Cerebrovasc Dis. 2001;10:128–131. doi: 10.1053/jscd.2001.25464. doi: 10.1053/jscd.2001.25464. [DOI] [PubMed] [Google Scholar]
- 27.Ciccone MM, Cicinelli E, Giovanni A, Scicchitano P, Gesualdo M, Zito A, et al. Ophthalmic artery vasodilation after intranasal estradiol use in postmenopausal women. J Atheroscler Thromb. 2012;19:1061–1065. doi: 10.5551/jat.13904. [DOI] [PubMed] [Google Scholar]
- 28.Ciccone MM, Scicchitano P, Gesualdo M, Fornarelli F, Pinto V, Farinola G, et al. Systemic vascular hemodynamic changes due to 17-β-estradiol intranasal administration. J Cardiovasc Pharmacol Ther. 2013;18:354–358. doi: 10.1177/1074248413484385. doi: 10.1177/1074248413484385. [DOI] [PubMed] [Google Scholar]
- 29.Aboa-Eboulé C, Mengue D, Benzenine E, Hommel M, Giroud M, Béjot Y, et al. How accurate is the reporting of stroke in hospital discharge data? A pilot validation study using a population-based stroke registry as control. J Neurol. 2013;260:605–613. doi: 10.1007/s00415-012-6686-0. doi: 10.1007/s00415-012-6686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulis G, Lapeyre-Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: What interest for medical research? Rev Med Interne. 2015;36:411–417. doi: 10.1016/j.revmed.2014.11.009. doi: 10.1016/j.revmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Erenus M, Karakoç B, Gürler A. Comparison of effects of continuous combined transdermal with oral estrogen and oral progestogen replacement therapies on serum lipoproteins and compliance. Climacteric. 2001;4:228–234. [PubMed] [Google Scholar]
- 32.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Krolewski AS, Rosner B, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 33.Scarabin-Carré V, Canonico M, Brailly-Tabard S, Trabado S, Ducimetière P, Giroud M, et al. High level of plasma estradiol as a new predictor of ischemic arterial disease in older postmenopausal women: the three-city cohort study. J Am Heart Assoc. 2012;1:e001388. doi: 10.1161/JAHA.112.001388. doi: 10.1161/JAHA.112.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]


