Summary of Recent Advances
Flagellar movement in Giardia, a common intestinal parasitic protist, is critical to its survival in the host. Each axoneme is unique in possessing a long, cytoplasmic portion as well as a membrane-bound portion. Intraflagellar transport (IFT) is required for the assembly of membrane-bound regions, yet the cytoplasmic regions may be assembled by IFT-independent mechanisms. Steady-state axoneme length is maintained by IFT and by intrinsic and active microtubule dynamics. Following mitosis and prior to their segregation, giardial flagella undergo a multigenerational division cycle in which the parental eight flagella migrate and reposition to different cellular locations; eight new flagella are assembled de novo. Each daughter cell thus inherits four mature and four newly synthesized flagella.
Introduction
Giardia intestinalis is a widespread zoonotic intestinal parasite and is one of the ten major parasites known in humans. Outbreaks of acute giardiasis occur commonly in areas with inadequate water treatment. An estimated one billion people are currently infected with Giardia worldwide, primarily in developing countries [1,2] where infection rates approaching 100% have been observed [3]. Despite the fact that giardiasis represents a global health concern, basic questions remain regarding the mechanisms of giardial invasion, attachment, cell division and pathogenesis. Giardia has two life cycle stages: a swimming, flagellated trophozoite form that attaches to the intestinal microvilli, and an infectious cyst form that persists in the environment [4,5]. Cysts are ingested from contaminated water or food, and may excyst in the small intestine of the animal host to become the flagellated trophozoite. Following excystation, trophozoites colonize the proximal small intestine, attaching to the intestinal villi using a unique microtubule (MT) structure termed the ventral disc [5,6]. Trophozoites that reach the colon encyst based on environmental cues and are released to infect new hosts [7].
Flagellar movements are important for giardial survival; specifically, for the initiation and maintenance of giardial infection. Giardia uses flagellar motility to find suitable sites for attachment to the intestinal villi [8]. Flagellar beating is also required for Giardia to complete cell division and cytokinesis [9,10], and may be necessary for encystation/excystation. Giardia is bilaterally symmetrical with a flattened teardrop shape (~15 μm long by 5 μm wide and 5 μm thick) and a complex internal ultrastructure [11]. The trophozoite has eight motile flagella (“9+2” microtubule arrangement) and complex axoneme-associated structures [12]. Although Giardia is well described in terms of disease, little is known about the assembly, division and functioning of the flagella and associated structures. The conservation of giardial flagellar structure and assembly mechanisms [13] illustrates the ancient evolution of flagellar axonemes and the mechanisms of motility, assembly and maintenance of flagellar length.
Flagellated protists comprise a significant fraction of eukaryotic diversity [14], and were likely among the first microbes visualized using a microscope. The discovery of Giardia, – and perhaps the discovery of flagella – is attributed to Antonie van Leewenhoek [15] who in 1681 observed Giardia from his own stool: “…Their bodies were somewhat longer than broad, and their belly, which was flattish, furnished with sundry little paws, wherewith they made such a stir in the clear medium and among the globules, that you might even fancy you saw a woodlouse running up against a wall; and albeit they made a quick motion with their paws, yet for all that they made but slow progress.” More than 300 years since that description, our understanding of giardial flagellar biology remains rudimentary, and advances have primarily involved improved cytological descriptions and more recently, genomic comparisons to other flagellated protists (reviewed in [16]), including the free-living amoeboflagellate Naegleria gruberi [17]. This review highlights both conserved and novel features of the giardial flagella and underscores the clinical and basic biological relevance of the study of flagellar assembly and division biology in Giardia.
Flagellar structure and flagellar-based movements
Giardia belongs to a phylogenetic group of protists termed diplomonads, whose defining characteristics are eight flagella and two nuclei [18]. The MT cytoskeleton of Giardia is quite complex. In addition to the eight motile flagella, there are several unique MT-based structures – the median body, ventral disc, and funis [11]. Despite Giardia’s divergence from many of the commonly studied flagellates, giardial axonemes possess the canonical structure of the eukaryotic motile flagellum [19]. Each of the eight giardial axonemes has a motile structure with radial spokes, dynein arms, and the nine outer doublet MTs and central microtubule pair [20,21]. Not all flagellar pairs have characteristic flagellar waveforms, however [8,22]. The eight flagella are organized into four bilaterally symmetrical flagellar pairs: the anterior, the caudal, the posteriolateral and the ventral (see Figure 1). The flagellar pairs differ in their cytological position within the trophozoite, the extent of their cytoplasmic length, and their association with ancillary structures. The coordinated and differential beating of Giardia’s eight motile flagella (Figure 2) results in complex movements that are essential for motility, cell division, and possibly attachment ([8] and reviewed in [11]).
Figure 1. Giardial axonemes are characterized by long cytoplasmic regions.
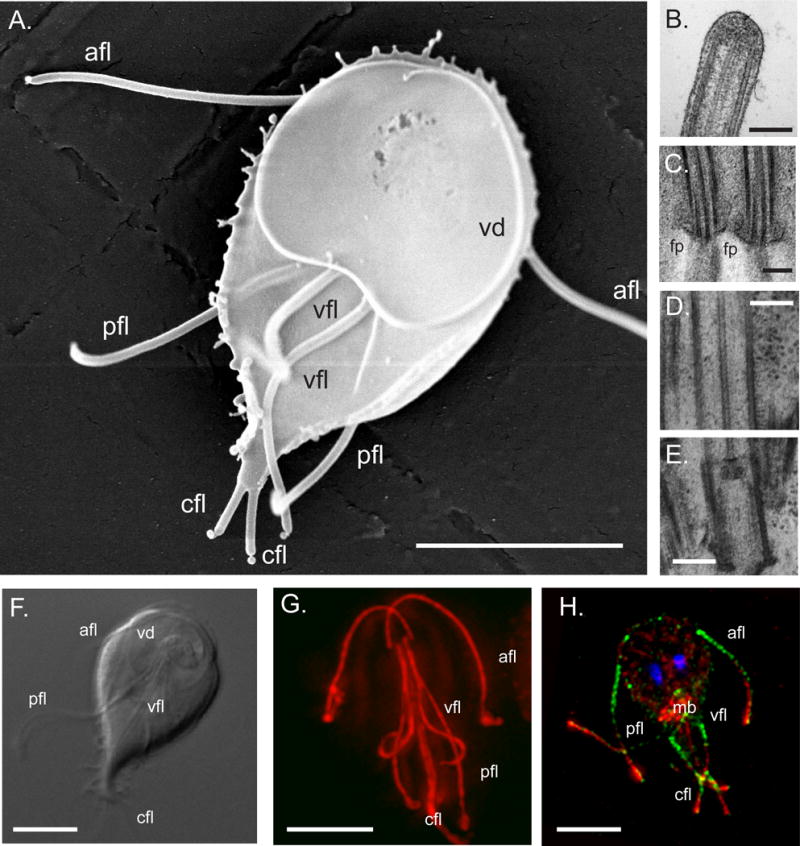
A ventral view of a Giardia trophozoite, visualized using SEM, is shown in (A) (scale bar = 5 μm). The characteristic teardrop shape and ventral disc (vd) as well as the four flagellar pairs (afl = anterior, pfl = posteriorlateral, vfl = ventral, cfl = caudal) are visible; image courtesy of Joel Mancuso, UC Berkeley. The distal flagellar tip (B) is shown in TEM and demonstrates the continuation of the A and B tubules close to the tip. In panel C, electron density around the flagellar pore (fp) region of the ventral axonemes is shown. A caudal axoneme basal body (E) and associated axonemal cytoplasmic region (D) shows the presence of the outer doublet MTS as well as the central pair MTs. Scale bars in B–E = 200 nm. The long cytoplasmic regions (F, G) and the membrane-bound portions (H) of all eight axonemes are visible using DIC and tubulin-immunostaining (F = DIC; G = anti-tubulin immunostaining, red; H = anti-alpha14-annexin which labels the membrane-bound axonemes [82] in green, red = anti-tubulin, blue = DAPI; mb = median body). Scale bars = 5 μm.
Figure 2. Flagellar contributions to complex movements in attached and unattached trophozoites.
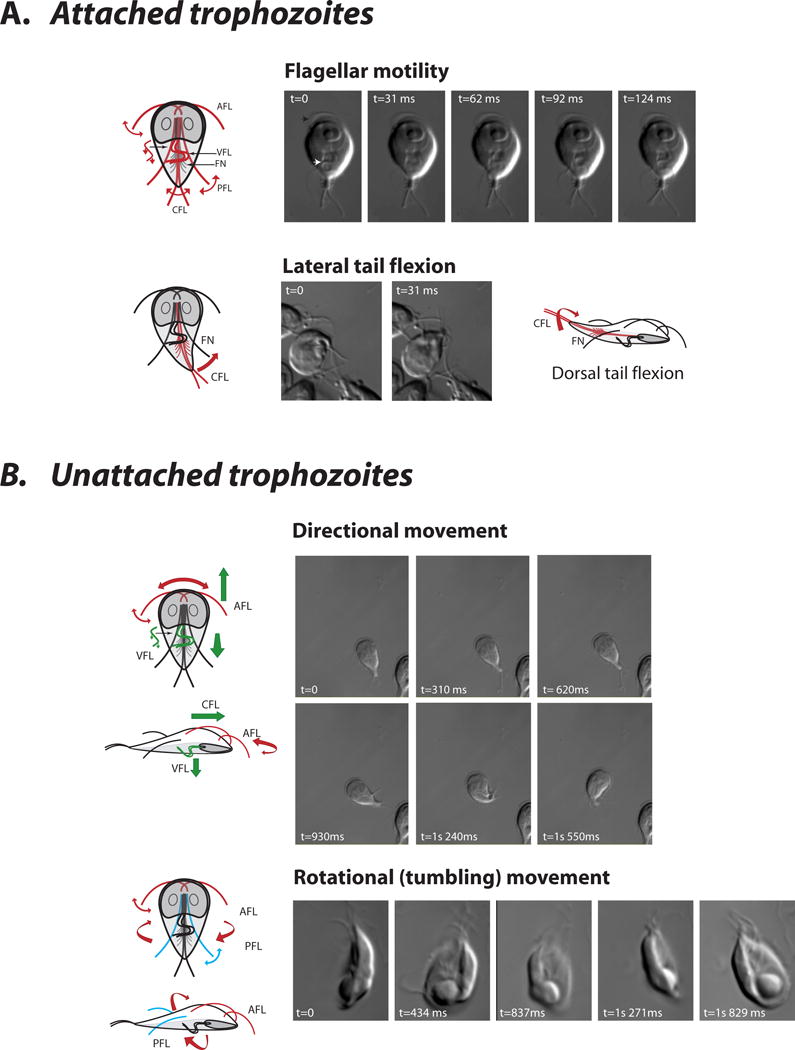
Each pair of giardial flagella (anterior = afl, posteriolateral = pfl, ventral = vfl, and caudal = cfl, fn = funis) contributes specifically to various modes of flagellar motility through differential movements and the action of axoneme-associated structures. In attached cells (A), flagellar motility is primarily evident in the ventral (white arrow) or anterior flagella (black arrow) (see Supplemental Movie 1). “Dorsal/lateral tail flexion”, or the lateral and/or dorsal flexing of the posterior end of the cell, has been attributed to the bending of the funis (i.e. caudal complex) that encircles the caudal flagella and may modulate their beating (see Supplemental Movie 2 and Supplemental Movie 3)[21,83]. Dorsal tail flexion has been associated with detachment essentially by breaking the “seal” of the ventral disc on a surface [84]. In unattached cells (B), left-right directional movement has been attributed to the anterior flagella, and forward or downward movement to the anterior and ventral flagella (see Supplemental Movie 3). Rotational or tumbling movement has been ascribed to anterior and/or posteriolateral flagellar beating, although ventral flagellar beating is during tumbling is also apparent and may contribute to these movements (see Supplemental Movie 4).
Giardia is unique in that each axoneme has a long, cytoplasmic region before it exits the cell body as a membrane-bound flagellum (Figure 1 and see [11]). The ratio of the length of the cytoplasmic region to the membrane-bound portion varies between each flagellar pair (i.e., over two-thirds of the length of the caudal axonemes is in the cytoplasmic region). The conserved “9+2” structure is present in both the cytoplasmic and membrane-bound regions (Figure 1). Eight flagellar basal bodies lie in between or in close proximity to the two nuclei, and are generally arranged with the six basal bodies of the ventral, caudal and posteriolateral axonemes positioned posteriorly below the two anterior basal bodies. The cytoplasmic regions are not transition zones, as transition zones are restricted to small regions proximal to the basal bodies [13].
Giardial flagellar architecture is also defined by the presence of novel structures that are associated with, and thus distinguish, each flagellar pair ([23] and Figure 3). These structures include the “marginal plate” that is associated with the anterior axonemes [23], the fin-like structures extending from the ventral axonemes [24], the electron dense material associated with the posteriolateral axonemes, and the MTs of the “caudal complex” or “funis” that both surround and extend from the caudal axonemes. None of these structures are homologous to the paraflagellar rod (PFR) that is physically attached to the axoneme of trypanosomes [25]. Extra-axonemal structures may confer on each flagellar pair a unique structural identity and, likely, a unique functional role in motility or even attachment [8].
Figure 3. Unique axoneme-associated structures in Giardia.
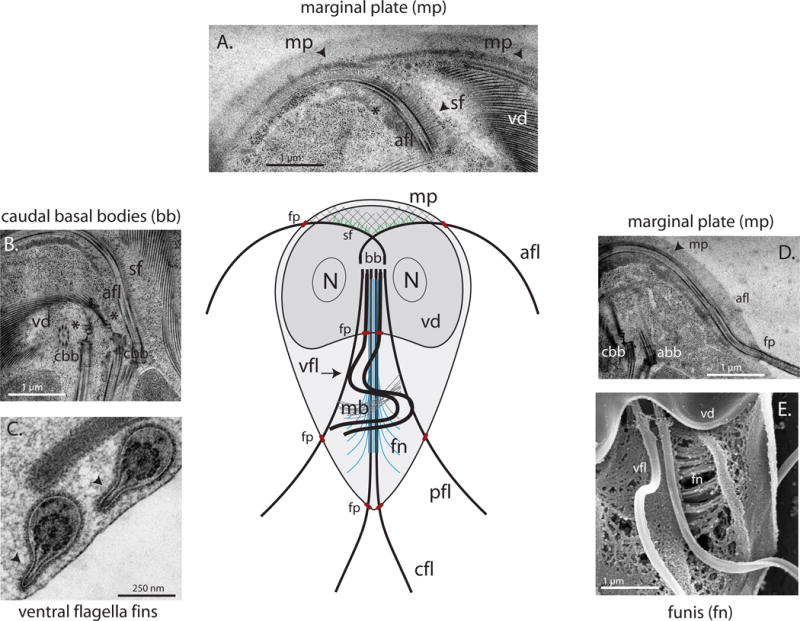
Panels A and D show transmission electron micrographs of the marginal plate (mp) and striated fibers (sf), repetitive structures of unknown function associated with the cytoplasmic regions of the anterior axonemes (afl) that may modulate attachment via the ventral disc (vd). In B, caudal axoneme basal bodies (cbb) are shown to nucleate the two spiral arrays of the ventral disc (B). Electron dense structures, i.e., “fins” (black arrows) associate with the membrane-bound portions of the ventral flagella (C); image courtesy of Cindi Schwartz, CU Boulder. In panel E, the MTs and fibers of the funis (fn) are shown radiating from the caudal axonemes toward the cell periphery (vfl = ventral flagella). The funis is proposed to modulate caudal axonemal beating resulting in dorsal/lateral tail flexion (see Figure 2).
Upwards of 500 proteins comprise the eukaryotic flagellum [26–29], although some flagellar components appear to be lineage-specific. Over eighty flagellar and basal body proteins identified through proteomic [27,28,30] and comparative genomic methods [31,32] are conserved in the giardial genome (see Supplementary Table 1). These include structural components such as the protofilament ribbons (Rib43a and Rib72), the central pair (PF16, PF20, and hydin), the radial spokes (rsp3 and rsp9), and nexin links (PF2). Basal body associated proteins (e.g., centrin, delta-tubulin and epsilon tubulin) and components of the BBSome are also present. Giardial homologs of flagellar genes involved in human ciliary-based genetic diseases have also been identified [33–35]. However, many flagellar and IFT homologs, including some IFT proteins, are not readily identifiable in the genome (and thus are not presented in Table 1). Some flagellar-associated proteins such as the annexins (e.g., alpha-giardins) appear specific to Giardia, although it is unclear whether they contribute to flagellar function or structure [36]. Flagellar proteomics in Giardia could contribute to our overall understanding of flagellar structure and evolution in eukaryotes.
Simultaneous IFT-dependent and IFT-independent assembly of giardial axonemes?
Axonemes are generally assembled by extension and elongation at the distal tip. Because the growing membrane-bound axoneme excludes ribosomes, complex and coordinated targeting and transport of components synthesized in the cytoplasm is required to transport flagellar building blocks to the distal elongating tip. Intraflagellar transport (IFT) ensures the delivery of axonemal building blocks from the cell body to the distal flagellar tips through the continuous and bidirectional movement of large proteinaceous particles (rafts) (originally described in [37] and reviewed recently in [38,39]). Links between proper flagellar function and human ciliary diseases such as polycystic kidney disease [40] and Bardet-Biedl syndrome [41] highlight the importance of understanding flagellar assembly dynamics in diverse eukaryotes.
Membrane-bound regions of giardial axonemes are assembled by IFT ([42] and Figure 4). The kinesin-II heterotrimeric complex [43] powers the anterograde movement of IFT proteinaceous rafts along the outer doublet of axonemes. The retrograde movement of rafts toward the cell body is mediated by cytoplasmic dynein 1b [44]. Giardial homologs of both the retrograde and anterograde IFT complexes (A and B), IFT complex B associated components (DYF-1, DYF-3, DYF-11 and DYF-13), the kinesin-II heterotrimeric complex and IFT dynein are present in the genome (Supplementary Table 1). Giardia does not contain homologs of the homodimeric OSM-3 complex found in both metazoans and ciliates. IFT complex A (IFT140) and complex B (IFT81) components and kinesin-2 motors localize to both the cytoplasmic and membrane-bound regions of axonemes, forming foci at the eight distal flagellar tips and the flagellar pores [13]. There is no significant localization of kinesin-2::GFP to the eight basal bodies. Giardial IFT81 and IFT140 GFP fusions also localize to cytoplasmic regions of axonemes, mainly to the posteriolateral pair. The basal body/transition zone region has been suggested as a docking site for the organization of IFT particles [45,46]; however, in Giardia both the IFT motors and raft particles likely dock on cytoplasmic portions of the axonemes, and accumulate at the flagellar pore regions ([13] and diagrammed in Figure 4). In contrast to trypanosome flagellar pockets, there is no evidence for localized endocytosis at the flagellar pore regions. IFT particle movement on both cytoplasmic and membrane-bound regions of axonemes has not been imaged in live Giardia, yet it is possible that the flagellar pores and distal tip regions of giardial axonemes represent the respective beginning and endpoints of the IFT pathways [13].
Figure 4. Putative IFT-dependent and IFT-independent mechanisms of flagellar assembly and maintenance.
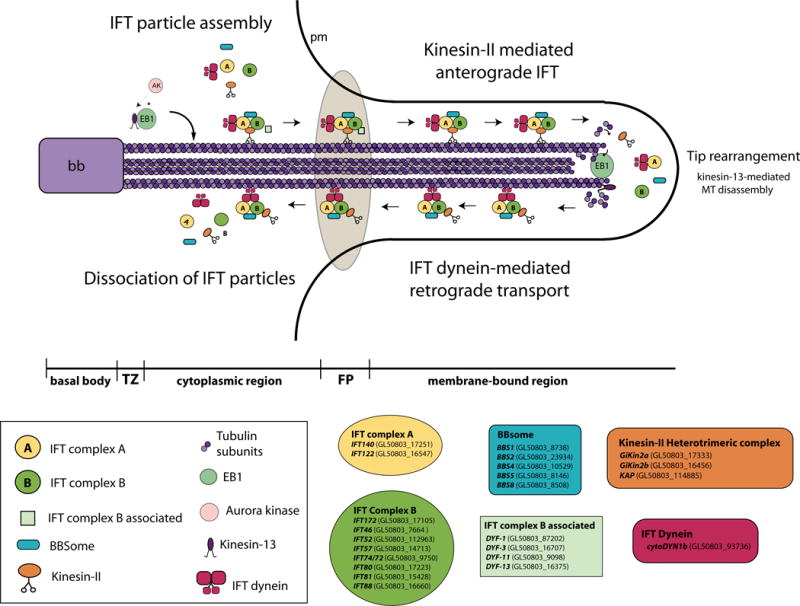
In terms of assembly of the membrane-bound axonemal regions, the anterograde kinesin-II complex likely assembles and loads on cytoplasmic axonemes rather than the basal bodies (BB) or transition zones (TZ), accumulates at the flagellar pore region (FP), and transports IFT raft particles (A and B complexes) as well as tubulin subunits to the flagellar tip. Retrograde transport is mediated by IFT dynein. As in other organisms, the giardial BBSome may be involved in linking the A and B raft complexes, and IFT-complex B associated proteins may facilitate assembly or docking at the flagellar pores. Cytoplasmic regions of axonemes may be assembled by IFT-independent mechanisms. The MT destabilizing kinesin-13 is present at the distal tips and promotes depolymerization of axonemes. Switching of anterograde to retrograde transport also occurs at the distal tip. EB1 is present at the distal tips and flagellar pores (and may mark microtubule plus ends and/or recruit kinesin-13 to tips). An aurora-like kinase (AK) may regulate axonemal disassembly through its regulation of kinesin-13 at the distal flagellar tips. Giardial homologs of IFT, BBSome or IFT-complex B associated proteins are indicated.
Cytoplasmic regions of giardial axonemes are not segregated from the site of protein synthesis. Is IFT required for assembly of these regions? While IFT motors and raft components localize to the cytoplasmic regions of axonemes, evidence suggests IFT-mediated assembly is required only for assembly of the membrane-bound portions. Disruption of the functioning of the anterograde IFT motor kinesin-2 results in severe inhibition of flagellar assembly in diverse eukaryotes, and flagella do not extend beyond the transition zone of the basal bodies [47,48]. Two studies recently investigated the role of kinesin-2 in IFT-mediated flagellar assembly in Giardia using two different methods to disrupt kinesin-2 function – interfering morpholinos and the overexpression of a dominant negative kinesin-2 [13,49]. Cytoplasmic axoneme length was unaffected using either strategy, yet a statistically significant decrease in the length of membrane-bound regions of six axonemes was observed. Interestingly, the anterior axonemes were not as affected [13,49]. Furthermore, anterior flagellar length was also less affected by MT drugs than the other flagellar pairs [50], which suggests the anterior flagella have a different rate of assembly than the other pairs. IFT then seems required for the assembly of membrane bound regions, yet not necessarily for the cytoplasmic regions of giardial axonemes. It is also possible that the different axonemes, such as the anterior pair, assemble at different rates. Thus, both IFT-mediated and non-IFT mediated assembly may occur simultaneously, as has been proposed in other eukaryotes [42,51]. IFT-mediated assembly is required in Drosophila for the assembly and maintenance of sensory neurons, but not for the assembly or function of Drosophila sperm flagella [51]. IFT particles and motors might assemble on cytoplasmic regions of axonemes rather than at basal bodies in Giardia. Future work should assess the initiation and maintenance of giardial infections in the mouse model of giardiasis using mutants that lack flagella.
Active and intrinsic flagellar length maintenance and dynamics
Eukaryotic flagella are dynamic structures with a continuous turnover of tubulin subunits at the distal flagellar tips [52]. Steady-state axonemal length is essentially controlled by the equilibrium between length-dependent rates of assembly, and length-independent rates of disassembly [52,53], as has been postulated in the “balance point model”. Thus, as in other flagellates, equilibrium flagellar length in Giardia is maintained by a balance of various hierarchical levels of flagellar length regulation. These include intrinsic microtubule dynamics, active IFT-mediated assembly (see above), and active axonemal MT disassembly by kinesin-13.
Assembly and maintenance of flagellar length requires IFT to provide building blocks to the distal tips of the giardial membrane-bound axonemes. Both active and intrinsic MT dynamics also contribute to steady-state axoneme length in giardial trophozoites. Intrinsic microtubule dynamics may make a more pronounced contribution to axonemal length in Giardia, however, than in other flagellates. In general, MT destabilizing drugs, such as nocodozole, sequester tubulin subunits and limit MT assembly. Nocodazole treatment has noticeable effects on the length of giardial axonemes, and effectively slows the IFT-mediated assembly of giardial axonemes. Thus nocodazole shifts the dynamic equilibrium flagellar length in favor of axonemal disassembly, resulting in shortened flagella [13]. Conversely, stabilization of axonemal MTs by Taxol increases the average length of giardial axonemes by 30–60%; thus, Taxol stabilization of MTs shifts the dynamic equilibrium flagellar length in favor of IFT-mediated axonemal assembly. As each flagellar pair has a different cytoplasmic and membrane-bound length, it is unclear how differences in lengths between flagella are maintained.
Disassembly of giardial axonemes is also actively regulated. Members of the kinesin-13 family regulate microtubule dynamics in interphase and mitotic arrays, particularly the establishment of proper kinetochore-microtubule attachments and mitotic progression [54–56]. Kinesin-13 is present at the distal tips of the giardial axonemes [50], as has been reported in trypanosomes [25]. Overexpression of a dominant negative kinesin-13 in Giardia results in significantly longer flagella [50]. Active MT destabilization by kinesin-13 at the distal axonemal tip may promote MT disassembly and turnover of tubulin subunits (Figure 4). Because the ultrastructure as well as the structural and regulatory molecular components of flagellar axonemes are conserved, active regulation of microtubule depolymerization at the flagellar tip by kinesin-13 may be a widespread and evolutionarily conserved mechanism important for flagellar length determination in many flagellates [25].
Kinesin-13 may be recruited to the distal flagellar tips by EB1, an evolutionarily conserved plus end MT binding protein (+TIP) that may modulate MT dynamics. EB1 could recruit proteins to the distal flagellar tip, promote dynamic MT instability, or mediate the transition from anterograde to retrograde IFT [57]. In Chlamydomonas, EB1 accumulates at the flagellar tips and at basal bodies [45,58]. In Giardia, EB1 also localizes to the distal tips of all eight flagella, and may aid in the localization of kinesin-13 to the microtubule plus ends at the distal flagellar tips. Kinesin-13 localization at distal flagellar tip could also be regulated by aurora kinase. Aurora kinases are known to localize and regulate the activity of kinesin-13 at the inner domain of the centromere [59]. In Chlamydomonas, an aurora kinase localizes to distal flagellar tips where it regulates microtubule disassembly [60]. Further studies in Giardia should elucidate the details of the mechanism of kinesin-13 localization and post-translational regulation.
Flagellar migration, duplication and maturation
Initial studies of giardial cell division indicated a lack of mitotic spindles or proposed novel, unprecedented mechanisms of chromosome segregation or nuclear division [61,62]. However, two extranuclear spindles have recently been reported in Giardia [63]. Giardia has a “semi-open” mitosis [64] with internal (presumably kinetochore) microtubules extending only a few microns into the nucleus from regions at the spindle poles, near the chromatin in late stage (anaphase B) nuclei. As Giardia is binucleate, the eight axonemal basal bodies are inherited by each daughter cell during a mitotic division that includes two spindles and four spindle poles. The eight flagella and basal bodies are not resorbed prior to cell division; instead, they are involved in the organization and positioning of the two spindles. Flagellar motility is also required to complete cell division, cytokinesis and possibly encystation/excystation [9,10].
In some flagellates, such as Chlamydomonas, flagella are resorbed at the onset of mitosis and the basal bodies (as centrioles) are recruited to function as part of the mitotic spindle poles [65]. Chlamydomonas flagellar basal bodies then organize the spindle microtubules in a bipolar array that may contribute to spindle positioning [66,67]. At the completion of mitosis and cytokinesis, each daughter cell inherits one basal body-daughter complex that functions as the flagellar basal bodies in the new cells [68,69]. This association of basal bodies with spindle poles may ensure equal segregation of basal body pairs to the daughter cells [70,71]. Unlike Chlamydomonas, both centrin localization [72,73] and ultrastructural studies [63] in Giardia indicate that all eight flagella are retained during mitosis, and the flagella and their associated basal bodies migrate to the four spindle poles.
In giardial mitosis, chromosome segregation and nuclear partitioning is followed by the duplication and repositioning of eight flagella in each of the daughter cells [10]. Dramatic flagellar migration and rearrangements coincide with prophase nuclear migration [9]. The migration of the nuclei to the center of the cell displaces the flagellar basal bodies that may then nucleate the four spindle poles. Spindle MTs radiate from one of the flagellar basal bodies near each spindle pole, forming a sheath around the nuclear envelope. Presumably one basal body at each pole acts as the central structural component of the MTOC of the mitotic spindles. It is unclear, however, which flagellar basal body nucleates each particular spindle pole. Gamma tubulin is most likely part of the spindle pole complex and has been detected at basal bodies both before and after nuclear division [74]. The association of flagellar basal bodies with spindle poles may also establish and maintain cell polarity in each generation. Specific combinations of two axonemes and associated basal bodies at each pole could confer a unique positional identity to each of the four spindle poles [63].
This process of flagellar migration and reorganization during mitosis has not been investigated at the molecular level, but likely involves microtubule motors such as kinesins and dyneins to generate forces for the repositioning of organelles during cell division. Flagellar motility and proper assembly is also required for the completion of cytokinesis, as daughter trophozoites complete division by swimming away from each other. Both morpholino knockdowns and dominant negative kinesin-2 mutants have defects in cell division, resulting in significant proportions of dividing cells in the “heart-shaped” or pre-cytokinetic phase of cell division [13,49].
Giardia flagella also have been proposed to undergo a multigenerational division cycle in which the parent flagella migrate and transform to different flagellar types and new flagella are assembled de novo prior to their segregation in daughter cells [9]. Flagella of some protists are known to undergo a similar maturation process that takes more than one cell cycle to complete [75], mirroring the behavior of centrioles in metazoans (reviewed in [76]). Based on immunostaining with a polyglycylated tubulin antibody to visualize parental axonemes and an acetylated tubulin antibody to visualize daughter axonemes, eight parental (old) flagella are retained and eight new flagella are synthesized each cell division cycle [9]. Before mitosis is completed, flagellar and basal body duplication occurs [9,63]. Flagellar regeneration begins in anaphase with short flagella (presumably the new ventral and posteriolateral pairs) emerging from the spindle poles [9,63]. While specific molecular markers have not been used to track each flagellar pair to confirm their identity during division [9], the full length parental anterior axonemes are proposed to become the right caudal axonemes in the new daughter cells. Parental right caudal axonemes are then proposed to become the left caudal axonemes. Thus each daughter cell inherits a full complement of eight flagella each generation – four parental flagella (old) and four newly duplicated flagella [9,63].
The division of the caudal axonemes and basal bodies also has notable implications for the de novo nucleation and assembly of the daughter discs. After the daughter nuclei have been partitioned and the caudal flagellar basal bodies have repositioned between the two nuclei [9], two new dorsal daughter discs are assembled in telophase. The parental ventral disc is not disassembled until later in the cell cycle. Thus the caudal basal bodies nucleate the caudal axonemes and also determine the site of disc assembly, establishing the polarity of the new daughter cells. The left caudal flagellum alone has been proposed to nucleate the spiral MT arrays that form the basis of the ventral disc [23]; however, both caudal basal bodies nucleate the ventral disc MTs_(see Figure 2). Live imaging using light microscopy is required to confirm flagellar migration during cell division in order to ultimately characterize the forces and mechanisms involved in flagellar maturation and daughter disc nucleation. The timing and mechanism by which the extra-axonemal-associated structures (e.g., marginal plate, caudal complex or funis) are assembled during cell division remains unclear [13].
Finally, flagellar movements or motility may be required for developmental transitions during encystation or excystation in Giardia. Flagella are internalized during cyst formation through an unknown mechanism, but do not completely resorb. Notably, internalized flagellar are known to continue to beat inside the newly formed cyst [77]. Flagellar motility has also been suggested to play a mechanical role in the initial opening of the cyst during excystation [78,79].
Conclusions
Flagellar functioning in Giardia is key to its survival in the host, and the eight flagella play critical roles in motility and cell division, and likely in attachment and encystation/excystation. Beyond cellular movement, the giardial axonemes create a stable scaffold for cell shape, cell polarization, and possibly, for intracellular protein trafficking [6] or chemotactic sensing [80]. Due to the conservation of flagellar structure in Giardia, giardial flagellar biology can inform general studies of eukaryotic flagellar structure and assembly in addition to parasitological roles of the flagella. Ultimately, molecular genetic analysis [49,50,81] and protein tagging of axonemal proteins combined with live imaging will be pivotal in assessing the role of giardial flagella in attachment, cell division, and encystation/excystation. Further investigations of flagellar mechanisms should emphasize: 1) flagellar motility in attachment; 2) flagellar biogenesis and nucleation of the ventral disc; 3) IFT-dependent or IFT-independent assembly and maintenance of the cytoplasmic regions of axonemes; 4) the high-resolution architecture and functioning of axoneme-associated structures; 5) the precise nature of the flagellar maturation cycle; and 6) motility and morphogenesis during encystation/excystation. IFT-mediated assembly of axonemes is a particularly interesting question to study in Giardia given the nature of the long cytoplasmic regions of axonemes. With current advances in molecular tools and techniques for microscopic investigations, Giardia’s “sundry little paws” are finally likely to receive more experimental attention.
Supplementary Material
Supplemental Movie 1. Flagellar motility, attached trophozoites.
Supplemental Movie 2. Lateral tail flexion, attached trophozoites.
Supplemental Movie 3. Directional movement, unattached trophozoites.
Supplemental Movie 4. Rotational (tumbling movement), unattached trophozoites.
The presence of both putative and known homologs of flagellar, BBSome, and basal body associated genes indicate that over 80 such proteins are present in the Giardia genome [85]. Homologs were identified by best mutual BLAST hits (e = 5 × 10−2) and compared to a hand-curated list of Chlamydomonas flagellar proteins that were discovered by biochemical, genetic, and bioinformatic methods [27]. Protein families are indicated by PFAM or Interpro designations. Shaded rows indicate giardial homologs of putative flagellar or basal body proteins that have only been identified in proteomic (Cre = Chlamydomonas reinhardtii [31,86]; Hs = Human [28]; Tet = Tetrahymena thermophile [30]) or bioinformatic studies (FABP) and await experimental confirmation. FlagellateCut (FlagCut) [32] were identified in a comparative genomic analysis of Chlamydomonas with other flagellated or non-flagellated eukaryotes. Flagellar associated proteins known in other organisms, such as some IFT proteins that are not readily identifiable in the genome, are not presented.
Acknowledgments
Kari Hagen and Moises de la Torre (UC Davis) and Lillian Fritz-Laylin (UC Berkeley) provided valuable editorial assistance and schematic diagrams. Several of the images presented here were graciously provided by colleagues at UC Berkeley (Joel Mancuso, Meredith Carpenter and Meredith Sagolla) and CU Boulder (Cindi Schwartz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006 doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, Jelinek T, Zeitz M, Fromm M, Schulzke JD. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut. 2007;56:328–335. doi: 10.1136/gut.2006.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan PA. Giardia–diagnosis, clinical course and epidemiology. A review. Epidemiol Infect. 1992;109:1–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Gillin FD, Reiner DS, McCaffery JM. Cell biology of the primitive eukaryote Giardia lamblia. Annu Rev Microbiol. 1996;50:679–705. doi: 10.1146/annurev.micro.50.1.679. [DOI] [PubMed] [Google Scholar]
- 5.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmendorf HG, Dawson SC, McCaffery JM. The cytoskeleton of Giardia lamblia. Int J Parasitol. 2003;33:3–28. doi: 10.1016/s0020-7519(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 7.Roxstrom-Lindquist K, Palm D, Reiner D, Ringqvist E, Svard SG. Giardia immunity–an update. Trends Parasitol. 2006;22:26–31. doi: 10.1016/j.pt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8*.Campanati L, Holloschi A, Troster H, Spring H, Souza WD, Monteiro-Leal LH. Video-microscopy observations of fast dynamic processes in the protozoon Giardia lamblia. Cell Motil Cytoskeleton. 2002;51:213–224. doi: 10.1002/cm.10026. This study uses live imaging to analyze the beating pattern and beat frequency of the eight flagella, with respect to each pair’s contribution to cellular movements. Based on observations of flagellar movement, the authors also propose that the ventral disc is the primary organelle in attachment, in contrast to the “hydrodynamic model”, which suggests an attachment role for the ventral flagella. [DOI] [PubMed] [Google Scholar]
- 9**.Nohynkova E, Tumova P, Kulda J. Cell Division of Giardia intestinalis: Flagellar Developmental Cycle Involves Transformation and Exchange of Flagella between Mastigonts of a Diplomonad Cell. Eukaryot Cell. 2006;5:753–761. doi: 10.1128/EC.5.4.753-761.2006. This cytological study visualized mature and newly synthesized Giardia flagella and proposed that the eight flagella undergo a multigenerational division cycle. Parent flagella are thought to migrate and transform to different flagellar types. New flagella are proposed to be assembled de novo prior to their segregation in daughter cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumova P, Kulda J, Nohynkova E. Cell division of Giardia intestinalis: assembly and disassembly of the adhesive disc, and the cytokinesis. Cell Motil Cytoskeleton. 2007;64:288–298. doi: 10.1002/cm.20183. [DOI] [PubMed] [Google Scholar]
- 11.Dawson SC. An insider’s guide to the microtubule cytoskeleton of Giardia. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01458.x. [DOI] [PubMed] [Google Scholar]
- 12**.Holberton DV. Attachment of Giardia-a hydrodynamic model based on flagellar activity. J Exp Biol. 1974;60:207–221. doi: 10.1242/jeb.60.1.207. Based on live imaging of recently harvested trophozoites, the author developed a model in which ventral flagellar beating is proposed to create a hydrodynamic current underneath the ventral disc, resulting in attachment. This hypothesis also proposes the existence of channels at the anterior end of the disc to draw the hydrodynamic current. [DOI] [PubMed] [Google Scholar]
- 13**.Hoeng JC, Dawson SC, House SA, Sagolla MS, Pham JK, Mancuso JJ, Lowe J, Cande WZ. High Resolution Crystal Structure and in vivo Function of a Kinesin-2 Homolog in Giardia intestinalis. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-11-1156. This study presents the crystal structure of the giardial kinesin-2 homolog, which is the first kinesin-2 structure from any organism. The authors also demonstrate that a dominant negative kinesin-2 results in shortened flagella, and that IFT proteins localize to both the cytoplasmic and the membrane-bound portions of all eight giardial axonemes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson DJ, Simpson AG, Weerakoon N. Free-living flagellates from anoxic habitats and the assembly of the eukaryotic cell. Biol Bull. 1999;196:381–383. doi: 10.2307/1542975. discussion 383–384. [DOI] [PubMed] [Google Scholar]
- 15.Dobell C. Antony van Leeuwenhoek and his “Little animals” being some account of the father of protozoology and bacteriology and his multifarious discoveries in these disciplines; collected, translated, and edited, from his printed works, unpublished manuscripts, and contemporary records. London: J. Bale sons & Danielsson ltd; 1932. [Google Scholar]
- 16.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz-Laylin LK, Prochnik SE, Ginger ML, Dacks JB, Carpenter ML, Field MC, Kuo A, Paredez A, Chapman J, Pham J, et al. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell. 2010;140:631–642. doi: 10.1016/j.cell.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 18.Feely DEH, D V, Erlandsen SL. The Biology of Giardia. In: Mayer EA, editor. Giardiasis. Vol. 3 Elsevier; 1990. pp. 11–49. Hum. Parasit. Dis. [Google Scholar]
- 19.Manton IaC, B An electron microscope study of teh spermatozoid of Sphagnum. Journal of Experimental Botany. 1952;3:265–275. [Google Scholar]
- 20.Clark JT, Holberton DV. Triton-labile antigens in flagella isolated from Giardia lamblia. Parasitol Res. 1988;74:415–423. doi: 10.1007/BF00535140. [DOI] [PubMed] [Google Scholar]
- 21*.Carvalho KP, Monteiro-Leal LH. The caudal complex of Giardia lamblia and its relation to motility. Exp Parasitol. 2004;108:154–162. doi: 10.1016/j.exppara.2004.08.007. This study uses TEM and 3D reconstruction to understand the structure of the caudal complex – a microtubule based structure associated with the caudal flagella. The authors propose that the caudal complex restricts the beating of the caudal flagella, resulting in dorso/lateral tail flexion. [DOI] [PubMed] [Google Scholar]
- 22*.Ghosh S, Frisardi M, Rogers R, Samuelson J. How Giardia swim and divide. Infect Immun. 2001;69:7866–7872. doi: 10.1128/IAI.69.12.7866-7872.2001. This study also uses live imaging to analyze the beating pattern and beat frequency in the eight flagella to determine the contribution of each flagellar pair to giardial motility. By following the movement of beads drawn under the ventral disc, the authors provide support for the “hydrodynamic model” of giardial attachment via currents created by the ventral flagella. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Friend DS. The fine structure of Giardia muris. Journal of Cell Biology. 1966;29:317–332. doi: 10.1083/jcb.29.2.317. This early TEM study of the giardial cytoskeleton provides some of the first detailed descriptions of the molecular architecture of the median body, the ventral disc, and novel axoneme-associated structures such as the striated fibers and marginal plate associated with the anterior axonemes in Giardia muris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulda J, Nohynkova E. Giardia in Humans and Animals. In: Kreier JP, editor. Parasitic Protozoa. Vol. 10 Academic Press, Inc; 1995. pp. 225–423. [Google Scholar]
- 25.Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol. 2007;17:778–782. doi: 10.1016/j.cub.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Dutcher SK. Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet. 1995;11:398–404. doi: 10.1016/s0168-9525(00)89123-4. [DOI] [PubMed] [Google Scholar]
- 27.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1:451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 29.Luck DJ. Genetic and biochemical dissection of the eucaryotic flagellum. J Cell Biol. 1984;98:789–794. doi: 10.1083/jcb.98.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilburn CL, Pearson CG, Romijn EP, Meehl JB, Giddings TH, Jr, Culver BP, Yates JR, 3rd, Winey M. New Tetrahymena basal body protein components identify basal body domain structure. J Cell Biol. 2007;178:905–912. doi: 10.1083/jcb.200703109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 32**.Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. This analysis of the genome of the green alga Chlamydomonas includes a comparative analysis of flagellar proteins in motile and non-motile eukaryotes, resulting in a comprehensive list of putative flagellar-associated proteins of use in comparative analyses with other protists such as Giardia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davy BE, Robinson ML. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum Mol Genet. 2003;12:1163–1170. doi: 10.1093/hmg/ddg122. [DOI] [PubMed] [Google Scholar]
- 34.Pfannenschmid F, Wimmer VC, Rios RM, Geimer S, Krockel U, Leiherer A, Haller K, Nemcova Y, Mages W. Chlamydomonas DIP13 and human NA14: a new class of proteins associated with microtubule structures is involved in cell division. J Cell Sci. 2003;116:1449–1462. doi: 10.1242/jcs.00337. [DOI] [PubMed] [Google Scholar]
- 35.Keller LC, Romijn EP, Zamora I, Yates JR, 3rd, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr Biol. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Vahrmann A, Saric M, Scholze H, Koebsch I. alpha14-Giardin (annexin E1) is associated with tubulin in trophozoites of Giardia lamblia and forms local slubs in the flagella. Parasitol Res. 2008;102:321–326. doi: 10.1007/s00436-007-0758-6. [DOI] [PubMed] [Google Scholar]
- 37.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 39.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 40.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snell WJ, Pan J, Wang Q. Cilia and flagella revealed: from flagellar assembly in Chlamydomonas to human obesity disorders. Cell. 2004;117:693–697. doi: 10.1016/j.cell.2004.05.019. [DOI] [PubMed] [Google Scholar]
- *42.Briggs LJ, Davidge JA, Wickstead B, Ginger ML, Gull K. More than one way to build a flagellum: comparative genomics of parasitic protozoa. Curr Biol. 2004;14:R611–612. doi: 10.1016/j.cub.2004.07.041. This comparative analysis shows the conservation of proteins involved in flagellar assembly across diverse flagellated protists including Giardia. [DOI] [PubMed] [Google Scholar]
- 43.Wedaman KP, Meyer DW, Rashid DJ, Cole DG, Scholey JM. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/95) complex. J Cell Biol. 1996;132:371–380. doi: 10.1083/jcb.132.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vashishtha M, Walther Z, Hall JL. The kinesin-homologous protein encoded by the Chlamydomonas FLA10 gene is associated with basal bodies and centrioles. J Cell Sci. 1996;109(Pt 3):541–549. doi: 10.1242/jcs.109.3.541. [DOI] [PubMed] [Google Scholar]
- 46.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starich TA, Herman RK, Kari CK, Yeh WH, Schackwitz WS, Schuyler MW, Collet J, Thomas JH, Riddle DL. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 1995;139:171–188. doi: 10.1093/genetics/139.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 49**.Carpenter ML, Cande WZ. Using morpholinos for gene knockdown in Giardia intestinalis. Eukaryot Cell. 2009;8:916–919. doi: 10.1128/EC.00041-09. This study is the first to show that morpholino oligonucleotides can be used to inhibit the expression of proteins in Giardia. The use of a morpholino targeted to the anterograde kinesin-2 motor resulted in significantly shortened flagella, but did not affect cytoplasmic axoneme length. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot Cell. 2007;6:2354–2364. doi: 10.1128/EC.00128-07. The authors show that both IFT-mediated assembly and intrinisic and active (kinesin-13 mediated) microtubule disassembly dynamics regulate flagellar length in Giardia. This study also shows the sensitivity of giardial axonemes to microtubule drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 52.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mennella V, Rogers GC, Rogers SL, Buster DW, Vale RD, Sharp DJ. Functionally distinct kinesin-13 family members cooperate to regulate microtubule dynamics during interphase. Nat Cell Biol. 2005;7:235–245. doi: 10.1038/ncb1222. [DOI] [PubMed] [Google Scholar]
- 55.Walczak CE, Mitchison TJ. Kinesin-related proteins at mitotic spindle poles: function and regulation. Cell. 1996;85:943–946. doi: 10.1016/s0092-8674(00)81295-7. [DOI] [PubMed] [Google Scholar]
- 56.Kline-Smith SL, Walczak CE. The microtubule-destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Mol Biol Cell. 2002;13:2718–2731. doi: 10.1091/mbc.E01-12-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr Biol. 2005;15:262–266. doi: 10.1016/j.cub.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen LB, Geimer S, Sloboda RD, Rosenbaum JL. The Microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr Biol. 2003;13:1969–1974. doi: 10.1016/j.cub.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 59.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 60.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 61.Solari AJ, Rahn MI, Saura A, Lujan HD. A unique mechanism of nuclear division in Giardia lamblia involves components of the ventral disk and the nuclear envelope. Biocell. 2003;27:329–346. [PubMed] [Google Scholar]
- 62.Benchimol M. Participation of the adhesive disc during karyokinesis in Giardia lamblia. Biol Cell. 2004;96:291–301. doi: 10.1016/j.biolcel.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 63**.Sagolla MS, Dawson SC, Mancuso JJ, Cande WZ. Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J Cell Sci. 2006;119:4889–4900. doi: 10.1242/jcs.03276. In addition to describing the overall mechanism of giardial mitosis, the authors use electron microscopy to show the interaction of the eight flagellar basal bodies with the four spindle poles. The authors also show the rearrangement of axonemes following mitosis and prior to cytokinesis. [DOI] [PubMed] [Google Scholar]
- 64.Raikov IB. The diversity of forms of mitosis in protozoa: A comparative review. European Journal of Protistology. 1994;30:252–269. [Google Scholar]
- 65.Ou Y, Rattner JB. The centrosome in higher organisms: structure, composition, and duplication. Int Rev Cytol. 2004;238:119–182. doi: 10.1016/S0074-7696(04)38003-4. [DOI] [PubMed] [Google Scholar]
- 66.Coss RA. Mitosis in Chlamydomonas reinhardtii basal bodies and the mitotic apparatus. J Cell Biol. 1974;63:325–329. doi: 10.1083/jcb.63.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehler LL, Holmes JA, Dutcher SK. Loss of spatial control of the mitotic spindle apparatus in a Chlamydomonas reinhardtii mutant strain lacking basal bodies. Genetics. 1995;141:945–960. doi: 10.1093/genetics/141.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall WF, Rosenbaum JL. How centrioles work: lessons from green yeast. Curr Opin Cell Biol. 2000;12:119–125. doi: 10.1016/s0955-0674(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 69.Dutcher SK. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic. 2003;4:443–451. doi: 10.1034/j.1600-0854.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 70.Lechtreck KF, Grunow A. Evidence for a direct role of nascent basal bodies during spindle pole initiation in the green alga Spermatozopsis similis. Protist. 1999;150:163–181. doi: 10.1016/S1434-4610(99)70019-2. [DOI] [PubMed] [Google Scholar]
- 71.Wright RL, Adler SA, Spanier JG, Jarvik JW. Nucleus-basal body connector in Chlamydomonas: evidence for a role in basal body segregation and against essential roles in mitosis or in determining cell polarity. Cell Motil Cytoskeleton. 1989;14:516–526. doi: 10.1002/cm.970140409. [DOI] [PubMed] [Google Scholar]
- 72.Meng T-C, Aley SB, Svard SG, Smith MW, Huang B, Kim J, Gillin FD. Immunolocalization and sequence of caltractin/centrin from the early branching eukaryote Giardia lamblia. Molecular and Biochemical Parasitology. 1996;79:103–108. doi: 10.1016/0166-6851(96)02636-9. [DOI] [PubMed] [Google Scholar]
- 73.Correa G, Morgado-Diaz JA, Benchimol M. Centrin in Giardia lamblia – ultrastructural localization. FEMS Microbiol Lett. 2004;233:91–96. doi: 10.1016/j.femsle.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 74.Nohynkova E, Draber P, Reischig J, Kulda J. Localization of gamma-tubulin in interphase and mitotic cells of a unicellular eukaryote, Giardia intestinalis. Eur J Cell Biol. 2000;79:438–445. doi: 10.1078/0171-9335-00066. [DOI] [PubMed] [Google Scholar]
- 75.Beech PL, Heimann K, Melkonian M. Development of the Flagellar Apparatus during the Cell Cycle in Unicellular Algae. Protoplasma. 1991;164:23–37. [Google Scholar]
- 76.Beisson J, Wright M. Basal body/centriole assembly and continuity. Curr Opin Cell Biol. 2003;15:96–104. doi: 10.1016/s0955-0674(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 77.Midlej V, Benchimol M. Giardia lamblia behavior during encystment: how morphological changes in shape occur. Parasitol Int. 2009;58:72–80. doi: 10.1016/j.parint.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Buchel LA, Gorenflot A, Chochillon C, Savel J, Gobert JG. In vitro excystation of Giardia from humans: a scanning electron microscopy study. J Parasitol. 1987;73:487–493. [PubMed] [Google Scholar]
- 79.Feely DE. A simplified method for in vitro excystation of Giardia muris. J Parasitol. 1986;72:474–475. [PubMed] [Google Scholar]
- 80.Ginger ML, Portman N, McKean PG. Swimming with protists: perception, motility and flagellum assembly. Nat Rev Microbiol. 2008;6:838–850. doi: 10.1038/nrmicro2009. [DOI] [PubMed] [Google Scholar]
- 81.Touz MC, Conrad JT, Nash TE. A novel palmitoyl acyl transferase controls surface protein palmitoylation and cytotoxicity in Giardia lamblia. Mol Microbiol. 2005;58:999–1011. doi: 10.1111/j.1365-2958.2005.04891.x. [DOI] [PubMed] [Google Scholar]
- 82.Szkodowska A, Muller MC, Linke C, Scholze H. Annexin XXI (ANX21) of Giardia lamblia has sequence motifs uniquely sdhared by giardial annexins and is specifically localized in the flagella. J Biol Chem. 2002;277:25703–25706. doi: 10.1074/jbc.M203260200. [DOI] [PubMed] [Google Scholar]
- 83*.Benchimol M, Piva B, Campanati L, de Souza W. Visualization of the funis of Giardia lamblia by high-resolution field emission scanning electron microscopy–new insights. J Struct Biol. 2004;147:102–115. doi: 10.1016/j.jsb.2004.01.017. This electron microscopic study shows the elaborate structure of the funis, a microtubule structure associated with the caudal axonemes. The funis is proposed to promote dorsal/lateral flexion in the posterior region of the Giardia trophozoite. [DOI] [PubMed] [Google Scholar]
- 84.Owen RL. The ultrastructural basis of Giardia function. Trans R Soc Trop Med Hyg. 1980;74:429–433. doi: 10.1016/0035-9203(80)90043-7. [DOI] [PubMed] [Google Scholar]
- 85.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 86.Keller LC, Marshall WF. Isolation and proteomic analysis of Chlamydomonas centrioles. Methods Mol Biol. 2008;432:289–300. doi: 10.1007/978-1-59745-028-7_20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. Flagellar motility, attached trophozoites.
Supplemental Movie 2. Lateral tail flexion, attached trophozoites.
Supplemental Movie 3. Directional movement, unattached trophozoites.
Supplemental Movie 4. Rotational (tumbling movement), unattached trophozoites.
The presence of both putative and known homologs of flagellar, BBSome, and basal body associated genes indicate that over 80 such proteins are present in the Giardia genome [85]. Homologs were identified by best mutual BLAST hits (e = 5 × 10−2) and compared to a hand-curated list of Chlamydomonas flagellar proteins that were discovered by biochemical, genetic, and bioinformatic methods [27]. Protein families are indicated by PFAM or Interpro designations. Shaded rows indicate giardial homologs of putative flagellar or basal body proteins that have only been identified in proteomic (Cre = Chlamydomonas reinhardtii [31,86]; Hs = Human [28]; Tet = Tetrahymena thermophile [30]) or bioinformatic studies (FABP) and await experimental confirmation. FlagellateCut (FlagCut) [32] were identified in a comparative genomic analysis of Chlamydomonas with other flagellated or non-flagellated eukaryotes. Flagellar associated proteins known in other organisms, such as some IFT proteins that are not readily identifiable in the genome, are not presented.


