Abstract
Objective:
The aim of the study was to investigate the associations of amino acids and other polar metabolites with metabolic syndrome (MetS) in postmenopausal women in a lean Asian population.
Methods:
The participants were 1,422 female residents enrolled in a cohort study from April to August 2012. MetS was defined according to the National Cholesterol Education Program Adult Treatment Panel III modified for Japanese women. Associations were examined between MetS and 78 metabolites assayed in fasting plasma samples using capillary electrophoresis-mass spectrometry. Replication analysis was performed to confirm the robustness of the results in a separate population created by random allocation.
Results:
Analysis was performed for 877 naturally postmenopausal women, including 594 in the original population and 283 in the replication population. The average age, body mass index, and levels of high- and low-density lipoprotein cholesterol of the entire population were 64.6 years, 23.0 kg/m2, 72.1 mg/dL, and 126.1 mg/dL, respectively. There was no significant difference in low-density lipoprotein cholesterol levels between women with and without MetS. Thirteen metabolites were significantly related to MetS: multiple plasma amino acids were elevated in women with MetS, including branched-chain amino acids, alanine, glutamate, and proline; and alpha-aminoadipate, which is generated by lysine degradation, was also significantly increased.
Conclusions:
Our large-scale metabolomic profiling indicates that Japanese postmenopausal women with MetS have abnormal polar metabolites, suggesting altered catabolic pathways. These results may help to understand metabolic disturbance, including in persons with normal body mass index and relatively high levels of high-density lipoprotein cholesterol, and may have clinical utility based on further studies.
Keywords: Amino acids, Aminoadipate, Branched-chain amino acids, Menopause, Metabolic syndrome, Metabolomics
Metabolic syndrome (MetS) is a cluster of conditions that increase the risk of development of cardiovascular disease (CVD) as a major residual risk factor beyond low-density lipoprotein cholesterol (LDL-C). Each of the five components of MetS, abdominal obesity, high blood pressure (BP), elevated triglycerides (TG), decreased high-density lipoprotein cholesterol (HDL-C), and elevated glucose is an independent predictor for CVD. Given that CVD accounts for one of every three deaths in the United States,1 tackling MetS is a key to CVD prevention.
The risk of CVD attributed to MetS is reported to be higher in women than in men.2-5 A large Japanese cohort study showed that MetS in women had a stronger association with future coronary artery disease than LDL-C levels.6 In women, loss of estrogen with menopause promotes accumulation of visceral fat and changes in lipid metabolism,7,8 putting women after menopause at greater risk of MetS and thus CVD. As the average life expectancy continues to increase around the globe, implementation of preventive strategies to decrease or delay MetS among postmenopausal women is crucial for graceful aging and maintenance of independent living without disability.
Recently, metabolomics has been increasingly applied to disease research, including CVD.9 Measurement of multiple small-molecule metabolites, such as sugars, lipids, fatty acids, amino acids, and cofactors, which are downstream products of genetic and environmental variations, provides an integrated measure of phenotypes, and thus leads to identification of mechanisms and biomarkers of many diseases. Low sample throughput and data preprocessing remain major hindrances, but improvements in sample preparation and automated data processing along with reductions in cost have allowed adaptation of metabolomics to large-scale epidemiological studies.10-14
Evidence from such studies has suggested that amino acids play key roles in obesity-related diseases. For example, amino acids are independently associated with CVD from conventional factors.15-20 Elevation of plasma amino acids precedes any alteration in insulin action detectable by standard measures, and their associations with future diabetes are stronger than those of age or body mass index (BMI).21 A recent metabolomics study highlighted the ability to distinguish between healthy obese and unhealthy obese participants after a caloric challenge using amino acid profiles.22 Interestingly, 6.2% of women in the United States were reported to have MetS even at normal weight, that is BMI of 18.5 to <25 kg/m2.23 This is referred to as “metabolically obese, normal weight (MONW)” and individuals with this condition have a higher CVD risk.24-27 Because MONW is often undetected and thus untreated for years because of the normal BMI, identifying individuals with MONW is important for early preventive measures. Most female participants in previous studies, however, have been overweight or obese, and whether such metabolic changes appear in women with normal weight has not been explored. In addition, these issues have not been investigated in postmenopausal women. There have been a few studies showing the association of altered amino acid metabolism with insulin resistance in normal-weight men.28,29
We thus performed a large-scale metabolomic profiling in Japanese postmenopausal women, which is a unique population with lean body mass, relatively high HDL-C, and a lower incidence of coronary artery disease than that in the Western populations.30 The goal of the study was to reveal the associations of amino acids and other polar metabolites with MetS. Capillary electrophoresis-mass spectrometry (CE-MS) was used for metabolomics measurements. There are several technologies used to analyze metabolites, including nuclear magnetic resonance, gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry, and CE-MS. Although CE-MS is performed less frequently compared with the other methods and has limitations of metabolome coverage, including water-insoluble lipids and neutral steroids, its superiority in measuring charged metabolites including amino acids simultaneously and its extremely high resolution made this method suitable for use in the current study.31
METHODS
Study population
The study base was the participants of the ongoing Tsuruoka Metabolomic Cohort Study, initiated in April 2012 (Yamagata Prefecture, Japan), comprising individuals aged 35 to 74 years who were recruited among attendees of annual municipal or worksite health check-up programs in the city. A total of 1,422 women consented to participate during the first few months of the baseline period, and plasma metabolomic profiling in these women was completed by the end of 2014. Only postmenopausal female participants were ultimately included in the analysis due to the small number of premenopausal women. Because there are known metabolic differences compared with natural menopause, participants with surgical or medical menopause were excluded.32 In addition, women who had used hormone therapy within 1 year or had undergone oophorectomy after menopause were excluded to eliminate the possible effect on the metabolic profile of administered estrogen and hormones excreted from postmenopausal ovaries.33 Menopause was defined as having ceased menstruation for at least 1 year. A detailed flow of the participants is shown in Figure 1. The study was approved by the Medical Ethics Committee of the School of Medicine, Keio University, Tokyo, Japan (Approval No. 20110264), and all participants gave voluntary written informed consent.
FIG. 1.
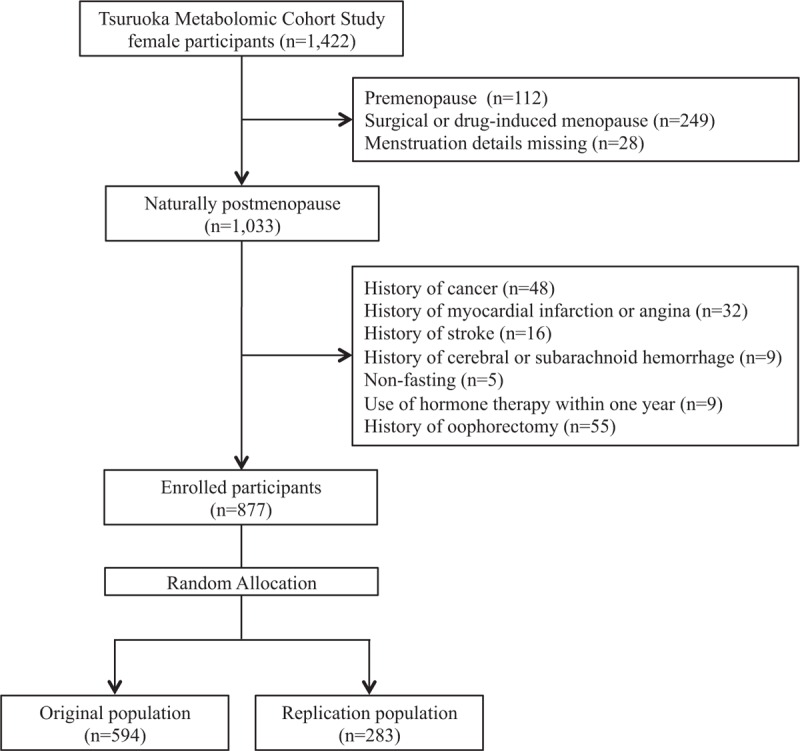
Flow diagram of included and excluded participants.
Data collection, anthropometric measurements, and biochemical examinations
All data and samples were obtained in the baseline study, including anthropometrics, clinical biochemistry, and blood specimens for metabolomic profiling. Detailed information was collected through a standardized self-administered questionnaire on medical history, including treatment of hypertension, dyslipidemia, and diabetes; gynecological and reproductive history, including menopause status (pre/peri/post), ages at menarche and menopause, reason for menopause (natural/medical/others), number of pregnancies and deliveries, age at first delivery, use of hormone therapy (if yes, number of years used and when it was used last), and whether oophorectomy was ever performed; and lifestyle parameters such as smoking habits, alcohol intake, diet, stress, and physical activity. Anthropometric measurements included body weight, height, and waist circumference (WC). WC was measured to the nearest 0.1 cm at the umbilicus at the end of a normal breath. If the umbilicus drooped down, the measurement was made midway between the inferior margin of the last rib and the top of the iliac crest in a horizontal plane. BP was measured twice on one occasion in the sitting position using an automated sphygmomanometer (Omron HBP-T105S-N), and the mean of the two measurements was used for analysis.
Blood samples were collected in the morning between 8:30 and 10:30 after overnight fasting to avoid variation due to fasting and circadian rhythm. Plasma samples were collected with ethylenediaminetetraacetic acid-2Na as an anticoagulant and kept at 4°C immediately after collection. The samples were centrifuged for 15 minutes (1,500g at 4°C) within 1 hour of collection, divided into aliquots, and kept for a maximum of 6 hours at 4°C until extraction of metabolites. Serum samples were collected with serum-separating medium and kept at room temperature after collection. Serum levels of total cholesterol, TG, and fasting plasma glucose were analyzed using enzymatic methods, and glycated hemoglobin (HbA1c) was determined by immunoassay. HDL-C values were measured by a direct method. LDL-C levels were calculated using the Friedwald equation.34
Definition of MetS
MetS was defined using the modified National Cholesterol Education Program Adult Treatment Panel III definition with 100 mg/dL as the cutoff for the glucose level.35 Because lower overall adiposity has been associated with an increased risk of medical conditions such as type 2 diabetes in Asian countries,36 the threshold for WC was set according to the recommendation for Asians established by several organizations, including the National Cholesterol Education Program.37,38 Specifically, central obesity was defined as WC at least 80 cm, high BP as mean systolic/diastolic BP at least 130/85 mm Hg or currently on antihypertensive therapy, high serum TG as at least 1.7 mmol/L (150 mg/dL), low HDL-C as less than 1.3 mmol/L (50 mg/dL), and high fasting plasma glucose as at least 5.5 mmol/L (100 mg/dL) or current use of antidiabetic medication. Women with three or more of these components were defined as having MetS.
Metabolomics measurement
Nontargeted mass spectrometry-based metabolomic profiling was performed with fasting plasma samples via capillary electrophoresis time-of-flight mass spectrometry. Metabolite extraction from plasma was completed within 6 hours after collection to minimize the effect of metabolic changes in plasma.39 The extraction method has been described in detail elsewhere.40 Capillary electrophoresis time-of-flight mass spectrometry analysis of cationic and anionic metabolites was performed as described previously.41,42 Raw data were processed using our proprietary software (MasterHands).43 As a preliminary study, we identified 290 metabolite peaks (131 cations and 159 anions) in plasma: 154 known with standard compounds and 136 unknown. We decided a priori to measure absolute concentrations of 115 metabolites (63 cations and 52 anions) that were expected to be observed stably in most human plasma samples and matched with standard compounds. To monitor the stability of metabolome analysis, quality control samples were injected every 10 samples and assessed at the start of the analytical run and at intervals throughout the analysis. The technical variability and between-subject variability for each metabolite assayed is shown in Supplementary Table 1.
Statistical analysis
Participants were randomly allocated into two groups (2:1) to create two independent “original” and “replication” populations. Analyses were first performed in the original population, and then the same analyses were performed in the replication population to confirm the robustness of the study outcome. The two groups were stratified for age and batch number to eliminate any confounding effects of age and analytical variability between multiple batches.
Out of 115 metabolites, 37 had plasma levels below the assay limits of detection (LOD) in more than 90% of the participants, and therefore were excluded from further analyses. The remaining 78 metabolites were analyzed. For samples with levels below LOD, which comprised less than 1% of all samples, values were input using half of the LOD.44 The distribution of each metabolite concentration was tested for normality using quantile-quantile plots. On the basis of the shape of the distribution, most of the metabolite concentrations were log-transformed.
The characteristics of participants with and without MetS were compared by t test and χ2 test for continuous and categorical variables, respectively. Age-adjusted Spearman correlation coefficients were calculated for each metabolite pair in the original population. Linear regression analysis was performed between the MetS and non-MetS groups with each metabolite concentration used as the outcome to examine the association between plasma metabolite concentrations and MetS. Analysis of covariance was performed to adjust for possible confounders using age, LDL-C levels, current smoking status, current alcohol consumption, physical activity, and dietary intake level. We calculated P values using the Benjamini and Hochberg false discovery rate method (α = 0.05) for analysis of the original population to adjust for independent multiple comparisons.45 Adjusted mean concentrations of each metabolite were calculated in both groups. Sensitivity analyses were performed by excluding (1) women with BMI at least 25 kg/m2 and (2) those taking medications for hypertension, dyslipidemia, and diabetes. Both sensitivity and replication analyses were conducted using the metabolites shown to be significantly associated with MetS in the original population (unadjusted false discovery rate P < 0.05). SAS 9.3 (SAS Institute Inc, Cary, NC) was used for all analyses.
RESULTS
Population characteristics
The final data set included 877 participants, with 594 women in the original population and 283 in the replication population. In the original population, 149 women were diagnosed with MetS and 445 women did not meet the criteria (non-MetS). In the replication population, 72 women were diagnosed with MetS and 211 were free of MetS. The ratio of MetS to non-MetS participants was similar in the two populations (1:3). The characteristics of the original population are shown in Table 1. Women with MetS were older than those without MetS (65.5 vs 64.1 y, P = 0.004), and had higher TG (111.0 vs 76.0 mg/dL) and lower HDL-C (64.8 vs 75.3 mg/dL) levels. There was no significant difference in serum LDL-C levels (126.1 vs 124.9 mg/dL) or in lifestyle characteristics (current smoking, current alcohol consumption, calorie intake, and physical activity) between the two groups. Most characteristics of the replication population were similar to those of the original population (data not shown). The only discrepancy between the two populations was for alcohol intake, with significantly fewer women with MetS having current alcohol intake in the replication population (7.0% vs 51.0%, P = 0.01). Thus, women with MetS in this population may have refrained from alcohol use.
TABLE 1.
Characteristics of the original study population (n = 594)
| Variables | non-MetS (n = 445) | MetS (n = 149) | Pf |
| Age, y | 64.1 (5.2) | 65.5 (5.1) | 0.004 |
| Age at menarche, y | 13.7 (1.5) | 14.0 (1.6) | N.S. |
| Age at menopause, y | 50.6 (3.2) | 50.3 (3.7) | N.S. |
| Years after menopause, y | 13.5 (6.3) | 15.2 (6.7) | 0.004 |
| No. of deliveries, times | 2.4 (0.6) | 2.4 (0.6) | N.S. |
| Age at first delivery, y | 23.8 (3.0) | 23.5 (3.5) | N.S. |
| Body mass index, kg/m2 | 22.1 (2.9) | 25.6 (3.0) | <0.0001 |
| Waist circumference, cm | 80.0 (8.2) | 90.1 (7.1) | <0.0001 |
| Systolic blood pressure, mm Hg | 124.4 (18.9) | 141.4 (18.0) | <0.0001 |
| Diastolic blood pressure, mm Hg | 71.5 (10.9) | 79.2 (9.8) | <0.0001 |
| Triglyceride, mg/dLa | 76.0 (27-226) | 111.0 (45-441) | <0.0001 |
| LDL-cholesterol, mg/dL | 124.9 (29.3) | 126.1 (31.5) | N.S. |
| HDL-cholesterol, mg/dL | 75.3 (16.3) | 64.8 (15.6) | <0.0001 |
| Non–HDL-cholesterol, mg/dLb | 141.4 (31.1) | 150.0 (33.3) | 0.004 |
| Fasting plasma glucose, mg/dLa | 95.0 (57-173) | 104.0 (77-230) | <0.0001 |
| Hemoglobin A1c (NGSP), %a | 5.6 (4.9-8.6) | 5.8 (5.1-9.9) | <0.0001 |
| On hypertensive medication, yesc | 109 (24.5%) | 80 (53.7%) | <0.0001 |
| On lipid-lowering medication, yesc | 118 (26.5%) | 55 (36.9%) | 0.02 |
| On diabetic medication, yesc | 18 (4.0%) | 30 (20.1%) | <0.0001 |
| Current smoker, yesc | 9 (2.0%) | 2 (1.3%) | N.S. |
| Any current alcohol intake, yesc,d | 89 (20.4%) | 33 (22.3%) | N.S. |
| Dietary energy intake, kcal/d | 1598.2 (249.9) | 1592.4 (249.0) | N.S. |
| Daily physical activity, METs × h/dc,e | 12.1 (0-79.5) | 11.1 (0-69.0) | N.S. |
Reported as mean (SD) unless stated otherwise.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; MetS, metabolic syndrome; METs, Metabolic Equivalent of Tasks; NGSP, National Glycohemoglobin Standardization Program; N.S., not significant.
aReported as median (range). Range (minimum-maximum).
bNon–HDL-cholesterol was calculated by subtracting HDL-cholesterol from total cholesterol.
cReported as numbers (percentage).
dInformation on alcohol intake was missing in nine participants.
eInformation on daily physical activity was missing in four participants.
fStudent's t test was used for comparisons of group means. Wilcoxon rank-sum test was used for comparisons of triglyceride, fasting plasma glucose, hemoglobin A1c, and daily physical activity. Fisher's exact test was used to compare proportions. P < 0.05 was considered significant.
Potential biomarkers of MetS in plasma metabolites
High correlations within groups of related metabolites were observed for amino acids and their metabolites, and for intermediates of the tricarboxylic acid (TCA) cycle, suggesting the importance of these metabolites in revealing the network of biochemical reactions in humans (Fig. 2). We discovered a total of 19 metabolites with plasma concentrations that differed significantly between MetS and non-MetS in the original population, of which 11 remained significant after adjusting for possible confounders (Table 2). Plasma levels of branched-chain amino acids (BCAAs) and their derivatives were 7% to 10% higher in MetS. Levels of phenylalanine and tyrosine tended to be slightly higher in MetS, but significance disappeared after adjustments. Other metabolites that showed significantly higher concentrations in MetS included alanine, glutamate, alpha-aminoadipate, cystine, and proline. A suggestive positive trend could also be seen for lactate and pyruvate (P = 0.06 and 0.07, respectively). 3-Hydroxybutyrate levels were 18% lower in the MetS group compared with the non-MetS group. The results for all metabolites are shown in Supplementary Table 2.
FIG. 2.
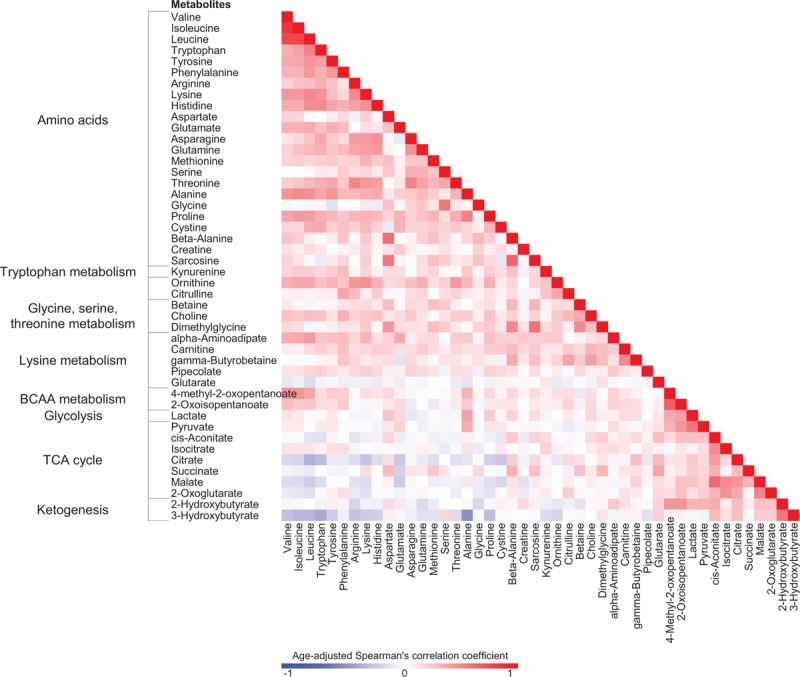
Correlation matrix for plasma metabolite concentrations in the original population (n = 594). Age-adjusted Spearman coefficients were calculated for each pair of metabolite levels in the original population. Metabolites with correlation coefficients more than 0.40 are listed. BCAA, branched-chain amino acid; TCA, tricarboxylic acid.
TABLE 2.
Associations of metabolites with metabolic syndrome
| Original population (n = 594) | Replication population (n = 283) | |||||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||
| Pathways | Metabolitesa | % Changeb | 95% CI | Pc | % Changed | 95% CI | Pc | % Changeb | 95% CI | Pe | % Changed | 95% CI | Pe | |
| Branched-chain amino acid metabolism | log | Valine | 10.3 | 6.5-14.3 | 1.1 × 10−6 | 10.7 | 6.8-14.8 | 5.3 × 10−6 | 12.1 | 6.6-17.9 | 1.1 × 10−5 | 11.8 | 6.1-17.8 | 3.6 × 10−5 |
| log | Isoleucine | 10.6 | 6.5-14.7 | 1.9 × 10−6 | 10.9 | 6.8-15.2 | 1.2 × 10−5 | 14.6 | 8.7-20.8 | 7.0 × 10−7 | 14.1 | 8.1-20.5 | 3.0 × 10−6 | |
| log | Leucine | 10.2 | 6.6-14.0 | 3.4 × 10−7 | 10.8 | 7.1-14.6 | 2.1 × 10−6 | 9.8 | 4.8-15.1 | 1.2 × 10−4 | 10.7 | 5.4-16.2 | 6.2 × 10−5 | |
| log | 4-Methyl-2-oxopentanoate | 7.4 | 3.2-11.8 | 0.004 | 8.0 | 3.7-12.5 | 0.009 | 15.2 | 8.8-21.9 | 1.9 × 10−6 | 15.1 | 8.5-22.1 | 4.1 × 10−6 | |
| 2-Oxoisopentanoate | 8.2 | 3.6-12.8 | 0.004 | 8.0 | 3.4-12.6 | 0.02 | 10.9 | 4.1-17.7 | 0.002 | 11.1 | 3.2-18.9 | 0.01 | ||
| Aromatic amino acid metabolism | log | Phenylalanine | 5.0 | 1.5-8.7 | 0.02 | 4.6 | 1.0-8.3 | 0.16 | 5.3 | 0.7-10.2 | 0.02 | 4.3 | −0.3 to 9.2 | 0.07 |
| log | Tyrosine | 5.6 | 2.2-9.0 | 0.007 | 4.3 | 1.0-7.8 | 0.15 | 4.8 | −0.1 to 9.8 | 0.05 | 3.6 | −1.3 to 8.6 | 0.15 | |
| Alanine, aspartate, and glutamate metabolism | log | Alanine | 13.3 | 8.9-17.8 | 8.5 × 10−8 | 12.2 | 7.8-16.8 | 3.7 × 10−6 | 19.9 | 13.5-26.6 | 3.3 × 10−10 | 19.4 | 12.9-26.3 | 1.7 × 10−9 |
| Alanine-glucose cycle | log | Glutamate | 26.2 | 16.8-36.4 | 2.6 × 10−7 | 26.8 | 17.1-37.3 | 2.1 × 10−6 | 28.2 | 14.4-43.6 | 2.3 × 10−5 | 28.0 | 13.8-44.0 | 4.7 × 10−5 |
| Glycine, serine, and threonine metabolism | log | Threonine | 5.2 | 1.3-9.2 | 0.04 | 5.2 | 1.2-9.3 | 0.15 | −3.3 | −8.5 to 2.1 | 0.22 | −2.7 | −7.9 to 2.8 | 0.33 |
| Lysine metabolism | log | Alpha-aminoadipate | 11.9 | 6.4-17.6 | 2.0 × 10−4 | 12.6 | 7.0-18.5 | 5.2 × 10−4 | 16.1 | 7.2-25.7 | 2.7 × 10−4 | 15.8 | 6.7-25.6 | 4.7 × 10−4 |
| Cysteine and methionine metabolism | log | Cystine | 10.3 | 6.6-14.2 | 4.4 × 10−7 | 9.8 | 6.1-13.7 | 1.2 × 10−5 | 4.4 | −1.0 to 10.1 | 0.11 | 3.4 | −2.0 to 9.2 | 0.22 |
| Proline and arginine metabolism | log | Proline | 12.4 | 6.3-18.9 | 4.0 × 10−4 | 11.8 | 5.7-18.4 | 0.006 | 11.5 | 3.3-20.4 | 0.005 | 12.8 | 4.5-21.8 | 0.002 |
| TCA cycle; glycolysis/gluconeogenesis; involved in metabolism of multiple amino acids | Pyruvate | 9.9 | 3.6-16.3 | 0.01 | 8.7 | 2.8-14.7 | 0.07 | 20.3 | 10.0-30.6 | 1.2 × 10−4 | 19.0 | 8.8-29.2 | 2.9 × 10−4 | |
| TCA cycle | log | cis-Aconitate | 7.2 | 1.8-12.8 | 0.04 | 5.8 | 0.5-11.3 | 0.28 | 8.8 | 1.0-17.3 | 0.03 | 6.6 | −1.3 to 15.0 | 0.10 |
| Glycolysis/gluconeogenesis, pyruvate metabolism | log | Lactate | 10.8 | 4.6-17.5 | 0.004 | 9.4 | 3.1-16.0 | 0.06 | 16.9 | 7.1-27.6 | 5.3 × 10−4 | 14.5 | 4.7-25.3 | 0.003 |
| Ketones | log | 3-Hydroxybutyrate | −17.9 | −28.4 to −5.7 | 0.02 | −19.8 | −30.2 to −7.8 | 0.048 | −20.6 | −35.4 to −2.3 | 0.03 | −24.8 | −39.0 to −7.5 | 0.007 |
| Glucuronic acid derivatives | log | Mucate | −9.3 | −14.4 to −3.8 | 0.007 | −8.9 | −14.2 to −3.3 | 0.05 | −14.8 | −26.4 to −1.3 | 0.03 | −12.7 | −25.1 to 1.7 | 0.08 |
| Uremic toxins | log | Guanidinosuccinate | −22.6 | −34.5 to −8.7 | 0.01 | −20.9 | −33.2 to −6.4 | 0.11 | −33.2 | −47.3 to −15.3 | 0.001 | −28.2 | −43.6 to −8.5 | 0.008 |
Model 1: unadjusted. Model 2: adjusted for age, LDL-C levels, current smoker or not, current alcohol drinker or not, physical exercise level (high/low), and calorie intake (high/low).
TCA, tricarboxylic acid.
aMetabolites labeled “log” were log-transformed.
bFor log-transformed metabolites, regression coefficients were back-transformed. For normal metabolites, regression coefficients were divided by the means of the non-MetS group.
cFalse discovery rate P values are shown. P < 0.05 is considered significant.
dSame as (b) for log-transformed metabolites. For normal metabolites, coefficients were divided by the adjusted means of the non-MetS group.
eRaw P values are shown. P < 0.05 is considered significant.
Out of the 19 metabolites, 16 (84.2%) could be replicated (Table 2), including those related to BCAA metabolism, aromatic amino acid metabolism, alanine, aspartate and glutamate metabolism, glucose-alanine cycle, lysine metabolism, proline and arginine metabolism, and the TCA cycle. Among these, 13 remained significant after adjustments. Comparisons of the adjusted means of the 13 metabolites between non-MetS and MetS in the original population are shown in Figure 3. Sensitivity analyses excluding (1) participants with BMI at least 25 kg/m2 and (2) those taking medications gave similar results (data not shown).
FIG. 3.
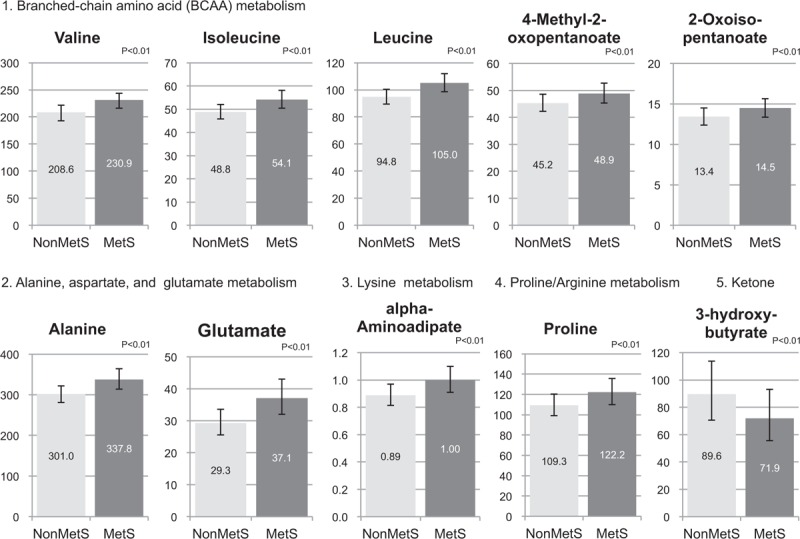
Comparisons of plasma metabolite adjusted mean concentrations in women with and without metabolic syndrome. The adjusted mean concentration of each metabolite was calculated using the fully adjusted model of the original population. Concentrations of metabolites are in μmol/L units. Numbers in the middle of each bar reflect the adjusted mean concentrations. Error bars reflect 95% confident intervals. False discovery rate P values are shown. MetS, metabolic syndrome.
DISCUSSION
To the best of our knowledge, this is the first population-based cross-sectional study to report associations between polar metabolites and MetS in postmenopausal women in a lean Asian population (average BMI 23 kg/m2) with average HDL-C levels of 72.1 mg/dL. Our data indicate that alterations in amino acid metabolism are present in both obese individuals and in women with normal BMI with MetS after menopause.
4-Methyl-2-oxopentaonate and 2-oxoisopentanoate, which are degradation products of BCAAs, were significantly elevated in MetS, in addition to BCAAs themselves. The significant increase of BCAAs in plasma in MetS is consistent with many prior experimental and physiological studies.28,46-49 In a study of lean and obese individuals, BCAAs accounted for the largest amount of variance in principal components analysis of metabolite data, along with derivatives from oxidation products of BCAAs.16 This increase in levels of downstream metabolites indicates that the associations with MetS are related to an alteration in the flux of BCAAs through this catabolic pathway.
A leading theory of the mechanism behind this physiological change is suppression of BCAA catabolism under insulin resistance. Diabetic rats and humans have significantly lower levels of branched-chain keto acid dehydrogenase (BCKDH) enzyme activity, which is the rate-limiting step in overall catabolism of BCAAs.45,48,50,51 Several theories have been proposed for the mechanisms underlying suppressed BCKDH activity, including increased activity of BCKDH kinase that inactivates BCKDH,48 and reduction of adiponectin signaling via an adenosine monophosphate-activated protein kinase α2-dependent pathway.47,49 The increase of BCAAs has also been explained based on amino acid breakdown products being released into blood because insulin resistance causes excess protein breakdown in skeletal muscle.52 A causal role for BCAAs in metabolic disease via the mammalian target of rapamycin pathway or inhibition of glucose transport/phosphorylation has also been suggested.53-55 Whether the increase in BCAAs reflects the consequence of diabetes pathogenesis or has a causal role in disease development has yet to be proven because many large epidemiological studies have had a cross-sectional or case-control design. Further work is needed to determine the mechanisms through which increased amino acid levels might contribute to metabolic diseases.
In our study, several metabolites other than amino acids were significantly associated with MetS in postmenopausal women. Alpha-aminoadipate levels were 6.4% to 17.6% higher in plasma of women with MetS compared with those without MetS. Aminoadipate was found to be the strongest biomarker of diabetes risk out of 70 metabolites screened in the Framingham Heart Study, in which participants with the highest quartile of plasma aminoadipate had fourfold higher odds of developing diabetes over a 12-year follow-up period compared with those in the lowest quartile.56 There were no correlations between aminoadipate and BCAAs. Because aminoadipate is generated by lysine degradation and may also serve as a substrate for enzymes downstream of tryptophan metabolism, the current and previous findings collectively suggest that the mechanism behind MetS and insulin resistance involves alterations in these metabolic pathways, distinct from pathways of BCAAs.
3-Hydroxybutyrate, a ketone body, was significantly lower in MetS compared with non-MetS participants (5.7%-28.4%). This is in contrast to previous studies showing that alpha-hydroxybutyrate is a positive predictor of type 2 diabetes.57-60 The reason for this discrepancy is unclear. Alpha-hydroxybutyrate is generated in the liver under increased oxidative stress; therefore, the different results and the suggestive trends in lactate and pyruvate concentrations may be indicative of down- and up-regulation of ketogenesis and its relationship to the TCA cycle. Another hypothesis is that the phenotype of MetS in our population differs from those in previous studies, especially with respect to the very high levels of HDL-C. A recent review article on BCAAs in insulin resistance described two types of obesity in rodents with regard to impairment of BCAA metabolism.61 Similarly, the alpha-hydroxybutyrate levels might be regulated in a more complex manner. Further studies of the mechanism behind the altered levels of 3-hydroxybutyrate are required.
One of the strengths of our study is the characteristics of the participants. To our knowledge, metabolomic profiling of MetS on a population of lean, older females has not been performed previously. The only study in women with normal BMI (average 24.4 kg/m2) was reported by Würtz et al62; however, the average age of the participants was 32.1 years. Metabolism changes significantly with age and data should be carefully interpreted, even with adjustment of statistical models for age. In addition, the average HDL-C level of our participants was much higher than those in other populations. HDL-C levels are indeed high among Japanese people in general, and have increased in the past few decades.63 As one of the components of MetS, decreased HDL-C is an independent risk factor for CVD, and the TG/HDL-C ratio has a positive correlation with the insulin resistance index.64,65 Our data suggest that alterations in plasma amino acids may precede the reduction of HDL-C levels in the development of insulin resistance.
The study has several limitations. When interpreting metabolic profiling data in a cross-sectional study, the temporality of cause-effect relationships is not assured and possible confounding of unmeasured factors may exist, even after adjustments. Equally of concern is that our data were derived from a single site, lacking test marker performance in an independent cohort. Therefore, follow-up and intervention studies along with those at different facilities are needed. Comparisons between pre- and postmenopausal women could not be performed because of the few premenopausal women enrolled in the study. Completion of a separate ongoing cohort with a working age population is awaited to investigate the impact of menopause on these metabolic changes. Measurement of WC at the umbilicus might also have affected the diagnosis of MetS. Other common sites for WC measurements include the midpoint between the lowest rib and the iliac crest; narrowest or widest WC; just below the lowest rib; and just above the iliac crest.66 However, there is no consensus on the optimal protocol for measurement of WC.66,67 An effort was made to eliminate conditions that could affect the metabolic profile, such as history of cancer and nonfasting status, but multiple drugs for treated participants may have acted as confounders. Various factors might also have influenced measurement variability in the metabolomics analysis, potentially causing nondifferential misclassification of metabolomics data. To minimize these variabilities, we set a uniform fasting condition for participants and standardized the quality control procedures for metabolomics analysis.39
CONCLUSIONS
This study shows that Japanese postmenopausal women who develop MetS may have elevated concentrations of multiple amino acids, including BCAAs, alanine, glutamate, lysine, proline, and other polar metabolites such as alpha-aminoadipate. These findings may not be translatable to populations of non-Japanese women without very high HDL-C levels, but identifying these metabolic changes may be useful for detection of high-risk individuals, including those with normal BMI and relatively high HDL-C levels. A follow-up survey is needed to examine whether these candidate metabolites provide better prediction than standard measures for future development of MetS.
Supplementary Material
Supplementary Material
Acknowledgments
We thank the residents of Tsuruoka City for their interest in our study and the members of the Tsuruoka Metabolomic Cohort Study team for their commitment to the project.
Footnotes
Funding/support: This study was supported in part by research funds from the Yamagata Prefectural Government and the city of Tsuruoka, and a Grant-in-Aid for Scientific Research (grant number 24390168) from the Japan Society for the Promotion of Science.
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Onat A, Ceyhan K, Başar O, Erer B, Toprak S, Sansoy V. Metabolic syndrome: major impact on coronary risk in a population with low cholesterol levels—a prospective and cross-sectional evaluation. Atherosclerosis 2002; 165:285–292. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Miettinen H, Stern MP. Relatively more atherogenic coronary heart disease risk factors in prediabetic women than in prediabetic men. Diabetologia 1997; 40:711–717. [DOI] [PubMed] [Google Scholar]
- 4.Alamgir MA, Javid RA, Hameed A, Mustafa I. Gender difference in components of metabolic syndrome among patients of type 2 diabetes. Pak J Med Sci 2015; 31:886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara A, Mangione CM, Kim C, et al. Translating Research Into Action for Diabetes Study Group Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2008; 31:69–74. [DOI] [PubMed] [Google Scholar]
- 6.Okamura T, Kokubo Y, Watanabe M, et al. A revised definition of the metabolic syndrome predicts coronary artery disease and ischemic stroke after adjusting for low density lipoprotein cholesterol in a 13-year cohort study of Japanese: the Suita study. Atherosclerosis 2011; 217:201–206. [DOI] [PubMed] [Google Scholar]
- 7.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann N Y Acad Sci 2000; 904:502–506. [DOI] [PubMed] [Google Scholar]
- 8.Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas 1990; 12:321–331. [DOI] [PubMed] [Google Scholar]
- 9.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases form and function. Circulation 2012; 126:1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn WB, Broadhurst D, Begley P, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 2011; 6:1060–1083. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto M, Kawakami M, Robert M, Soga T, Tomita M. Bioinformatics tools for mass spectroscopy-based metabolomic data processing and analysis. Curr Bioinform 2012; 7:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirayama A, Tomita M, Soga T. Sheathless capillary electrophoresis-mass spectrometry with a high-sensitivity porous sprayer for cationic metabolome analysis. Analyst 2012; 137:5026–5033. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M, Hirayama A, Robert M, Abe S, Soga T, Tomita M. Prediction of metabolite identity from accurate mass, migration time prediction and isotopic pattern information in CE-TOFMS data. Electrophoresis 2010; 31:2311–2318. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto M, Kikuchi S, Arita M, Soga T, Nishioka T, Tomita M. Large-scale prediction of cationic metabolite identity and migration time in capillary electrophoresis mass spectrometry using artificial neural networks. Anal Chem 2005; 77:78–84. [DOI] [PubMed] [Google Scholar]
- 15.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969; 281:811–816. [DOI] [PubMed] [Google Scholar]
- 16.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9:311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012; 125:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009; 32:1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol 2009; 53:2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Würtz P, Tiainen M, Mäkinen VP, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 2012; 35:1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badoud F, Lam KP, Perreault M, Zulyniak MA, Britz-McKibbin P, Mutch DM. Metabolomics reveals metabolically healthy and unhealthy obese individuals differ in their response to a caloric challenge. PLoS One 2015; 10:e0134613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003; 163:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen I. Heart disease risk among metabolically healthy obese men and metabolically unhealthy lean men. CMAJ 2005; 172:1315–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr 2009; 18:280–284. [PubMed] [Google Scholar]
- 26.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes 1998; 47:699–713. [DOI] [PubMed] [Google Scholar]
- 27.Hashemipour S, Esmailzadehha N, Hamid H, Oveisi S, Yakhchaliha P, Ziaee A. Association of metabolic syndrome components with insulin resistance in normal weight population: the Qazvin Metabolic Diseases study. J Endocrinol Invest 2015; 38:1111–1115. [DOI] [PubMed] [Google Scholar]
- 28.Yamada C, Kondo M, Kishimoto N, et al. Association between insulin resistance and plasma amino acid profile in non-diabetic Japanese subjects. J Diabetes Investig 2015; 6:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010; 53:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008; 118:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuehnbaum NL, Kormendi A, Britz-McKibbin P. Multisegment injection-capillary electrophoresis-mass spectrometry: a high-throughput platform for metabolomics with high data fidelity. Anal Chem 2013; 85:10664–10669. [DOI] [PubMed] [Google Scholar]
- 32.Farahmand M, Ramezani Tehrani F, Bahri Khomami M, Noroozzadeh M, Azizi F. Surgical menopause versus natural menopause and cardio-metabolic disturbances: a 12-year population-based cohort study. J Endocrinol Invest 2015; 38:761–767. [DOI] [PubMed] [Google Scholar]
- 33.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab 2007; 92:3040–3043. [DOI] [PubMed] [Google Scholar]
- 34.Friedwald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 35.Enkhmaa B, Shiwaku K, Anuurad E, et al. Prevalence of the metabolic syndrome using the Third Report of the National Cholesterol Educational Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) and the modified ATP III definitions for Japanese and Mongolians. Clin Chim Acta 2005; 352:105–113. [DOI] [PubMed] [Google Scholar]
- 36.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 39.Hirayama A, Sugimoto M, Suzuki A, et al. Effects of processing and storage conditions on charged metabolomic profiles in blood. Electrophoresis 2015; 36:2148–2155. [DOI] [PubMed] [Google Scholar]
- 40.Hirayama A, Nakashima E, Sugimoto M, et al. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem 2012; 404:3101–3109. [DOI] [PubMed] [Google Scholar]
- 41.Soga T, Sugimoto M, Honma M, et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol 2011; 55:896–905. [DOI] [PubMed] [Google Scholar]
- 42.Soga T, Igarashi K, Ito C, Mizobuchi K, Zimmermann H-P, Tomita M. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal Chem 2009; 81:6165–6174. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010; 6:78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hornunga RW, Reeda LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990; 5:46–51. [Google Scholar]
- 45.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 1995; 57:289–300. [Google Scholar]
- 46.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 2007; 293:e1552–e1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saha AK, Xu XJ, Lawson E, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 2010; 59:2426–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuzuya T, Katano Y, Nakano I, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun 2008; 373:94–98. [DOI] [PubMed] [Google Scholar]
- 49.Lian K, Du C, Liu Y, et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 2015; 64:49–59. [DOI] [PubMed] [Google Scholar]
- 50.Doisaki M, Katano Y, Nakano I, et al. Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem Biophys Res Commun 2010; 393:303–307. [DOI] [PubMed] [Google Scholar]
- 51.Lackey DE, Lynch CJ, Olson KC, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 2013; 304:E1175–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007; 27:293–310. [DOI] [PubMed] [Google Scholar]
- 53.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem 2001; 276:38052–38060. [DOI] [PubMed] [Google Scholar]
- 54.Tremblay F, Jacques H, Marette A. Modulation of insulin action by dietary proteins and amino acids: role of the mammalian target of rapamycin nutrient sensing pathway. Curr Opin Clin Nutr Metab Care 2005; 8:457–462. [DOI] [PubMed] [Google Scholar]
- 55.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002; 51:599–605. [DOI] [PubMed] [Google Scholar]
- 56.Wang TJ, Ngo D, Psychogios N, et al. 2-aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 2013; 123:4309–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013; 62:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varvel SA, Pottala JV, Thiselton DL, et al. Serum α-hydroxybutyrate (α-HB) predicts elevated 1 h glucose levels and early-phase (β-cell dysfunction during OGTT. BMJ Open Diabetes Res Care 2014; 2:e000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varvel SA, Voros S, Thiselton DL, et al. Comprehensive biomarker testing of glycemia, insulin resistance, and beta cell function has greater sensitivity to detect diabetes risk than fasting glucose and HbA1c and is associated with improved glycemic control in clinical practice. J Cardiovasc Transl Res 2014; 7:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013; 62:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014; 10:723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Würtz P, Soininen P, Kangas AJ, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013; 36:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yokoyama S. Unique features of high-density lipoproteins in the Japanese: in population and in genetic factors. Nutrients 2015; 7:2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007; 357:1301–1310. [DOI] [PubMed] [Google Scholar]
- 65.He J, He S, Liu K, Wang Y, Shi D, Chen X. The TG/HDL-C ratio might be a surrogate for insulin resistance in Chinese nonobese women. Int J Endocrinol 2014; 2014:105168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity (Silver Spring) 2007; 15:1061–1067. [DOI] [PubMed] [Google Scholar]
- 67.Ross R, Berentzen T, Bradshaw AJ, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev 2008; 9:312–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


