Abstract
Objective:
Hypertension causes adverse remodeling and vasomotor alterations in coronaries. Hormones such as estrogen may help counterbalance some of these effects. The aim of this study was to analyze the effects of ovariectomy and estrogen therapy in a rat model of menopausal hypertension induced by angiotensin II (AII).
Methods:
We investigated diameter, tone, and mechanics of intramural coronaries taken from ovariectomized female rats (n = 11) that received chronic AII treatment to induce hypertension, and compared the results with those found in female rats that were also given estrogen therapy (n = 11). The “hypertensive control” group (n = 11) underwent an abdominal sham operation, and received AII. After 4 weeks of AII treatment, side branches of left anterior descendent coronary (approximately 200 μm in diameter) were isolated, cannulated with plastic microcannulas at both ends, and studied in vitro in a vessel chamber. The inner and outer diameter of the arteries were measured by microangiometry, and spontenuous tone, wall thickness, wall cross-sectional area, tangential stress, incremental distensibility, circumferential incremental elastic modulus, thromboxane agonist-induced tone, and bradykinin-induced dilation were calculated.
Results:
In hypertension, intramural small coronaries show inward eutrophic remodeling after ovariectomy comparing with hypertensive controls. Estrogen therapy had an opposite effect on vessel diameter. Hormone therapy led to an increase in spontaneous tone, allowing for greater dilatative capacity.
Conclusions:
Estrogen may therefore be considered to counterbalance some of the adverse changes seen in the wall of intramural coronaries in the early stages of chronic hypertension.
Keywords: Coronary, Contractility, Ovariectomy, Estrogen therapy, Angiotensin II, Menopausal hypertension
It is well established that chronic hypertension leads to hyperthrophic remodeling of the vessel wall, dysfunction of the endothelium, and an alteration of smooth muscle reactivity. The characteristics of remodeling are determined by several factors, including hypertensive stimuli, sex, and hormonal effects. Alterations in vasoconstriction and/or vasodilation are detectable in most cases.1,2 The effects of ovariectomy and estrogen therapy on vascular remodeling are well documented in normotension.3 One of our previous studies also describes the specific effects of hypertension on intramural coronaries in women4; however, little is known about female hormone status-related remodeling under chronic hypertensive conditions.
Alterations of the intramural coronary arteries in the left ventricle are of the utmost importance concerning target organ damage in hypertensive and ischemic heart disease. As it is technically demanding to dissect these coronary branches, more data are available on peripheral branches and epicardial coronaries.5,6 Intramural arteries are rarely investigated in vitro.7 Compared with epicardial coronaries, intramural vessels demonstrate unique flow conditions. This is hypothesized to be the effect of the characteristic vascular bed created by the muscle environment.8,9
In this article we studied a rat menopausal hypertension model—the effects of ovariectomy followed by estrogen therapy on the vasoconstrictive and endothelium-dependent dilatative capability of the small intramural arteries of the left ventricle in hypertension. Specimens were taken from female rats, subjected to angiotensin-induced chronic hypertension. This was achieved by implantation of an osmotic minipump (100 ng/kg per min, SC). In this model we aimed to reach that common clinical condition where the lack of sexual steroids occurs nearly parallel with hypertension. In the therapeutic regime used in this study the lack of female sexual steroids occurs immediately, and the hypertension stabilizes gradually within 2 to 3 weeks.10,11 The rationale behind this study was that direct vascular effects of different sexual steroids might be different in normotensives and hypertensives.
METHODS
Drugs
Pentobarbital (Nembutal; Phylaxia-Sanofi, Budapest, Hungary) was used for anesthesia. A prophylactic dose of 100,000 IU of intramuscular penicillin (TEVA-Biogal, Debrecen, Hungary) was administered after the subcutaneous surgical implantation of the device that released the angiotensin. The osmotic minipump implanted (Alzet, ML4; Durect Co, Cupertino, CA) was filled with angiotensin II (AII) acetate from Sigma-Aldrich Co (St. Louis, MO and Budapest, Hungary). Details of the protocol to induce angiotensin-dependent hypertension is described in detail elsewhere.10,11 Composition of the normal Krebs-Ringer (nKR) solution used in these in vitro studies was (in mmol/L): NaCl 119, KCl 4.7, NaH2PO4 1.2, MgSO4 1.17, NaHCO3 24, CaCl2 2.5, glucose 5.5, and EDTA 0.034. The Ca2+-free Krebs solution contained (in mmol/L): NaCl 92, KCl 4.7, NaH2PO4 1.18, MgCl2 20, MgSO4 1.17, NaHCO3 24, glucose 5.5, EGTA 2, and EDTA 0.025. The temperature of the solution was kept at 37°C, and it was bubbled with 5% CO2, 20% O2, 74% N2 that stabilized the pH at 7.4. U46619, a thromboxane (Tx) A2-receptor agonist, and bradykinin (BK) were obtained from Sigma-Aldrich Co. Drugs were prepared the day of the experiment with nKR solution.
Animals
A total of 33 sexually mature, virgin female Sprague-Dawley rats were used (Charles River Laboratories, Wilmington, MA), weighing 210 to 240 g at the beginning of the study. All of these rats were subjected to subcutaneous implantation of osmotic minipump under anesthesia (Nembutal 45 mg/kg) and in sterile conditions. The osmotic pump infused 100 ng/kg/min AII subcutaneously. Previous studies described that this dose leads to a chronic elevation in blood pressure in 2 to 3 weeks, without acute pressure effects. This is a model to study early hypertensive vessel alterations.10,11 Twenty-two of these animals also underwent surgical ovariectomy, half of them (n = 11) received continuous estrogen therapy during the chronic experiment (450 μg/kg estradiol-proprionate IM, weekly). The rest of the animals received vehicle material only. The remaining 11 animals served as the “hypertensive control” group (“control” hypertensive group, n = 11). They underwent an abdominal sham operation procedure without oophorectomy, and an osmotic minipump was implanted for AII treatment. They received vehicle of estrogen only. No medical or surgical complications were observed. Conventional rat chow and tap water were provided ad libitum. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was accepted by the University's Animal Care Commission and Hungarian authorities.
In vitro biomechanical and pharmacological reactivity of the intramural coronary artery
After 4 weeks of treatment, animals were reanesthetized (Nembutal 45 mg/kg IM), and blood pressure was measured directly by cannulation of the carotid artery. After opening the chest, the heart was removed and placed into cold, oxygenized, normal nKR solution. Intramural coronary arteries (approximately 200 μm in diameter, secondary branches of the left anterior descending coronary artery) were isolated as previously described.3,7,12,13 Then the chosen segment was excised and placed into a nKR-filled vessel chamber, cannulated with plastic microcannulas at both ends, and extended to its in vivo length. Both cannulas were connected to pressure-servo system (Living Systems, Burlington, VT) and the arteries were pressurized under no-flow conditions.
The outer diameter of the arteries was measured by microangiometry. In this setup the glass-bottomed tissue bath was positioned in the light path of a microscope. A magnified image of the vessel was recorded with a video camera (Philips LDH 0702/20) and a monitor (Philips Computer Monitor 80), and a microcomputer, developed for this purpose, evaluated the signals coming from the camera and automatically positioned two light markers to the contours of the vessel. The distance between the two light spots, namely the outer diameter of the segment, was measured continuously. Inner diameter was also simultaneously measured. Intraluminal pressure was measured at both sides of the segments (Gould pressure transducer). Pressure and diameter signals were digitized by an A/D converter (PCL 7/8; Advantech Corporation, Milpitas, CA) and transmitted into an IBM Pentium PC computer for data storage and further processing.
Coronary arteries were allowed to equilibrate for 30 minutes at 50 mm Hg intraluminal pressure in nKR solution. After this, incubation pressure was decreased to 2 mm Hg and then increased first to 30 mm Hg, then up to 90 mm Hg in 20 mm Hg pressure increments. The steady state diameter was measured at each step. The pressure load cycle was repeated with U46619, a TxA2-receptor agonist (10−6 M), and then with BK (10−6 M). Both were administered as continuous flow superfusion into the vessel chamber. They were infused separately, one after another, allowing 10 minutes of incubation after each drug at 50 mm Hg intraluminal pressure. Finally, passive diameter was obtained in Ca2+-free Krebs solution. The segments were incubated for 20 minutes, then intraluminal pressure was increased incrementally as before, and the passive diameter of the arteries was measured at each pressure level.
Biomechanical calculations
From the original calibrated pressure-diameter plots, the following geometrical and biomechanical parameters were computed for each intraluminal pressure level14: tangential stress was computed according to the Laplace equation: σθ = p × ri/h, where σθ is the tangential (circumferential) wall stress, p is the intraluminal pressure, ri is the inner radius, and h is the wall thickness (h = ro−ri, where ro is the outer radius).
Incremental distensibility Dinc = ΔV/V × ΔP, where Dinc is the incremental distensibility and ΔV is the change in vessel lumen volume in relation to the initial volume of V in response to pressure change of ΔP.
The circumferential incremental elastic modulus was computed from the following equation: Einc = (Δp/Δro) × 2ri2 × ro/(ro2−ri2), where Einc is the incremental elastic modulus, ri is the inner, ro is the outer radius, and Δro is the change in outer radius in response to intraluminal pressure change of Δp.
Spontaneous tone of the vessels was expressed as an active strain, quantified for each intraluminal pressure level: TnKR = (ri Ca-free−ri nKR)/ri Ca-free, where ri Ca-free and ri nKR are the inner radii measured in calcium-free Krebs solution and in nKR solution, respectively.
TxA2-induced tone was also expressed as an active strain, quantified for each intraluminal pressure level: TTxA2 = (ri Ca-free−ri TxA2)/ri Ca-free, where ri Ca-free and ri TxA2 are the inner radii measured in calcium-free Krebs solution and U46619/TxA2-agonist, respectively.
BK-induced tone in nKR solution was also expressed as active strain, quantified for each intraluminal pressure level as follows: TBK = (ri Ca-free−ri BK)/ri Ca-free, where ri Ca-free and ri BK are the inner radii measured in calcium-free Krebs solution and BK, respectively. In active strain parameters the size of the vascular lumen does not influence the value (%) of vascular reactivity. BK-induced relaxation compared with tone in nKR solution was calculated with the following formula: BK relaxation = (ri BK−ri nKR)/ri nKR.
Statistical analysis
For statistical analysis data were compared by two-way analysis of variance (ANOVA). In vitro parameters were plotted as a function of intraluminal pressure between groups and were compared by two-way ANOVA. Paired comparisons were made according to treatment groups to create the graphs. Tukey's test was used as a post hoc test. P < 0.05 was uniformly accepted as significant difference. Data are represented as mean ± SEM.
RESULTS
Mean arterial pressure
The mean arterial pressure of the control hypertensive group was 130 ± 5 mm Hg, the ovariectomized hypertensives had pressures of 134 ± 6 mm Hg, and the ones given estrogen therapy was 142 ± 5 mmHg. There was no significant difference among the groups either in mean arterial pressure or heart weight (Table 1). The elevation after AII treatment was, however, significant compared with healthy controls of the same strain, 96 ± 2 mm Hg, meaning a hypertensive state was successfully established.4
TABLE 1.
Mechanical parameters of the vessels and relative heart weight
| Parameter | Angiotensin II (n = 11) | Angiotensin II-OVX (n = 11) | Angiotensin II-OVX-estrogen (n = 11) |
| Tangential wall stress, kPa | 16.32 ± 1.75 | 18.33 ± 2.58 | 19.15 ± 2.50 |
| Distensibility, 1/kPa | 0.0288 ± 0.0079 | 0.0282 ± 0.0093 | 0.0375 ± 0.0087 |
| Elastic moduli, lgPa | 5.48 ± 0.15 | 5.50 ± 0.10 | 5.29 ± 0.14 |
| Cross-sectional area, μm2 | 30,137 ± 3,795 | 25,586 ± 2,596 | 29,550 ± 3,801 |
| Wall-to-lumen ratio, 1/μm | 0.461 ± 0.050 | 0.428 ± 0.050 | 0.409 ± 0.048 |
| Relative heart weight, g/100g bw | 0.386 ± 0.009 | 0.365 ± 0.021 | 0.390 ± 0.011 |
Mechanical parameters were calculated on P = 50 mm Hg. Relative heart weights were normalized and calculated per 100 g of body weight. There was no significant difference in these parameters between the groups. bw, body weight; OVX, ovariectomy.
Effects of AII, ovariectomy, and estrogen therapy on the vessel geometry of intramural coronary arterioles
Estrogen treatment resulted in largest vessel lumens (E, 284 ± 24 μm vs AII, 270 ± 14 μm vs ovariectomized (OV), 254 ± 14 μm, at P = 50 mm Hg; Fig. 1A). Difference in wall thickness did not reach the preset level of statistical difference between the groups (P = 0.06 between AII and OV; AII 41 ± 4 μm vs OV 31 ± 3 μm vs E 36 ± 3 μm, on P = 50 mm Hg); cross-sectional areas of vessel wall did not differ among the groups (Table 1).
FIG. 1.
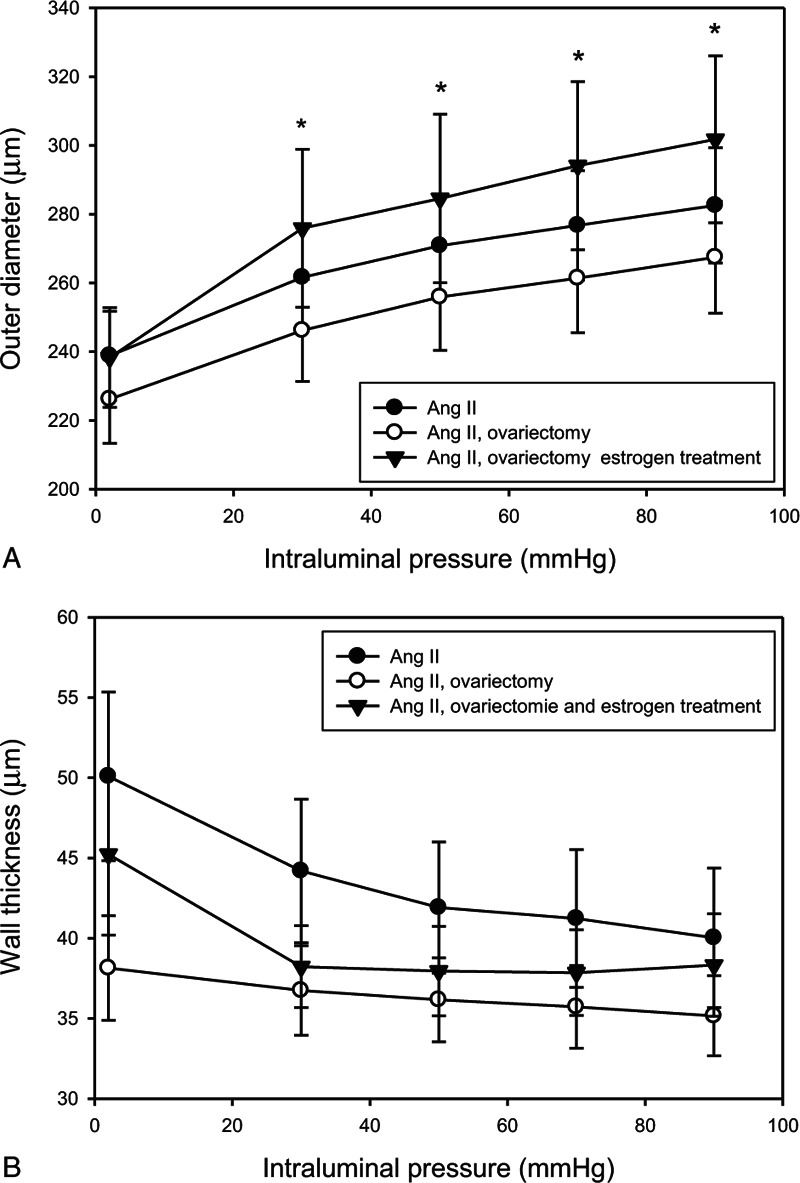
(A) Outer radius; (B) wall thickness; values from the control angiotensin II-treated group (AII; full circles, n = 11), ovariectomy (OVX; empty circles, n = 11), and estrogen-treated (full triangles, n = 11) groups are shown. Mean ± SEM values. Asterisk indicates statistical significance (P < 0.05) between control AII and estrogen-treated groups versus OVX group. Estrogen treatment resulted in largest vessel lumens. Difference in wall thickness did not reach the preset level of statistical difference between the groups; cross-sectional areas of vessel wall did not differ among the groups.
Effects of AII, ovariectomy, and estrogen therapy on the contractility of intramural coronary arterioles
Spontaneous myogenic tone was higher in the estrogen-treated group compared with the ovariectomized group (Fig. 2A). There was no difference in U46619-induced tone between the groups (Fig. 2B). Remaining tone was significantly higher in the estrogen-treated and control AII hypertensive group compared with the ovariectomized animals (E 11.1 ± 2.1%, AII 9.9 ± 2.8%, and OV 6.6 ± 2.0%, on P = 50 mm Hg) in BK-induced relaxation. Comparing with spontaneous tone, there was no significant relaxation in the OV group; however, estradiol treatment restored nitric oxide (NO)-dependent, BK-induced relaxation to the hypertensive control level (Fig. 3A, B).
FIG. 2.
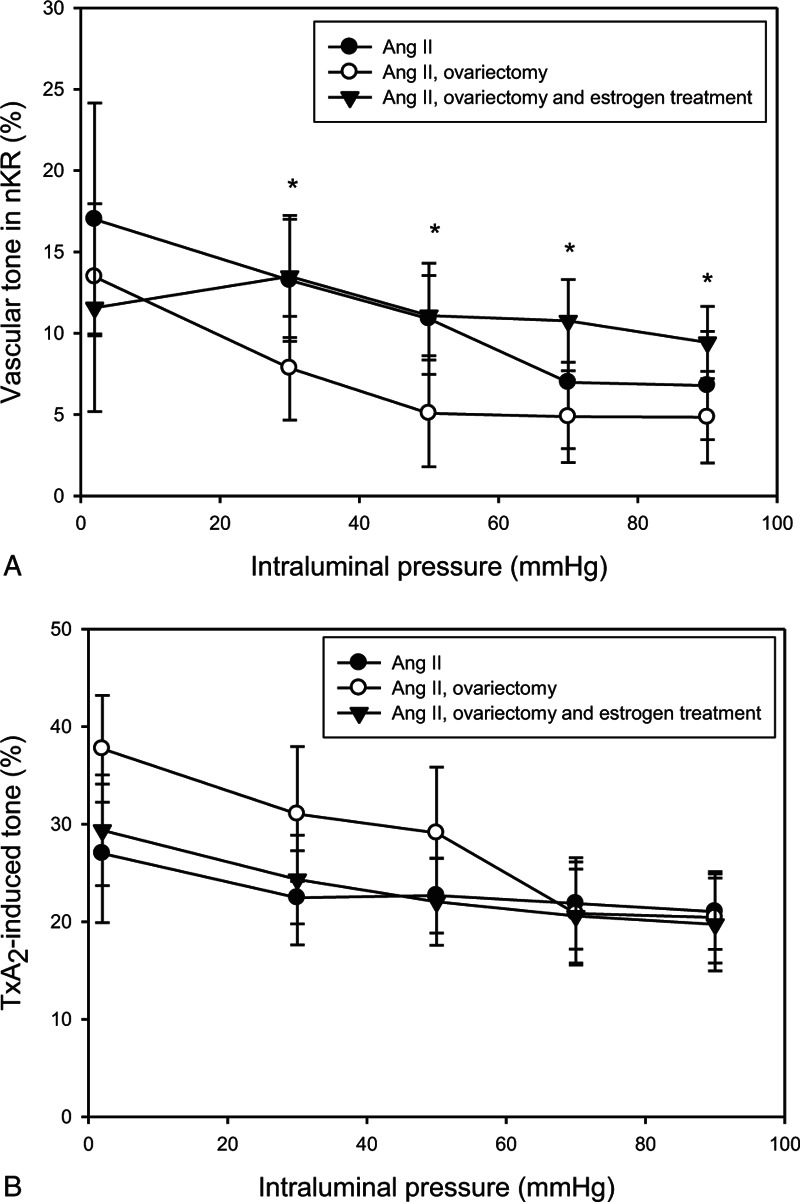
(A) Myogenic (spontaneous) tone, (B) TxA2-induced tone of rat intramural coronary arterioles as a function of intraluminal pressure. Values from the control angiotensin II-treated group (AII; full circles, n = 11), ovariectomy (OVX; empty circles, n = 11), and estrogen-treated (full triangles, n = 11) groups are shown. Mean ± SEM values. Asterisk indicates statistical significance (P < 0.05) between control AII and estrogen-treated groups versus OVX group. Spontaneous myogenic tone was higher in the estrogen-treated group compared with the ovariectomized group. There was no difference in U46619-induced tone between the groups.
FIG. 3.
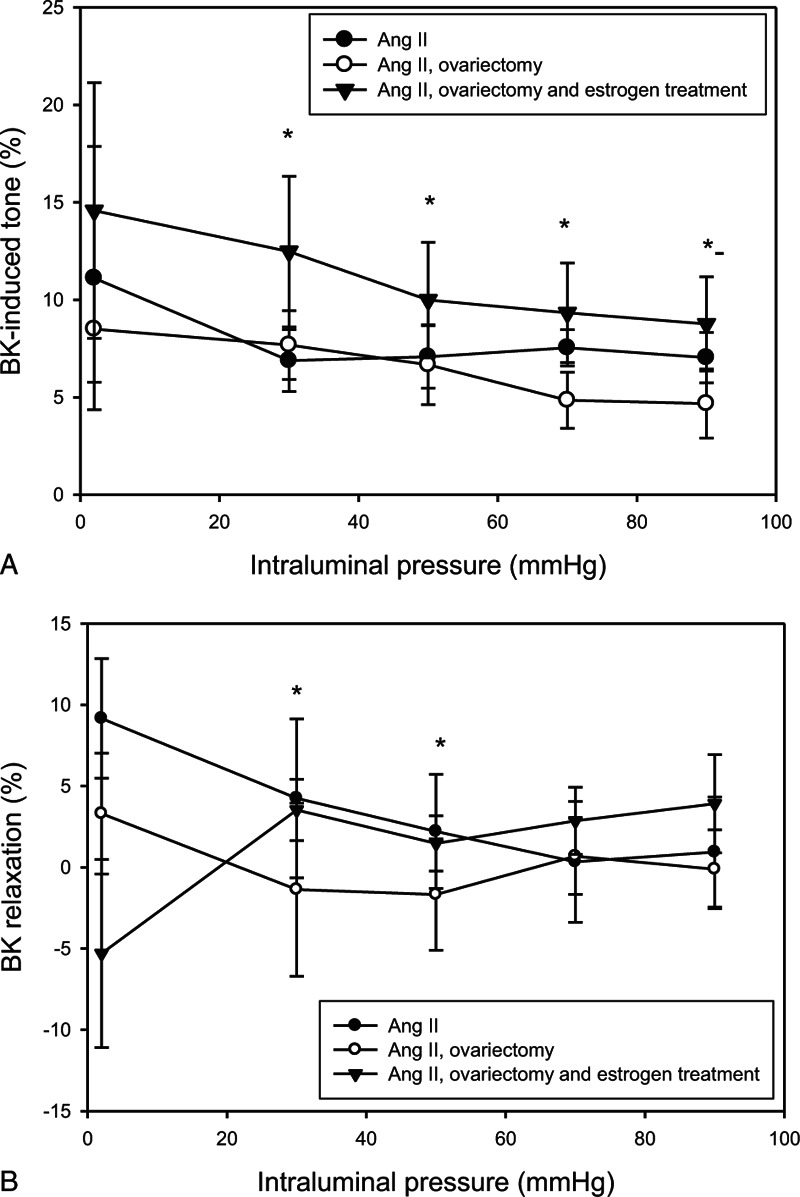
Bradykinin (BK)-induced tone of the rat intramural coronary arterioles was expressed as active strain and a function of intraluminal pressure (10−6 M BK). Values from the control angiotensin II-treated group (AII; full circles, n = 11), ovariectomy (OVX; empty circles, n = 11), and estrogen-treated (full triangles, n = 11) groups are shown. Mean ± SEM values. Asterisk indicates statistical significance (P < 0.05) between estrogen-treated group versus OVX group in respect of BK-induced tone (A) and control AII and estrogen-treated groups versus OVX group in respect of BK relaxation (B). Remaining tone was significantly higher in the estrogen-treated and control AII hypertensive group compared with the ovariectomized animals in BK-induced relaxation. Comparing with spontaneous tone, there was no significant relaxation in the ovariectomized group; however, estradiol treatment restored nitric oxide-dependent, BK-induced relaxation to the hypertensive control level.
Effects of AII on the biomechanical parameters of intramural coronary arterioles
No difference was found in terms of wall stress, distensibility (Table 1), and elastic moduli (Fig. 4) among the groups.
FIG. 4.
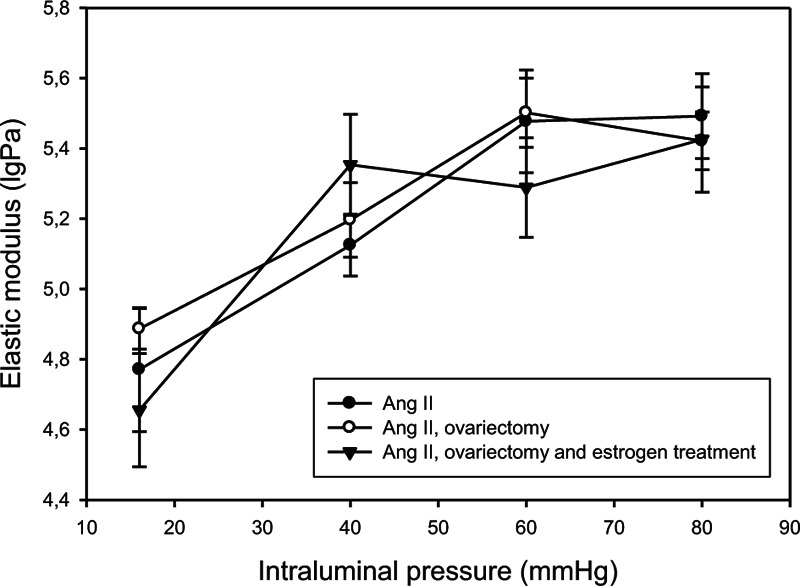
Elastic moduli in intramural coronaries. Values from the control angiotensin II-treated group (AII; full circles, n = 11), ovariectomy (OVX; empty circles, n = 11), and estrogen-treated (full triangles, n = 11) groups are shown. No difference was found in terms of elastic moduli among the groups.
DISCUSSION
Chronic AII treatment raised mean arterial pressure significantly, modeling the early stages for AII-induced hypertension as expected. Adverse vascular changes have been demonstrated to follow.4 In this study we focused on sexual steroid-related vascular adaptation in hypertension. Menopausal hypertension, however, might lead to different vascular adaptational mechanisms compared with those observed earlier in normotension.3
Geometry
Unwelcome changes in vessel geometry, such as a decrease in coronary lumen, have been apparent in the early stages of AII-induced hypertension. Even though lumen of the AII + OV group was significantly narrower, the cross-section of vessel wall did not differ that corresponds to eutrophic remodeling.15 Estrogen therapy, however, even with still maintained hypertension leads to an increase in coronary vessel lumen. The eutrophic wall remodeling of AII hypertension was counteracted by estrogen. Estrogen has been proven to counterbalance adverse vascular effects of ovariectomy in other regions, such as the hypothalamus.16
Contractility
Key alterations happened in coronary contractility. Earlier work demonstrated that U46619 constrictions were elevated in the AII hypertensive group compared with controls. Stabilization of this contracted lumen can be one mechanism being responsible for the observed eutrophic remodeling.4 In this series we could not observe any difference in U46619-induced tone between the ovariectomized and hormone therapy groups (Fig. 3); however, spontaneous myogenic tone and remaining tone after BK-induced dilation were higher in the control hypertensive and estrogen-treated groups compared with the ovariectomized group. This elevated spontaneous tone if exists in vivo allows for a greater functional range, and a greater capacity for vasodilation, and can be considered a cardioprotective mechanism. Vasodilation to BK practically disappeared in the ovariectomy group, meaning that this key vasodilatative mechanism was lost in this group, although it is still close to intact in the hypertensive control, and even most importantly, it is retained in the estrogen therapy group.
Biomechanical parameters
In a previous study we found that tangential wall stress and elastic modulus decreased significantly in hypertensive animals compared with normal, at high-pressure levels. In the present series, no further difference, however, was found in terms of wall stress, distensibility, and elastic moduli between ovariectomized and hormone therapy groups.
Potential clinical value
The goal of this animal model was to understand local morphological and functional adaptations in a key vascular segment (intramural coronaries are mainly responsible for the blood supply of the heart) that is difficult to examine in humans. Hypertension is a common pathological state in postmenopausal women; the geometrical, contractile, and mechanical properties of intraluminal coronary resistance arteries play a paramount role in hypertensive and ischemic heart disease. Preventing adverse remodeling in these vessels could have cardioprotective effects. Ovariectomy—lack of estrogen—in combination with hypertension has a dangerous impact on intramural coronaries. Estrogen therapy, however, may counterbalance some of the adverse effects of chronic hypertension on these vessels.
CONCLUSIONS
Estrogen therapy has an opposite effect on vascular lumen as ovariectomy and AII treatment, which produces eutrophic remodeling. Estrogen may therefore be considered to counterbalance the adverse changes seen in the vascular wall of intraluminal coronaries in the early stages of chronic hypertension. As intraluminal coronaries play a key role in hypertensive and ischemic heart disease, preventing adverse remodeling in these vessels cannot be overemphasized.
NO-mediated dilation is a cardioprotective mechanism. If this capacity is decreased, the vessels become more rigid, which is an adverse phenomenon. In the absence of estrogen this NO-mediated dilation becomes vulnerable in the face of early chronic hypertension, opening the gate to further remodeling and vascular damage.
Estradiol therapy leads to an elevation of spontaneous tone, allowing for greater range for vasomotion, meaning a greater capacity for dilation. It also restored NO-dependent reactivity of the coronaries to BK. This made the behavior of the vessel similar to that seen in the control group, meaning again that in this series estrogen therapy has counterbalanced the adverse effect of AII-induced hypertension.
We hope that our animal study can aid the first step in understanding some clinical phenomena related to the altered effects of renin-angiotensin system and consequential changes in prostanoid and BK effects in the heart and perhaps in other organs also.17,18
Acknowledgments
We thank the expert technical assistance of Ms Ildiko Oravecz.
Footnotes
Our original article is not published and not under review elsewhere.
M.M. and J.R.H. contributed equally to this work.
Funding/support: This work was supported by the National Research, Development and Innovation Office OTKA32019 and the Hungarian Society of Hypertension.
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1.London GM, Safar ME. Arterial wall remodelling and stiffness in hypertension: heterogeneous aspects. Clin Exp Pharmacol Physiol 1996; 23:S1–S5. [DOI] [PubMed] [Google Scholar]
- 2.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci 2002; 17:105–109. [DOI] [PubMed] [Google Scholar]
- 3.Mericli M, Nadasy GL, Szekeres M, et al. Estrogen replacement therapy reverses changes in intramural coronary resistance arteries caused by female sex hormone depletion. Cardiovasc Res 2004; 61:317–324. [DOI] [PubMed] [Google Scholar]
- 4.Matrai M, Szekacs B, Mericli M, et al. Biomechanics and vasoreactivity of female intramural coronaries in angiotensin II induced hypertension. Acta Physiol Hung 2010; 97:31–40. [DOI] [PubMed] [Google Scholar]
- 5.Monnink SH, van Haelst PL, van Boven AJ, et al. Endothelial dysfunction in patients with coronary artery disease: a comparison of three frequently reported tests. J Investig Med 2002; 50:19–24. [DOI] [PubMed] [Google Scholar]
- 6.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation 2000; 101:1653–1659. [DOI] [PubMed] [Google Scholar]
- 7.Nadasy GL, Szekeres M, Dezsi L, Varbiro S, Szekacs B, Monos E. Preparation of intramural small coronary artery and arteriole segments and resistance artery networks from the rat heart for microarteriography and for in situ perfusion video mapping. Microvasc Res 2001; 61:282–286. [DOI] [PubMed] [Google Scholar]
- 8.Toyota E, Ogasawara Y, Hiramatsu O. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am J Physiol Heart Circ Physiol 2005; 288:H1598–H1603. [DOI] [PubMed] [Google Scholar]
- 9.Vis MA, Bovendeerd PH, Sipkema P, Westerhof N. Effect of ventricular contraction, pressure, and wall stretch on vessels at different location in the wall. Am J Physiol 1997; 272 (6 pt 2):H2963–H2975. [DOI] [PubMed] [Google Scholar]
- 10.Simon G, Abraham G, Cserep G. Pressor and subpressor angiotensin II administration. Two experimental models of hypertension. Am J Hypertens 1995; 8:645–650. [DOI] [PubMed] [Google Scholar]
- 11.Varbiro S, Nadasy GL, Monos E, et al. Effect of ovariectomy and hormone replacement therapy on small artery biomechanics in angiotensin-induced hypertension in rats. J Hypertens 2000; 18:1587–1595. [DOI] [PubMed] [Google Scholar]
- 12.Szekeres M, Dezsi L, Nadasy GL, Kaley G, Koller A. Pharmacologic inhomogeneity between the reactivity of intramural coronary arteries and arterioles. J Cardiovasc Pharmacol 2001; 38:584–592. [DOI] [PubMed] [Google Scholar]
- 13.Szekeres M, Nadasy GL, Dezsi L, Orosz M, Tokes A, Monos E. Segmental differences in geometric, elastic and contractile characteristics of small intramural coronary arteries of the rat. J Vasc Res 1998; 35:332–344. [DOI] [PubMed] [Google Scholar]
- 14.Cox RH. Three-dimensional mechanics of arterial segments in vitro: methods. J Appl Physiol 1974; 36:381–384. [DOI] [PubMed] [Google Scholar]
- 15.Mulvany MJ. Vascular remodelling of resistance vessels: can we define this? Cardiovasc Res 1999; 41:9–13. [DOI] [PubMed] [Google Scholar]
- 16.Szelke E, Mersich T, Szekacs B, Sandor P, Komjati K, Varbiro S. Effects of estrogen and progestin on the CO2 sensitivity of hemispheric cerebral blood volume. Menopause 2008; 15:346–351. [DOI] [PubMed] [Google Scholar]
- 17.Maric-Bilkan C, Gilbert EL, Ryan MJ. Impact of ovarian function on cardiovascular health in women: focus on hypertension. Int J Womens Health 2014; 6:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriprasert I, Beydoun H, Barnabei V, Nassir R, LaCroix AZ, Archer DF. Incidence of endometrial spotting or bleeding during continuous-combined estrogen-progestin therapy in postmenopausal women with and without hypertension. Menopause 2015; 22:1067–1075. [DOI] [PubMed] [Google Scholar]


