Abstract
Background:
Studies have explored the risk for and impact of respiratory syncytial virus (RSV) infection requiring hospitalization among healthy preterm infants born at 29–35 weeks of gestational age not given RSV immunoprophylaxis. We performed a systematic review and qualitative synthesis of these studies.
Methods:
Two experienced reviewers used prespecified inclusion/exclusion criteria to screen titles/abstracts and full-text studies using MEDLINE, Embase, BIOSIS and Cochrane Library (January 1, 1985, to November 6, 2014). We abstracted data on risk factors for RSV hospitalization, incidence and short- and long-term outcomes of RSV hospitalization. Using standard procedures, we assessed study risk of bias and graded strength of evidence (SOE).
Results:
We identified 4754 records and reviewed 27. Important risk factors for RSV hospitalization included young age during the RSV season, having school-age siblings and day-care attendance, with odds ratios >2.5 in at least one study (high SOE). Incidence rates for RSV hospitalizations ranged from 2.3% to 10% (low SOE). Length of hospital stays ranged from 3.8 to 6.1 days (low SOE). Recurrent wheezing rates ranged from 20.7% to 42.8% 1 to 2 years after RSV hospitalization (low SOE).
Conclusions:
Young chronological age and some environmental risk factors are important clinical indicators of an increased risk of RSV hospitalization in healthy preterm infants 32 to 35 weeks of gestational age. SOE was low for estimates of incidence of RSV hospitalizations, in-hospital resource use and recurrent wheezing in this population. Studies were inconsistent in study characteristics, including weeks of gestational age, age during RSV season and control for confounding factors.
Keywords: preterm infants, risk factors, respiratory syncytial virus, hospitalization, infectious disease
Respiratory syncytial virus (RSV) infection is an important cause of childhood morbidity from acute lower respiratory tract infections worldwide. An estimated 33.8 million new episodes occur in children under 5 years of age, and 3.4 million RSV episodes require hospital admission.1 Mortality from RSV is rare in developed countries.1 Chronological age is the main risk factor for RSV hospitalization in term infants; most hospitalized episodes occur in infants under 1 year of age, with incidence rates of hospitalization decreasing during the first year of life.2 Degrees of prematurity, chronic lung disease and some forms of congenital heart disease are generally considered additional risk factors; they may characterize subsets of children at heightened risk for RSV hospitalization.3,4
Currently, no vaccine is available to prevent RSV infection in infants. In 1998, the US Food and Drug Administration approved immunoprophylaxis with the monoclonal antibody palivizumab to prevent RSV infection. The US Food and Drug Administration specified the indication “for the prevention of serious lower respiratory tract disease caused by RSV in children at high risk of RSV disease.”5 Since the approval of palivizumab, the American Academy of Pediatrics (AAP) has produced several clinical practice guidelines for its use, focusing on subgroups of children who are at high risk of RSV infection.
In 2014, the AAP issued an updated guidance on palivizumab prophylaxis in high-risk infants and young children.3,4 In this guidance, the AAP Committee on Infectious Diseases and Bronchiolitis and the Guidelines Committee concluded, based on their literature review, that preterm infants born at 29 to 35 weeks of gestational age (WGA) without chronic lung disease, hemodynamically significant congenital heart disease or other coexisting conditions have only a small risk (<5%) of RSV hospitalization. The guideline recommended that this subpopulation of premature infants not be offered palivizumab prophylaxis.
Two additional reviews presented estimates of the risk of RSV hospitalization in healthy preterm infants.6,7 Neither these recent reviews nor the AAP guidance document presented their study selection criteria or assessed study characteristics or risk of bias. We have performed a systematic review and qualitative synthesis of the published evidence to elucidate further the risk of RSV hospitalization and its outcomes in healthy preterm infants born at 29 to 35 WGA.
METHODS
The target population for our review was preterm infants born at 29 to 35 WGA who did not receive RSV immunoprophylaxis and who did not have chronic lung disease or other major coexisting conditions (including hemodynamically significant congenital heart disease, anatomic pulmonary abnormalities, neuromuscular disorders, Down syndrome or cystic fibrosis).
We sought to answer the following 4 questions for the target population:
What are the risk factors for RSV hospitalization?
What is the incidence of RSV hospitalization?
What are the short-term outcomes during the RSV hospitalization?
What are the long-term outcomes after an RSV hospitalization?
Short-term outcomes associated with RSV hospitalization include case fatality rate, length of hospital stay, intensive care unit (ICU) admission and length of stay and need for and duration of mechanical ventilation. Long-term outcomes after RSV hospitalization in infancy include childhood asthma and prolonged and recurrent wheezing up to 6 years of age.
Following a study protocol with prespecified search terms, an experienced research librarian conducted electronic searches to identify studies with publication dates from January 1, 1985, to the day of the search, November 6, 2014. We placed no limitations on publication language or geography. We searched the following electronic databases:
MEDLINE and MEDLINE In-Process (using PubMed platform)
Embase (using Elsevier Platform)
BIOSIS (using Dialog platform)
The Cochrane Library, including the following:
The Cochrane Central Register of Controlled Trials
The Cochrane Database of Systematic Reviews
Database of Abstracts of Reviews of Effectiveness.
We screened publications for inclusion based on prespecified inclusion/exclusion criteria, summarized in Table 1. Senior members of the research team responsible for the title/abstract (level 1) and full-text (level 2) screening included a pediatrician epidemiologist and a health economist. They also searched reference lists of selected studies in the level 2 screening, including all studies selected for data abstraction and recent systematic reviews and meta-analyses. Twenty-four articles were reviewed at level 2 in languages other than English. Disagreements were resolved by consensus or consultation with a third experienced researcher. We documented the inclusion and exclusion process using a spreadsheet, including completion of a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.8
Table 1.
Summary of Study Inclusion Criteria
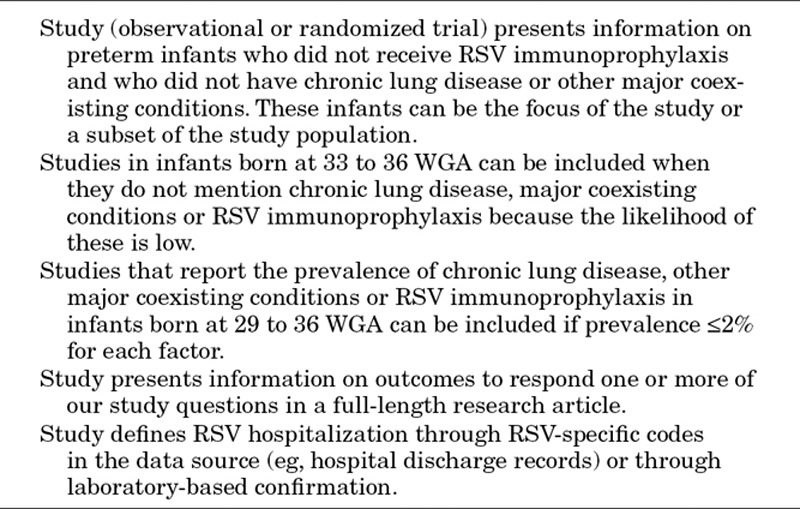
One senior reviewer abstracted data from included studies into detailed evidence tables; a second researcher checked all abstractions against the original source. Data in evidence tables included information on study authors, year, country, funding source, infant population(s) studied, data source(s) used, study characteristics and key study endpoints presented. In addition to evidence tables, we prepared 4 summary tables presenting key information from the included studies for each research question and qualitatively synthesized study characteristics and results for each research question.
For each study, we assessed the risk of bias using an adapted version of the RTI Item Bank.9 We characterized the risk of bias of each included study as low, medium or high based on the average score of the 11 items in the tool, defined as follows: 0 to <1.0 = high risk of bias; 1.0 to 1.5 = medium risk of bias; and 1.6 to 2.0 = low risk of bias.
We graded the strength of the body of evidence for each study question using the 5 domains recommended by the Agency for Healthcare Research and Quality Evidence-based Practice Center program10: study limitations, directness of evidence, consistency of evidence, precision of results and reporting bias. The 4 possible grades of evidence using the Agency for Healthcare Research and Quality approach are high, moderate, low and insufficient.
RESULTS
Of a total 4754 records, we included 454 studies for level 2 screening of full-text studies. After full-text review, we included 27 studies in our review (Fig. 1). Reasons for exclusion included data not presented for target population, RSV not laboratory confirmed or with diagnosis code, outcomes not relevant and study type.
FIGURE 1.
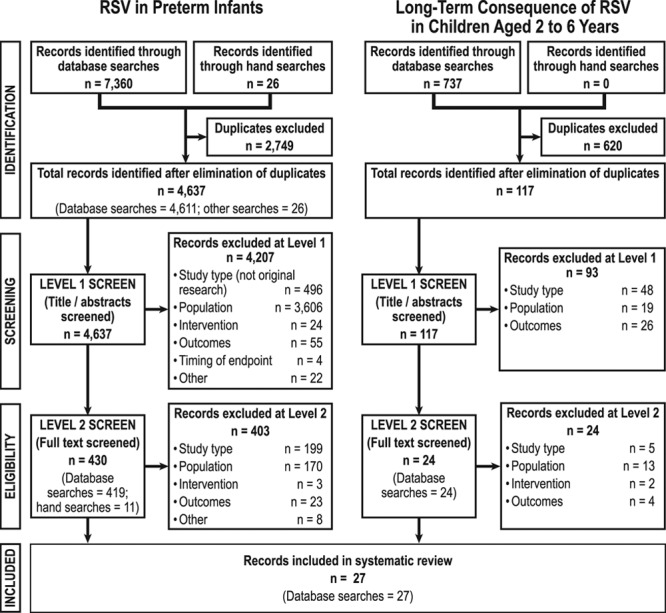
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram
Risk Factors for RSV Hospitalization
Six studies estimated risk factors for RSV hospitalization for the target population (N = 557 to 4761 infants; Table 2).11–16 Of these 6 studies, 5 were prospective cohort studies and 1 was a case–control study;11 risk of bias was low in all 6 studies. All 6 studies estimated risk factors associated with RSV hospitalization in an infant’s first RSV season and included only preterm infants born between 32 or 33 and 35 WGA; thus, they did not provide any information for infants born between 29 and 32 WGA.
Table 2.
Significant Risk Factors for RSV Hospitalization in Target Population
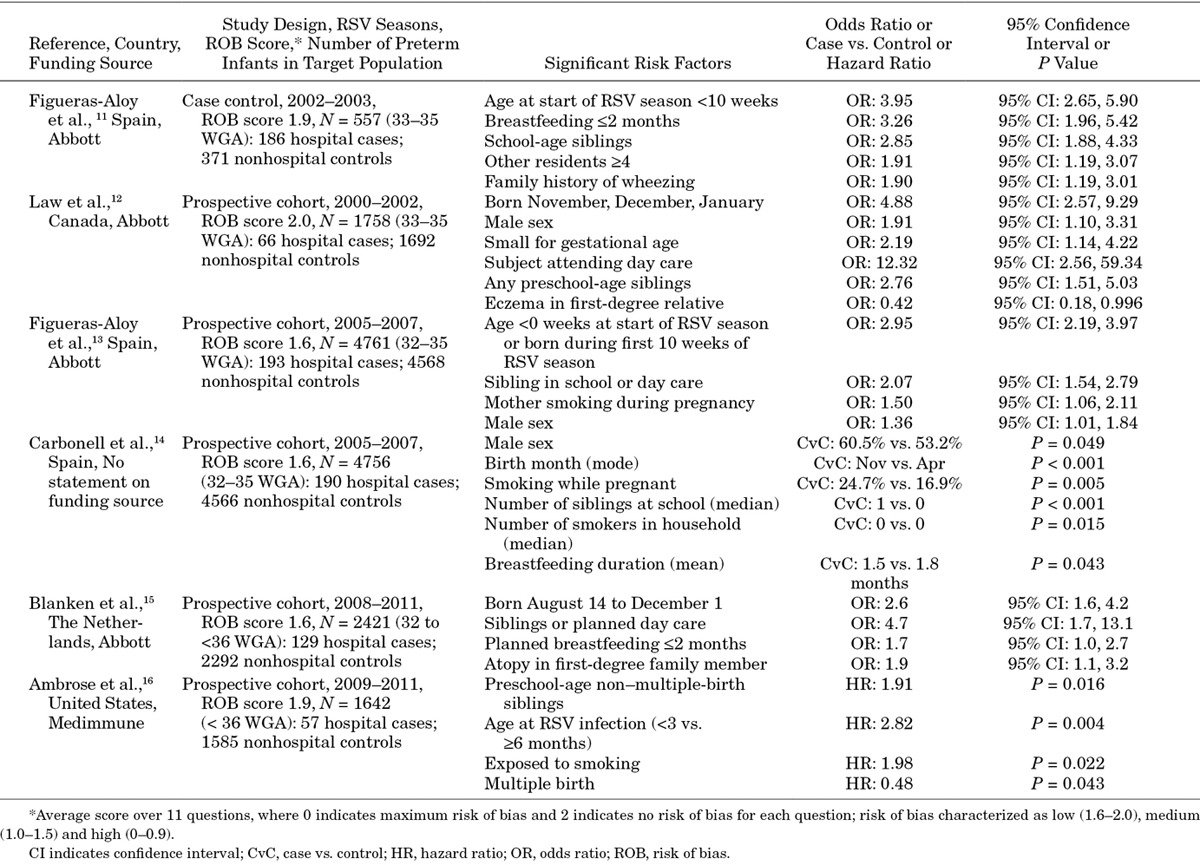
Risk factors examined in the different studies were similar but not identical. All studies that included age showed that young chronological age at the start of or during the RSV season or birth month were statistically significant predictors of RSV hospitalization (odds ratios [OR] = 2.60–4.88). The presence of siblings of preschool or school age was also a significant predictor of RSV hospitalization (ORs = 1.91–2.85). Other family and environment factors that significantly increased the risk of RSV hospitalization in at least 1 study included infant day-care attendance, exposure to smoking, duration of breastfeeding, small for gestational age and family history of wheezing, atopy or eczema. The results were generally consistent even though the studies spanned 10 years, included data from 4 countries and used varying RSV testing strategies.
The strength of evidence (SOE) was high for those in the target population with WGA 32 to 35 for most of the risk factors in the analyses. Risk factors with the most impact on the risk of RSV hospitalization (OR > 2.0 in at least 1 study) were young chronological age during the RSV season, having school-age siblings, day-care attendance, breastfeeding less than 2 months and small for gestational age.
Incidence of RSV Hospitalization
Ten studies presented estimates of the incidence of RSV hospitalization in our target population (N = 182 to 5184); incidence ranged from 2.3% to 10.0% (Table 3).12,16–24 Two studies were randomized controlled trials, 2 were prospective cohort studies and 6 were retrospective cohort studies. Studies covered from 1 to 10 RSV seasons. Risk of bias was low in 6 studies and medium in 4 studies.
Table 3.
Incidence of RSV Hospitalization in Target Population
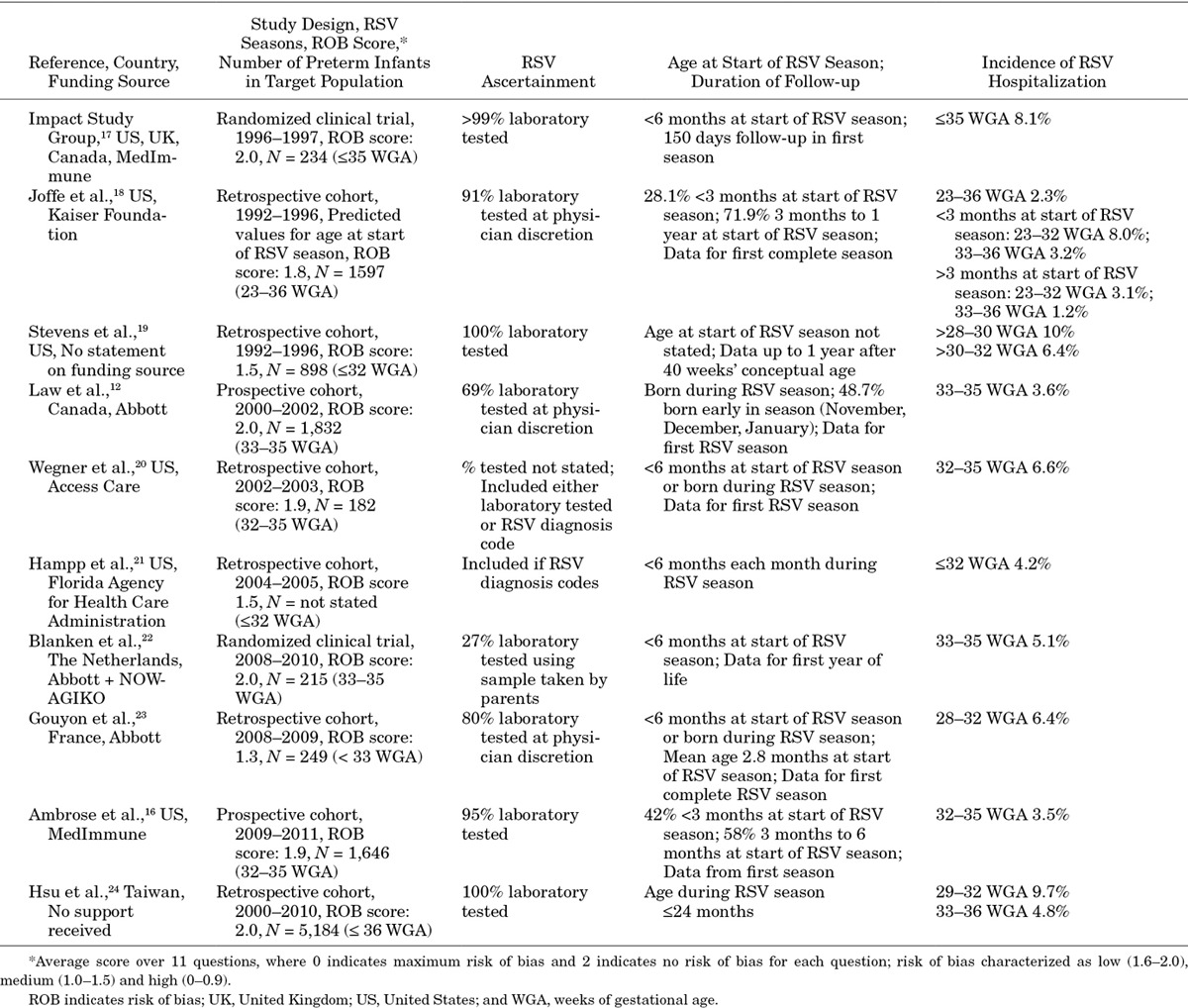
Study characteristics differed substantially among these 10 studies. Differences included range of WGA in infants who otherwise met our inclusion criteria, duration and timing of the observation period, age of the infant during the observed RSV season and ascertainment rates for RSV hospitalizations. We examined the extent to which these study characteristics affected the study results.
We found no consistent relationships in these studies between WGA range and estimated incidence of RSV hospitalizations. Thus, the 6 studies presenting incidence rates for infants born between 32/33 and 35/36 WGA12,16,18,20,22,24 estimated incidence of RSV hospitalizations ranging from 3.2% to 6.6%; incidence rates in the 2 studies that presented estimates for all preterm infants with ≤35 or <36 WGA17,18 ranged from 2.3% to 8.1%; and incidence rates in the 5 studies that presented estimates for those with <33 WGA18,19,21,23,24 ranged from 4.2% to 10.0%.
We saw no consistent relationship between estimated incidence of RSV hospitalizations and duration and timing of the observation period for RSV for each infant. Some studies estimated the incidence of RSV hospitalizations for the first year of life (5.1%–10.0%)19,22 or the first 2 years of life (4.8%–9.7%).24 Others estimated incidence of RSV hospitalizations in the first complete RSV season after birth (2.3%–8.1%)17,18,23 or in infants born either before or during a single RSV season (2.3%–6.6%)16,18,20,21 or only in those born during an RSV season (3.6%).12
Chronological age of preterm infants has been associated with risk of RSV hospitalization.11–16 Ages of infants during the observation period in these 10 studies ranged from born during the observation period to up to 2 years. Only 1 study presented estimates for infants with different chronological ages during the observed RSV season. Joffe et al18 estimated the lowest overall incidence of RSV hospitalization (2.3%); they included estimates for all infants born up to 1 year before the RSV season, during which the infants were observed. However, only 28.1% of these infants were less than 3 months of age at the beginning of the observed RSV season; for these infants, estimated RSV hospitalization rates were 3.2% (33–36 WGA) and 8.0% (23–32 WGA).
More complete ascertainment of RSV infections was not consistently related to higher incidence of hospitalization. One study conducted RSV testing for all infants hospitalized with a respiratory tract infection19; 3 studies conducted RSV testing in >90% of potentially eligible hospitalizations.16–18 Nevertheless, in these 4 studies, incidence rates ranged from 2.3% to 10.0%. Two studies tested for RSV at the physician’s discretion in 60%–80% of potentially eligible hospitalizations,12,23 and another study had parents take and send samples to the laboratory, resulting in testing of only 27% of inpatient and outpatient respiratory episodes.22 In these 3 studies, the incidence rates for hospitalization ranged from 3.6% to 6.4%.
Most studies reported rates in which the numerator included only the tested RSV-positive or RSV-coded hospitalizations, and the denominator included all infants in the study. RSV hospitalization rates may be underestimated in such studies if not all infants hospitalized with respiratory tract infections were tested. One study conducted sensitivity analyses to explore the impact of having tested only 80% of potentially eligible hospitalizations.23 For infants born at 28–32 WGA, hospitalization rates varied from 6.4% (numerator included only RSV-positive cases among those tested) to 9.6% (assuming all non–RSV-tested cases were RSV-positive).
Because hospitalization rates might vary according to the risk of bias as well as in studies across countries because of different treatment patterns, and over time, we looked at the rates in subgroups of our identified studies. Among the 7 studies with a low risk of bias,12161718202224 the hospitalization rates ranged between 2.3% and 9.7%. In the 6 US studies,161718192021 the hospitalization rates ranged between 2.3% and 10.0%. Similarly, looking only at studies published since 2010, the rates ranged between 3.5% and 9.7%. For all the different subgroups, however, the included studies had different characteristics; moreover, for the studies with low risk of bias and for the US studies, publication dates ranged from 1998 to 2014.
Based on our review, the SOE for incidence of RSV hospitalization in the target population is low. Estimates are inconsistent and not clearly related to variability in study characteristics, including the WGA group ranges included, duration of observation, age during the observation period, RSV ascertainment method and study country and publication date.
Short-Term Outcomes During RSV Hospitalization
Eleven studies presented estimates of the short-term outcomes during an RSV hospital stay in the target population (N = 28 to 378 infants; Table 4).19,20,23,25–32 Of these, 1 was a prospective survey, 1 was a prospective cohort study and 9 were retrospective cohort studies. The number of RSV seasons studied ranged from 1 to 4. The studies included different ranges of WGA in infants who otherwise met our inclusion criteria. Risk of bias was low in 6 studies and medium in 5 studies.
Table 4.
Short-Term Outcomes During RSV Hospitalization in the Target Population

Outcomes included case fatality rate in the hospital and hospital service use; the latter covered total length of stay, percentage with care in the ICU, mean length of stay in the ICU, percentage on mechanical ventilation and mean duration on mechanical ventilation. The need for ICU care and mechanical ventilation are particularly important outcomes because they are indicators of more severe disease.
The estimated in-hospital mortality rate for all healthy infants <36 WGA ranged from 0% to 2.9% in 3 studies (published between 1992 and 2000), with a range of 0.95% to 2.9% in the 2 studies with a low risk of bias.27,29,30 Moler et al,28 in a study with low risk of bias, included only infants requiring mechanical ventilation enrolled in a clinical trial of ribavirin; they reported a mortality rate of 1.7% for healthy preterm infants whether or not they were treated with ribavirin.
Of these 11 studies, 10 estimated length of stay in the hospital for the target population. In the 6 studies with a low risk of bias,20,26,28–31 the mean length of stay for all infants ranged from 3.75 to 6.1 days; for those requiring mechanical ventilation, the mean length of stay was 12.7 days.28 Six studies included US patients. Five of these studies estimated a mean length of stay for all infants ranging between 3.75 and 6.1 days19,20,25,26,30; the sixth study estimated a mean length of stay of 12.7 days for those requiring mechanical ventilation.28 Two Canadian studies estimated a median length of stay of 7.0 and 5.0 days for all infants.27,29 Studies in France23 and Tunisia32 estimated a mean length of stay of 7.2 and 9.1 days, respectively. In a subgroup analysis, Stevens et al19 showed a longer length of stay for those between 30 and 32 WGA than for those between 28 and 30 WGA.
The percentage of healthy preterm infants admitted to the ICU ranged from 13.3% to 60.7% in the 7 studies reporting this outcome.19,23,25–27,30,32 In the 2 studies with a low risk of bias,26,30 the percentage admitted to the ICU ranged from 12.6% to 38%; both studies were more than 10 years old but included infants with similar WGA. Four studies included US patients. Of these studies, 3 included all preterm infants,25,26,30 and the estimated percentages admitted to the ICU ranged from 12.6% to 38%. Stevens et al19 included only infants with ≤32 WGA; they estimated ICU admissions to be 13.3% for those with 28–30 WGA and 26.3% for those with 30–32 WGA. One Canadian study (medium risk of bias) estimated that 33.6% of preterm infants with ≤36 WGA were admitted to the ICU.27 A US study by Wegner et al20 (low risk of bias) estimated mean ICU stay of 1.08 days for all hospitalized infants with 32–35 WGA. This length of stay in the ICU is similar to the estimate by Meert et al25 (medium risk of bias) of 0.9 days for infants with ≤37 WGA.
Dougherty and Meissner30 (low risk of bias) estimated a mean ICU length of stay of 7 days for infants with ICU admissions; Moler et al28 (low risk of bias) estimated a mean length of stay of 8.2 days for those admitted to the ICU and requiring mechanical ventilation.
The percentage of all healthy preterm infants hospitalized who required mechanical ventilation ranged from 11.8% to 24.0% in the 2 studies with low risk of bias26,30 and in the 3 US studies25,26,30 and the 1 Canadian study27 that presented these estimates. One other study with low risk of bias estimated a 33% mechanical ventilation rate in preterm infants with 29–32 WGA admitted to the pediatric ICU in Israel.31 Meert et al25 (medium risk of bias) estimated a mean duration of 0.7 days of mechanical ventilation for all hospitalized infants; Dougherty and Meissner30 and Moler et al28 estimated means of 10 and 6.3 days, respectively, for those requiring mechanical ventilation.
The SOE for short-term outcomes of RSV hospitalization in the target population is low. Five of the studies had a medium risk of bias. Moreover, estimates across the studies were inconsistent overall and for the risk of bias or country-specific subgroups analyzed. In addition, 9 of the studies were published before 2005 and so do not capture any recent changes in treatment patterns.
Long-Term Outcomes After RSV Hospitalization
Five studies33–37 presented estimates of the long-term outcomes after an RSV hospitalization in our target population (N = 14 to 408 hospitalized for RSV; N = 154 to 20,250 infants not hospitalized for RSV; Table 5). Two studies were prospective cohort studies; 3 were retrospective cohort studies. Follow-up after the index RSV hospitalization ranged from 1 year to a mean of 2.1 years. Risk of bias was low in 1 study36 and medium in 4 studies.33,35,37 Outcomes estimated in these studies included recurrent wheezing, asthma, respiratory procedures and health care service use.
Table 5.
Long-Term Outcomes After RSV Hospitalization in Target Population
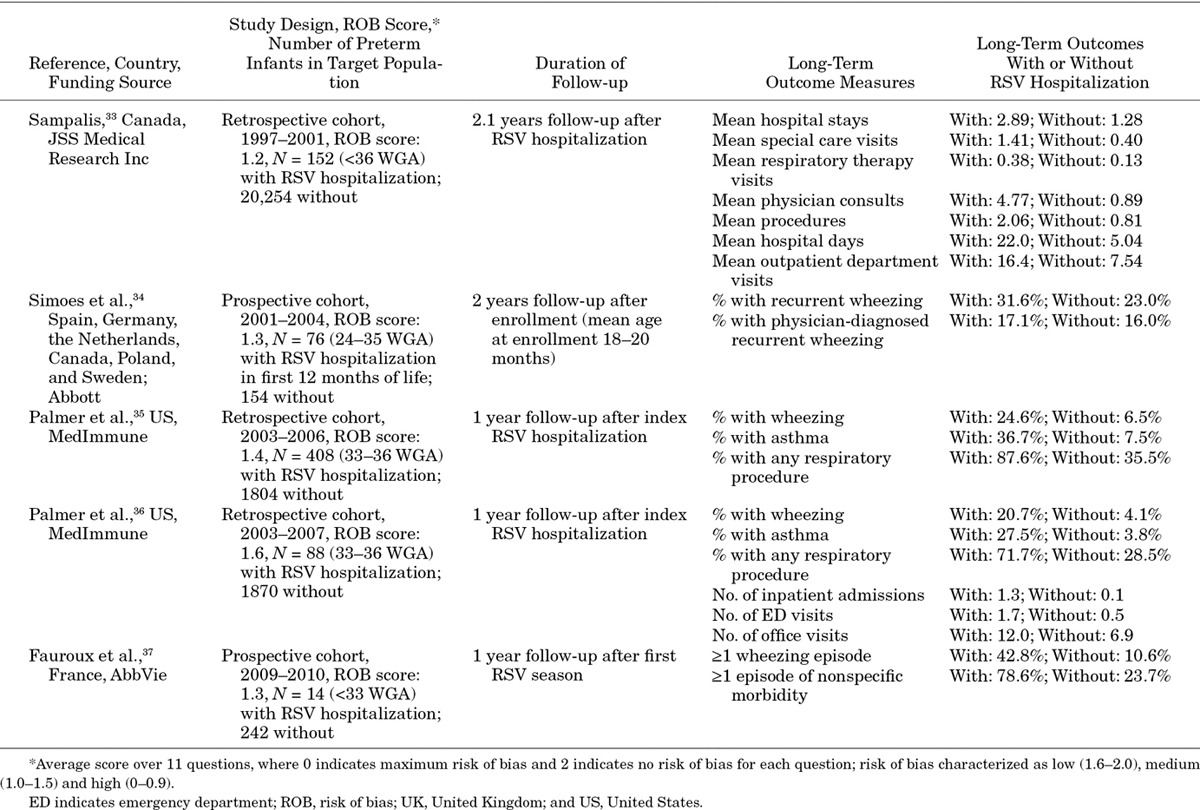
Four studies with 1 to 2 years’ follow-up34353637 estimated higher rates of clinical outcomes for the healthy preterm infants with an RSV hospitalization than for those without such a hospitalization. Wheezing rates ranged from 20.7% to 42.8% for the target population with an RSV hospitalization and from 4.1% to 23.0% for those without an RSV hospitalization. In the study with a low risk of bias, the wheezing rate was 20.7% for those with an RSV hospitalization and 4.1% for those without a hospitalization. In addition, Sampalis33 (medium risk of bias) followed preterm infants with 32 to <36 WGA for a mean time of 2.1 years after RSV hospitalization; rates of use of respiratory-related health care services were higher for this group than for a matched population of healthy preterm infants without a proven or probable RSV hospitalization.
The SOE for long-term outcomes of RSV hospitalization in the target population is low. Four of the 5 studies had a medium risk of bias. Moreover, no study controlled for confounding factors that might be correlated with both risk of RSV hospitalization and recurrent wheezing and childhood asthma.
DISCUSSION
The results of our systematic review clarify the strength of the published evidence for the risk of RSV hospitalization and its consequences in preterm infants born between 29 and 35 WGA who do not have chronic lung disease or other major coexisting conditions and who did not receive RSV immunoprophylaxis (the target population). We found that strong evidence exists for the risk factors associated with hospitalization for RSV. However, estimates of the magnitude of the incidence rates of hospitalization and the short- and long-term outcomes from hospitalization are inconsistent or inadequately controlled.
With respect to the risk of RSV hospitalization in our target population, 6 studies were relatively homogeneous in the gestational ages and risk factors investigated. More important for clinicians and parents, they suggested that factors with the most impact (OR >2.0 in at least 1 study) are young chronological age during the RSV season, having school-age siblings, day-care attendance, breastfeeding less than 2 months and small for gestational age (all high SOE). These are the most definitive findings from our study; they should provide practitioners and others with important indicators of risk of RSV hospitalization in healthy preterm infants.
We identified 10 studies that estimated the incidence of RSV hospitalization and 11 that estimated short-term outcomes of these hospitalizations. Estimates in these studies were inconsistent even when only considering studies with a low risk of bias (low SOE). Inconsistency in the results might be explained partially by differences in study characteristics. For example, studies estimating the incidence of RSV in the target population included infants with different WGA ranges, different ages and lengths of follow-up during the observed RSV season, and different rates of ascertainment of RSV infection. In addition, the date of the study might influence hospitalization rates or short-term outcomes because of changes in practice patterns. For example, changes in the recommendations for the use of continuous pulse oximetry in hospitalized infants in the AAP bronchiolitis guidelines might influence length of stay in the hospital for RSV hospitalizations for all infants in the United States.38
We identified 5 studies that reported long-term impacts of RSV hospitalizations. None of these studies controlled for potential confounders such as family history, underlying pulmonary physiology and environmental factors. Other studies have shown that these factors all increase the risk of both an RSV hospitalization and recurrent wheezing.39,40
Three recent reviews summarize RSV hospitalization rates for our target population.4,6,7 Although we identified all the studies in these reviews and reviewed the full-text articles, we ultimately excluded many because they did not meet our eligibility criteria. For instance, they did not require laboratory confirmation of RSV, included infants with chronic lung disease or other major coexisting conditions (or did not explicitly exclude them), and included infants who were given immunoprophylaxis.2,41–57 In addition, several articles in our review14,16–18,20–24 were not included in at least one of the published reviews. Despite these differences, the ranges of estimated incidence rates for RSV hospitalization in the target population in the 3 reviews were similar to those that we reported.
Our study was not designed to compare outcomes between our target population and other infant subgroups. However, many studies in our review presented data for other infant subgroups. These studies generally showed that the incidence of RSV hospitalization in the target population was higher than for those receiving immunoprophylaxis or for term infants but lower than for preterm infants with chronic lung disease or congenital heart disease.A recent large US study published after the date of our searches58 has also shown higher hospitalization rates for healthy preterm infants 33–36 WGA than for healthy term infants.
In studies looking at short-term outcomes, the target population tended to use more hospital resources than healthy term infants. The Helfrich et al58 study also showed longer hospital length of stay and greater need for respiratory support for healthy preterm infants 33–36 WGA than for healthy term infants. Our included studies showed fewer hospital resources for those in our target population hospitalized with RSV than for those with chronic lung disease or congenital heart disease in most cases; however, these results were not seen in all studies. In particular, 2 studies estimated higher ICU use for the target population than for either infants with chronic lung disease19 or those with bronchopulmonary dysplasia.30 Finally, for infants with RSV hospitalizations, rates of long-term wheezing for those in the target population were similar to rates for term infants in one study.35
Our included studies varied widely in their characteristics, particularly in the WGA range for the infants included in the study and the calendar age of the infants during their first RSV season. Risk-factor studies clearly demonstrated that calendar age during the first RSV season and other risk factors were significant predictors of hospitalization for RSV infection. Given this finding, we suggest that future studies to determine the incidence of hospitalizations in our target population focus on infants born less than 3 months before the RSV season or during the season, both with and without other risk factors. This will allow investigators to assess whether the incidence of hospitalization in these infants is high enough to consider prophylaxis. In addition, studies comparing wheezing rates for those with and without an RSV hospitalization should always control for other factors associated with long-term wheezing to ensure that differences in the 2 populations are accounted for in the analysis. Finally, we would like to emphasize the importance of thorough reporting of all characteristics of the included population and analyses that allow the impact of different population characteristics to be determined for understanding the impact of RSV on our target population.
Strengths and Limitations of Our Review
We implemented a formal systematic review that followed rigorous and prespecified processes for library searches; screening of titles, abstracts and full-text articles; assessment of risk of bias; and grading of evidence. Our eligibility criteria were designed to include only studies with laboratory-confirmed RSV or an RSV diagnosis code (to avoid overestimating the incidence of RSV hospitalization), but we did include studies reflecting broad geographic locations. Our qualitative synthesis of results discussed key study characteristics and their relationship to study results.
The evidence base ultimately had several important drawbacks. All but 2 studies were observational studies and thus subject to issues of bias and confounding that affect observational research. Observational studies do reflect routine care in the general population more closely than randomized studies and so may be quite relevant for our research questions. In the studies presenting estimates of the incidence of RSV hospitalizations, ages of the preterm infants during the RSV season studied varied; ages ranged from “born during the RSV season” to 2 years. Some studies included only a subset of our target population (eg, infants born at 32–35 WGA), and some studies included all preterm infants who otherwise met our criteria (eg, <36 WGA). Studies used different definitions of chronic lung disease, coexisting conditions, risk factors and both short- and long-term outcomes; they also did not present information on disease severity, which can affect hospital length of stay and outcomes. Yet other limitations were the broad span of time covered (15 years), during which clinical practices might have changed, and small sample sizes (eg, fewer than 50 infants in the target population). All these differences made qualitative synthesis challenging.
Of the 27 studies we included, 44% reported receiving funding from industry, 7% from the government and 15% from other nonindustry sources or no funding; 33% did not specify funding source in the publication. In this particular field of inquiry, relatively few teams are conducting research; of these, many are funded by the manufacturer of palivizumab.
Conclusions
Evidence from our systematic literature review was limited for the target population: preterm infants born 29–35 WGA and without chronic lung disease, congenital heart disease or other significant comorbidity and not receiving immunoprophylaxis. Estimates of incidence of RSV hospitalization, in-hospital resource use and recurrent wheezing varied widely, possibly because of inconsistencies in study characteristics, especially the characteristics of the study population. This heterogeneity in the study populations and in the results severely limited our ability to draw clinical or policy conclusions for the target population for these outcomes. By contrast, studies that estimated risk factors for RSV hospitalizations included similar populations, and their results consistently indicated that young chronological age during the RSV season, having school-age siblings, day-care attendance, breastfeeding less than 2 months and small for gestational age are significant risk factors for RSV hospitalization. We regard these as important clinical indicators for practitioners caring for preterm infants.
ACKNOWLEDGMENTS
We acknowledge Outi Ahdesmaki, BSc, Sarah Mitchell, BSc, Emily Reese, PhD, and Nuria Riera, PhD, for their assistance during the literature searches, level 1 and 2 screening, data abstraction and risk of bias assessment. We acknowledge editorial assistance by S. Daniel Siepert, MA.
APPENDIX
Copies of the review protocol, reasons for study exclusion, details of quality and bias assessments and detailed extraction tables are available at www.rtihs.org/RSVsupplement.
Footnotes
All authors are employees of RTI International; none has a financial interest in the sales of palivizumab, which is manufactured by AstraZeneca/Medimmune who provided an unrestricted grant to RTI International to perform the review. AstraZeneca/Medimmune had no role in the development of the protocol, performance of the literature searches, abstraction of the data or preparation of the qualitative report and appendices. The authors have no other conflicts of interest.
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winterstein AG, Knox CA, Kubilis P, et al. Appropriateness of age thresholds for respiratory syncytial virus immunoprophylaxis in moderate-preterm infants: a cohort study. JAMA Pediatr. 2013;167:1118–1124. doi: 10.1001/jamapediatrics.2013.2636. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics (AAP) Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics (AAP) Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Technical report: updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 5.Synagis (palivizumab) injection for intramuscular use [prescribing information]. March 2014. Available at: http://www.medimmune.com/docs/default-source/pdfs/prescribing-information-for-synagis.pdf. Accessed July 29, 2015.
- 6.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30:510–517. doi: 10.1097/INF.0b013e3182184ae7. [DOI] [PubMed] [Google Scholar]
- 7.Resch B, Kurath S, Manzoni P. Epidemiology of respiratory syncytial virus infection in preterm infants. Open Microbiol J. 2011;5:135–143. doi: 10.2174/1874285801105010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan M, Berkman ND, Dryden DM, Hartling L. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI Item Bank. Report No.: 13-EHC106-EF. [web site] http://www.ncbi.nlm.nih.gov/books/NBK154461/. Accessed June 16, 2015. [PubMed] [Google Scholar]
- 10.Berkman ND, Lohr KN, Morgan LC, et al. Interrater reliability of grading strength of evidence varies with the complexity of the evidence in systematic reviews. J Clin Epidemiol. 2013;66:1105–1117.e1. doi: 10.1016/j.jclinepi.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Figueras-Aloy J, Carbonell-Estrany X, Quero J IRIS Study Group. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J. 2004;23:815–820. doi: 10.1097/01.inf.0000136869.21397.6b. [DOI] [PubMed] [Google Scholar]
- 12.Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23:806–814. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 13.Figueras-Aloy J, Quero-Jiménez J, Fernández-Colomer B, et al. Grupo IRIS. [Usefulness of different risk factor associations in predicting admissions due to respiratory syncytial virus in premature newborns of 32 to 35 weeks gestation in Spain]. An Pediatr (Barc) 2009;71:47–53. doi: 10.1016/j.anpedi.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Carbonell X, Fullarton JR, Gooch KL, et al. The evolution of risk factors for respiratory syncytial virus-related hospitalisation in infants born at 32-35 weeks’ gestational age: time-based analysis using data from the FLIP-2 study. J Perinat Med. 2012;40:685–691. doi: 10.1515/jpm-2011-0248. [DOI] [PubMed] [Google Scholar]
- 15.Blanken MO, Koffijberg H, Nibbelke EE, et al. Dutch RSV Neonatal Network. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: a multicenter birth cohort study. PLoS One. 2013;8:e59161. doi: 10.1371/journal.pone.0059161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrose CS, Anderson EJ, Simões EA, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J. 2014;33:576–582. doi: 10.1097/INF.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 18.Joffe S, Escobar GJ, Black SB, et al. Rehospitalization for respiratory syncytial virus among premature infants. Pediatrics. 1999;104(4 Pt 1):894–899. doi: 10.1542/peds.104.4.894. [DOI] [PubMed] [Google Scholar]
- 19.Stevens TP, Sinkin RA, Hall CB, et al. Respiratory syncytial virus and premature infants born at 32 weeks’ gestation or earlier: hospitalization and economic implications of prophylaxis. Arch Pediatr Adolesc Med. 2000;154:55–61. [PubMed] [Google Scholar]
- 20.Wegner S, Vann JJ, Liu G, et al. Direct cost analyses of palivizumab treatment in a cohort of at-risk children: evidence from the North Carolina Medicaid Program. Pediatrics. 2004;114:1612–1619. doi: 10.1542/peds.2004-0959. [DOI] [PubMed] [Google Scholar]
- 21.Hampp C, Kauf TL, Saidi AS, et al. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med. 2011;165:498–505. doi: 10.1001/archpediatrics.2010.298. [DOI] [PubMed] [Google Scholar]
- 22.Blanken MO, Rovers MM, Bont L Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze. N Engl J Med. 2013;369:782–783. doi: 10.1056/NEJMc1307429. [DOI] [PubMed] [Google Scholar]
- 23.Gouyon JB, Rozé JC, Guillermet-Fromentin C, et al. Hospitalizations for respiratory syncytial virus bronchiolitis in preterm infants at <33 weeks gestation without bronchopulmonary dysplasia: the CASTOR study. Epidemiol Infect. 2013;141:816–826. doi: 10.1017/S0950268812001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CH, Lin CY, Chi H, et al. Prolonged seasonality of respiratory syncytial virus infection among preterm infants in a subtropical climate. PLoS One. 2014;9:e110166. doi: 10.1371/journal.pone.0110166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meert K, Heidemann S, Lieh-Lai M, et al. Clinical characteristics of respiratory syncytial virus infections in healthy versus previously compromised host. Pediatr Pulmonol. 1989;7:167–170. doi: 10.1002/ppul.1950070309. [DOI] [PubMed] [Google Scholar]
- 26.Meert K, Heidemann S, Abella B, et al. Does prematurity alter the course of respiratory syncytial virus infection? Crit Care Med. 1990;18:1357–1359. doi: 10.1097/00003246-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Navas L, Wang E, de Carvalho V, et al. Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. J Pediatr. 1992;121:348–354. doi: 10.1016/s0022-3476(05)90000-0. [DOI] [PubMed] [Google Scholar]
- 28.Moler FW, Steinhart CM, Ohmit SE, et al. Effectiveness of ribavirin in otherwise well infants with respiratory syncytial virus-associated respiratory failure. Pediatric Critical Study Group. J Pediatr. 1996;128:422–428. doi: 10.1016/s0022-3476(96)70294-9. [DOI] [PubMed] [Google Scholar]
- 29.Law BJ, Wang EE, MacDonald N, et al. Does ribavirin impact on the hospital course of children with respiratory syncytial virus (RSV) infection? An analysis using the pediatric investigators collaborative network on infections in Canada (PICNIC) RSV database. Pediatrics. 1997;99:E7. doi: 10.1542/peds.99.3.e7. [DOI] [PubMed] [Google Scholar]
- 30.Dougherty NN, Meissner HC. Respiratory syncytial virus immunoprophylaxis: impact on epidemiology. Paediatr Drugs. 2000;2:127–132. doi: 10.2165/00148581-200002020-00005. [DOI] [PubMed] [Google Scholar]
- 31.Prais D, Schonfeld T, Amir J Israeli Respiratory Syncytial Virus Monitoring Group. Admission to the intensive care unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use. Pediatrics. 2003;112(3 pt 1):548–552. doi: 10.1542/peds.112.3.548. [DOI] [PubMed] [Google Scholar]
- 32.Fodha I, Vabret A, Ghedira L, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol. 2007;79:1951–1958. doi: 10.1002/jmv.21026. [DOI] [PubMed] [Google Scholar]
- 33.Sampalis JS. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr. 2003;143(5 suppl):S150–S156. doi: 10.1067/s0022-3476(03)00513-4. [DOI] [PubMed] [Google Scholar]
- 34.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab Long-Term Respiratory Outcomes Study Group. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42, 42.e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 35.Palmer L, Hall CB, Katkin JP, et al. Healthcare costs within a year of respiratory syncytial virus among Medicaid infants. Pediatr Pulmonol. 2010;45:772–781. doi: 10.1002/ppul.21244. [DOI] [PubMed] [Google Scholar]
- 36.Palmer L, Hall CB, Katkin JP, et al. Respiratory outcomes, utilization and costs 12 months following a respiratory syncytial virus diagnosis among commercially insured late-preterm infants. Curr Med Res Opin. 2011;27:403–412. doi: 10.1185/03007995.2010.542744. [DOI] [PubMed] [Google Scholar]
- 37.Fauroux B, Gouyon JB, Roze JC, et al. Respiratory morbidity of preterm infants of less than 33 weeks gestation without bronchopulmonary dysplasia: a 12-month follow-up of the CASTOR study cohort. Epidemiol Infect. 2014;142:1362–1374. doi: 10.1017/S0950268813001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralston SL, Lieberthal AS, Meissner HC, et al. American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 39.Thomsen SF, van der Sluis S, Stensballe LG, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 40.Zomer-Kooijker K, Uiterwaal CS, van der Gugten AC, et al. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. Eur Respir J. 2014;44:666–674. doi: 10.1183/09031936.00009314. [DOI] [PubMed] [Google Scholar]
- 41.Boyce TG, Mellen BG, Mitchel EF, Jr, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 42.Liese JG, Grill E, Fischer B, et al. Munich RSV Study Group. Incidence and risk factors of respiratory syncytial virus-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003;162:230–236. doi: 10.1007/s00431-002-1105-7. [DOI] [PubMed] [Google Scholar]
- 43.Nachman SA, Navaie-Waliser M, Qureshi MZ. Rehospitalization with respiratory syncytial virus after neonatal intensive care unit discharge: A 3-year follow-up. Pediatrics. 1997;100:E8. doi: 10.1542/peds.100.6.e8. [DOI] [PubMed] [Google Scholar]
- 44.Doering G, Gusenleitner W, Belohradsky BH, et al. The risk of respiratory syncytial virus-related hospitalizations in preterm infants of 29 to 35 weeks’ gestational age. Pediatr Infect Dis J. 2006;25:1188–1190. doi: 10.1097/01.inf.0000246978.58565.b5. [DOI] [PubMed] [Google Scholar]
- 45.Clark SJ, Beresford MW, Subhedar NV, et al. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Arch Dis Child. 2000;83:313–316. doi: 10.1136/adc.83.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deshpande SA, Northern V. The clinical and health economic burden of respiratory syncytial virus disease among children under 2 years of age in a defined geographical area. Arch Dis Child. 2003;88:1065–1069. doi: 10.1136/adc.88.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carbonell-Estrany X, Quero J, Bustos G, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J. 2000;19:592–597. doi: 10.1097/00006454-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Carbonell-Estrany X, Quero J IRIS Study Group. Hospitalization rates for respiratory syncytial virus infection in premature infants born during two consecutive seasons. Pediatr Infect Dis J. 2001;20:874–879. doi: 10.1097/00006454-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Heikkinen T, Valkonen H, Lehtonen L, et al. Hospital admission of high risk infants for respiratory syncytial virus infection: implications for palivizumab prophylaxis. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F64–F68. doi: 10.1136/adc.2003.029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99(1):93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 51.Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, et al. IRIS Study Group. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatr Infect Dis J. 2003;22:823–827. doi: 10.1097/01.inf.0000086403.50417.7c. [DOI] [PubMed] [Google Scholar]
- 52.McCormick J, Tubman R. Readmission with respiratory syncytial virus (RSV) infection among graduates from a neonatal intensive care unit. Pediatr Pulmonol. 2002;34:262–266. doi: 10.1002/ppul.10169. [DOI] [PubMed] [Google Scholar]
- 53.Singleton R, Dooley L, Bruden D, et al. Impact of palivizumab prophylaxis on respiratory syncytial virus hospitalizations in high risk Alaska Native infants. Pediatr Infect Dis J. 2003;22:540–545. doi: 10.1097/01.inf.0000069768.34383.18. [DOI] [PubMed] [Google Scholar]
- 54.Resch B, Pasnocht A, Gusenleitner W, et al. Rehospitalisations for respiratory disease and respiratory syncytial virus infection in preterm infants of 29-36 weeks gestational age. J Infect. 2005;50:397–403. doi: 10.1016/j.jinf.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Resch B, Gusenleitner W, Müller WD, et al. Observational study of respiratory syncytial virus-associated hospitalizations and use of palivizumab in premature infants aged 29-32 weeks. Eur J Clin Microbiol Infect Dis. 2006;25:120–122. doi: 10.1007/s10096-005-0082-y. [DOI] [PubMed] [Google Scholar]
- 56.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. IRIS Study Group. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27:788–793. doi: 10.1097/INF.0b013e3181710990. [DOI] [PubMed] [Google Scholar]
- 57.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 58.Helfrich AM, Nylund CM, Eberly MD, et al. Healthy Late-preterm infants born 33-36+6 weeks gestational age have higher risk for respiratory syncytial virus hospitalization. Early Hum Dev. 2015;91:541–546. doi: 10.1016/j.earlhumdev.2015.06.009. [DOI] [PubMed] [Google Scholar]


