Abstract
Infants with the triad (neurologic dysfunction, subdural hematoma [SDH], and retinal hemorrhage) are often diagnosed as victims of shaken baby syndrome. Medical conditions/predisposing factors to developing the triad are often dismissed: short falls, birth-related SDH that enlarges, macrocephaly, sinus/cortical vein thrombosis, and others. Six infants with the triad are described in which child abuse was diagnosed, but parents denied wrongdoing. All 6 had either macrocephaly or enlarging head circumference, which suggested medical explanations. Three infants incurred short falls, 1 had a difficult delivery in which there was likely a rebleed of a birth-related SDH, 1 had a spontaneous SDH associated with increased extra-axial fluid spaces, and 1 had a sinus thrombosis. Following legal proceedings, all 6 infants were returned to their parents, and there has been no child maltreatment in follow-up, suggesting child abuse never happened. The results indicate that alternative medical explanations for causing the triad should be considered and that macrocephaly or an enlarging head circumference raises the possibility of a medical explanation.
Key Words: shaken baby syndrome, abusive head trauma, cortical/sinus thrombosis, child abuse, macrocephaly, increased extra-axial fluid spaces
The triad consists of neurologic dysfunction, subdural hematoma (SDH), and retinal hemorrhage (RH). Infants with the triad in which parents/caregivers have no apparent explanation and in which there is no significant prior trauma are often diagnosed as having shaken baby syndrome (SBS) or abusive head trauma (AHT). The authors have noticed that in some cases of the triad there is impressive macrocephaly or enlarging head circumference that may indicate a medical cause of the triad and not child abuse. Here we present 6 such cases in which the infant was returned to the parents after legal proceedings and without any future evidence of child maltreatment on follow-up.
METHODS
Two of the authors have been involved in the evaluation of infants with the triad in which the parents were initially accused of child abuse. We reviewed such cases in which there was macrocephaly at birth or an enlarging head circumference during the first few months of life prior to the presentation with the triad, observations that suggest alternative medical diagnoses. The following information was obtained for each case: birth history, medical history, family history, head circumference growth, head imaging studies, outcome of legal proceedings, and long-term disposition of child including any evidence of child abuse. The following 6 cases are of infants in which SBS or AHT was initially diagnosed, and the infant was eventually returned to the parents. The authors contacted the attorneys and families for follow-up to determine how the child was doing since being returned to his/her parents. This study had institutional review board approval.
Patient 1 (Male)
He was the 4260-g product of 40-week-gestational-age pregnancy and vaginal delivery born in October 1995. Birth length was 55.3 cm, and birth head circumference was 39.0 cm (97th percentile). Because of his large head size, he had a head ultrasound at 6 months of age, which showed mild increase of the extra-axial fluid spaces (IEAFS). He rolled over at 7 months and began walking at 11 months.
At 11.5 months, he fell off the edge of a bed about 1.5 to 2.0 f. high while his mother was next to him. The mother saw the fall coming and was able to put her hand under his shoulders to try and break the fall. His buttocks hit the floor first, and then his occiput hit the floor. He started flailing his arms and became incoherent and consequently was taken to the hospital. He quickly improved, and the emergency department (ED) evaluation diagnosed a breath-holding spell after minor head trauma.
The following day, he began vomiting, and a head computed tomography (CT) scan showed bilateral IEAFSs. During the following week, his mother noted increased sleepiness and intermittent fussiness. One week after the initial fall, he was walking around the hardwood dining room floor in the presence of his grandparents who insisted he put socks on his bare feet. Because of the low friction between his socks and the smooth hardwood floor, he slipped and fell forward hitting his head just off the midline. He cried and then fell asleep. The following day, the mother noted increased lethargy and sleepiness. He was taken to the ED where a head CT scan showed SDH, which a head magnetic resonance imaging (MRI) confirmed. Bilateral RHs were also present.
The child advocacy pediatrician diagnosed child abuse. Author M.M. thought the history of the falls in conjunction with the macrocephaly associated with IEAFSs was a plausible alternative explanation and defended this alternative explanation on behalf of the parents in civil proceedings to determine the disposition of the child. The court returned him to his parents, and 17 years later, there has been no evidence of child maltreatment.
Figure 1 shows his head circumference growth curve in which his occipital frontal circumference (OFC) accelerates after birth and reaches the 99th percentile (±5 SDs) by 6 months of age.
FIGURE 1.
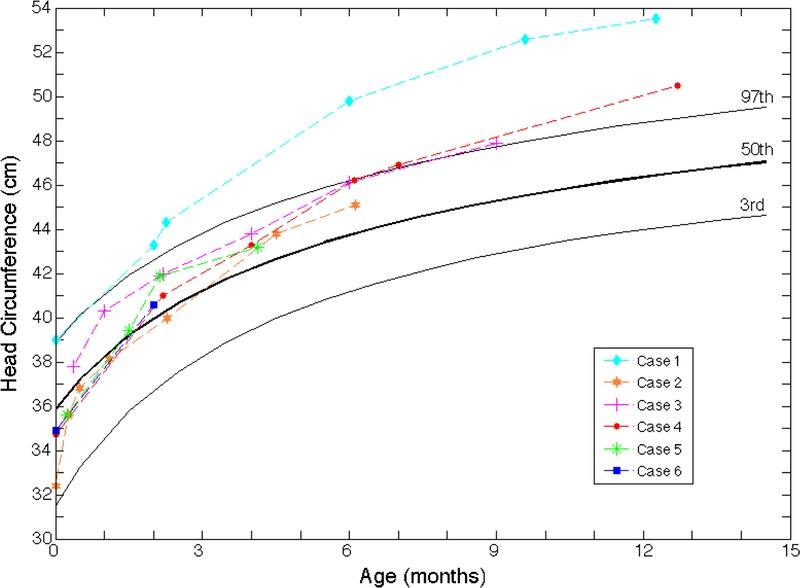
Head circumference (OFC) growth curves. The head circumference growth of all 6 cases showed either macrocephaly or an enlarging head circumference over time.
Patient 2 (Male)
He was the 3370-g product of a 39-week-gestational-age pregnancy and cesarean delivery due to a failure to progress born in November 2009. Birth length was 53.3 cm, and head circumference was 32.4 cm (5th percentile).
At 8 months of age, the father placed him on a bed to take a nap and then heard a thump. The father found he had fallen onto a carpeted floor. He vomited about 90 minutes later and was taken to the ED by his parents. An evaluation showed a viral illness. He was sent home, but 2 days later, he again fell out of bed and developed vomiting, sleepiness, and possible seizures. He was readmitted to the hospital where evaluation revealed the following:
-
Head CT scan showed SDH in the interhemispheric fissure over the right cerebral convexity and in the left frontal region.
Head imaging studies are shown in Figure 2, A to D.
Ophthalmology examination showed unilateral RHs on the right side.
Head MRI showed bilateral proteinaceous hemispheric subdural collections of fluids consistent with IEAFS.
Skeletal survey showed no fractures.
FIGURE 2.
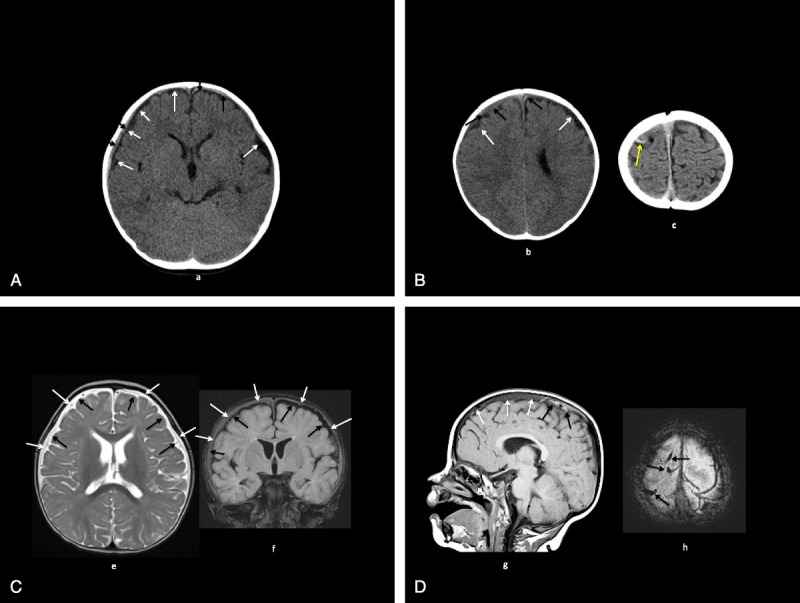
A, Patient 2: CT (a) shows bilateral mildly moderately large low-density inner subarachnoid collections (white arrows), small isohyperdense outer subdural collections (black arrows). B, Patient 2: CT (b, c) shows bilateral mildly moderately large low-density inner subarachnoid collections (white arrows, b), small isohyperdense outer subdural collections (black arrows, b), high-density cortical vein (yellow arrow, c). C, Patient 2: MRI axial T2 (e), coronal fluid attenuated inversion recovery (FLAIR) (f); bilateral moderately large T2 high-intensity, FLAIR low-intensity inner subarachnoid collections (black arrows) with T2 high-intensity, FLAIR isointense outer subdural collections (white arrows). D, Patient 2: MRI sagittal T1 (g), axial T2* (h: bilateral T1 isohypointense extracerebral collections (white arrows) with more recent T1 hyperintense, T2* hypointense hemorrhages, venous thromboses (black arrows).
The child advocacy pediatrician diagnosed AHT as the cause of the triad and claimed the findings were not consistent with a short, uncomplicated fall. The father was accused of child abuse and denies wrongdoing. Psychological evaluation of the father revealed no evidence of any mental health diagnoses. The parents both passed polygraph tests showing no evidence of deception.
A hearing was held in superior court in which the medical records were reviewed by a pediatric neuroradiologist (author P.B.), pediatric neurologist, ophthalmologist, and biomedical engineer. The reviews supported the father’s innocence, and all charges were dismissed. The family then went through family court and received custody of him in September 2012, after having had their son removed from them for 6 weeks. He is presently in good health with no signs of maltreatment 20 months after being placed back with his parents.
Figure 1 illustrates an accelerated growth in the circumference of his head beginning at the 5th percentile at birth and reaching the 85th percentile by 6 months of age. The father has a large head circumference as well at 59.1 cm (99th percentile; ±2.3 SDs).
Patient 3 (Male)
He was the 3880-g product of a 40-week-gestational-age pregnancy and cesarean delivery due to a failure to progress after 12 hours born in August 2010. Birth length was 53.3 cm, and head circumference was 37.8 cm at 11 days (72th percentile).
At 9 months of age, he fell from his bed and hit his head on the carpeted concrete floor. He vomited 3 times during the night, and thus his parents took him to the local hospital. Computed tomography and MRI scans showed bilateral SDHs. Head imaging studies and a picture of the infant showing the macrocephaly are shown in Figure 3, A to C. A pediatric ophthalmologist found preretinal and intraretinal hemorrhages in both eyes with no signs of increased intracranial pressure (ICP).
FIGURE 3.
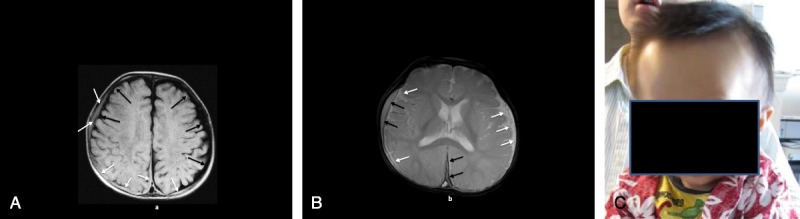
a, Patient 3: MRI axial T1 image (A) shows bilateral large lower-intensity subarachnoid spaces (black arrows) with superimposed higher-intensity bilateral convexity and interhemispheric subdural collections (white arrows). B, Patient 3: MRI axial T2* image (B) shows bilateral large high-intensity subarachnoid spaces (white arrows) with superimposed lower-intensity right convexity and interhemispheric subdural collections (black arrows). C, Patient 3: at 9 months of age showing frontal bossing and macrocephaly.
The Children, Youth, and Families Department removed him from the parents’ custody for 6 weeks and pressed charges against the father. The case, however, was dropped when a group of potential expert witnesses affirmed the macrocephaly and fall were likely the basis of the findings. He was returned to his parents in July 2011, and there has been no evidence of child maltreatment.
Figure 1 shows his head circumference growth curve. He exhibited macrocephaly and showed an accelerated increase in head circumference from birth. At 11 days old, he had a head circumference of 37.8 cm (72th percentile), and at 9 months, 2 weeks prior to the fall, his head circumference was 47.9 cm (98th percentile). A photograph of him demonstrating his macrocephaly is shown in Figure 1.
Family history shows that the father has a head circumference of 61.5 cm (99th percentile). Both parents are Chinese, and the father has a PhD in physics.
Patient 4 (Male)
He was the 3810-g product of a 38 5/7 week-gestational-age pregnancy and vaginal birth after a cesarean delivery with failed vacuum extraction twice and forceps use during delivery, born in April 2009. At birth, there was a cephalohematoma and head molding. Mother breast-fed him, and he had an ALTE (apparent life-threatening event) at 4 days of age, which was thought to be related to the hydromorphone (Dilaudid) the mother was taking. Mother had difficulty breast-feeding as the infant would not latch on. She then pumped her breast and bottle fed him the breast milk. He slept about 22 hours a day. He was diagnosed with reflux at 2 months of age because of continuous vomiting and arching of the back.
At 22 weeks of age, he started vomiting while in the care of the babysitter. The vomiting persisted for another day at which time the mother noticed he was lethargic and took him to the pediatrician who immediately called 911. He was taken to the ED where a head MRI revealed subdural effusions and RHs. The child abuse team suspected the injury was perpetrated by an individual, but no specific individual was named. The family was advised by the children’s services to change child care providers, which they did.
On April 18, 2010, at 1 year of age, he bumped his head at his birthday party, and then again on April 26, 2010, while in his aunt’s care. On April 27, 2010, he started vomiting intermittingly and was diagnosed with a possible viral infection. On May 17, 2010, he had a seizure and was immediately taken to the hospital. A head MRI revealed an SDH behind the bump on his forehead and another in the occipital region, as well as RHs. Head imaging studies are shown in Figure 4, A–D.
FIGURE 4.
A, Patient 4: initial MRI sagittal T1 image (A) shows inner low-intensity subarachnoid spaces (black arrows) and outer gray intensity subdural collections (white arrows). B, Patient 4: initial MRI axial T2 image (B) shows inner high-intensity subarachnoid spaces (black arrows) and outer high-intensity subdural collections (white arrows) separated by intervening low-intensity membranes and veins. C, Patient 4: MRI 6 months later, sagittal T1 image (D) shows inner low-intensity subarachnoid spaces (black arrows) and outer gray intensity enlarging subdural collections (white arrows) plus smaller high-intensity more recent hemorrhage (yellow arrow). D, Patient 4: MRI 8 weeks later, sagittal T1 image (C) shows inner low-intensity subarachnoid spaces (black arrows) and outer gray intensity subdural collections (white arrows).
He was removed from his parents’ care for 3 months. During legal proceedings, the parents admitted no wrongdoing, and the court allowed him to be returned to the parents with supervision by the paternal grandmother who lived with the family. The supervision lasted 14 months, and ever since he has been living with his parents with no evidence of maltreatment since October 2011.
Figure 1 shows his head circumference growth curve. He exhibited rapid head circumference growth from 34.8 cm at birth (25th percentile) to 47.0 cm at 7 months (98th percentile). The father has a head circumference of 59.5 cm (99th percentile; ±3.5 SDs), and he has a PhD in physics.
Patient 5 (Male)
He was the 3410-g, 51-cm product of a term pregnancy and cesarean delivery born in April 2007. On August 7, 2007, at 4 months of age, he was evaluated by his pediatrician for constipation. On August 13, 2007, he was evaluated at an urgent care for crying, sleeping only for 20 minutes at a time, and vomiting. No diagnosis was made, and he was released home, but he continued to vomit and the same day was diagnosed with herpangia and started on ibuprofen (Motrin).
He continued to appear sick with increased irritability, decreased feeding, and occasional vomiting. Two days later, he woke up from a nap crying, was inconsolable, and went limp. He was in the care of his father who called 911 and gave him rescue breaths. He was transported to the ED where a CT scan revealed subacute bilateral SDHs. A thorough physical examination revealed no outward sign of injury of any kind. He had bilateral RHs. Follow-up imaging confirmed SDHs, and other tests revealed no evidence of bone fractures or other injuries.
His treating professionals believed his condition was explained by external hydrocephalus, whereas a pediatrician and an ophthalmologist believed his condition was due to SBS. His case was adjudicated in juvenile court. The child was returned under the supervision of the court, and after 6 months, the case was terminated and dismissed with no signs of parental abuse. Parents divorced reportedly over the stress of the child abuse allegations and since August 2008 have had shared custody of the child, and there has been no evidence of maltreatment.
Family history shows his sister has macrocephaly with IEAFS. Figure 1 shows his head circumference, which accelerated from the 50th percentile at 1.5 months of age to the 75th percentile at 4 months of age.
Patient 6 (Female)
She was the 3798-g (75th percentile) product of a term pregnancy and repeat cesarean delivery born in February 2011. Her head circumference at birth was 34.9 cm (50th percentile). Apgar scores were 9 and 9 at 1 and 5 minutes, respectively. She went home at 3 days of age.
At 2 weeks of age, she was seen for conjunctivitis and lacrimal duct obstruction. At 4.5 weeks, she was seen for parental concerns about fussiness and spitting up. She was diagnosed with probable formula intolerance and a Candida diaper dermatitis. She was switched to a soy-based formula. At her 2-month check-up, her parents noted increasing fussiness and increasing number of vomiting episodes.
Her head circumference had increased rapidly to 40.6 cm (90th percentile). The mother noted she had a bruise on her right cheek from when her sister accidentally hit her with a block. A right otitis media was diagnosed, and she was administered amoxicillin. A complete blood count was performed, which showed the following:
white blood cells = 12.4 × 109 cells/L (reference range, 3.1–10.0)
hemoglobin = 11.5 g% (reference range, 12–16 g%)
platelets = 578,000 platelets/mcL (reference range, 128,000–434,000)
A day after her 2-month check-up, she again presented with persistent vomiting. A week after her 2-month check-up, she was seen at the hospital for lethargy and was noted to have a tense and bulging fontanel. An evaluation revealed the following:
-
head CT scan:
a. bilateral IEAFSs causing generalized flattening of the gyri over both convexities
b. thrombosis in the left transverse sinus
c. possible infarcts in the right frontal and right posterior parietal lobes
serum sodium = 121 mmol/L (reference range, 134–143 mmol/L)
platelets = 1,078,000 platelets/mcL (150,000–400,000)
bilateral RHs
normal skeletal survey
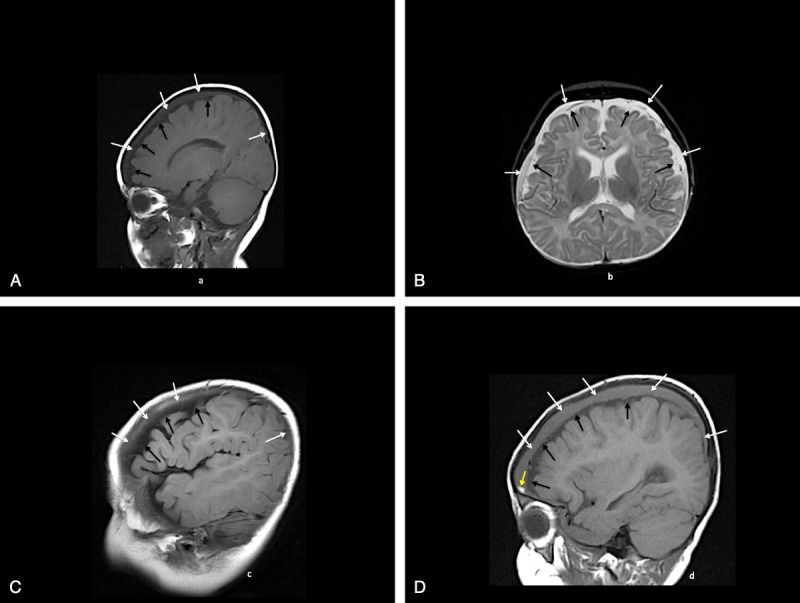
Head imaging studies are shown in Figure 5, A to C. After the evaluation at the local hospital, she was immediately transported to a children’s hospital where emergent burr holes were placed to relieve the increased ICP. The surgical note indicates: “We encountered immediate return of chronic bloody subdural fluid, which was clearly under pressure squirting some 18 to 24 inches from the child’s head.” The infant recovered.
FIGURE 5.
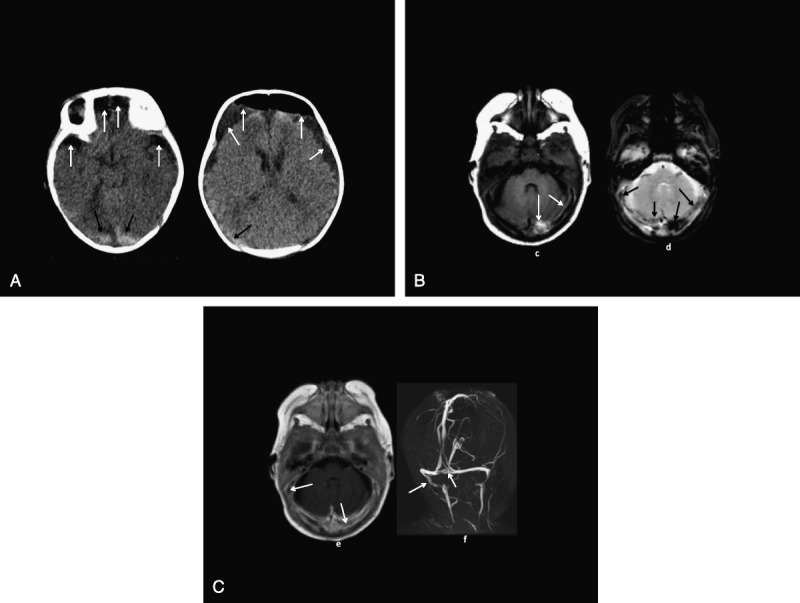
A, Patient 6: CT (A, B) shows postsurgery frontal extracerebral low-density air-fluid collections (white arrows) and high-density hemorrhages or thromboses along the tentorium (black arrows). B, Patient 6: MRI axial T1 (C) and T2* (D) images show T1 high plus T2* low-intensity hemorrhages and/or thromboses along the transverse dural venous sinuses (arrows). C, Patient 6: MRI axial gadolinium T1 (E) and posterior MRV (F) images show asymmetric left transverse and right sigmoid dural venous sinus flow gaps (arrows) consistent with thromboses.
Child abuse was alleged, and parents denied wrongdoing. The father was initially accused of 2 felony charges and faced up to 100 years in prison. When the defense presented the sinus thrombosis as an alternative explanation of the SDHs, a plea agreement was reached where the child was returned to the parents, and the father did not go to prison. The child was returned to the parents in October 2012, and there has been no evidence of child maltreatment.
Figure 1 shows the head circumference growth curve that indicates that at birth her head circumference was at the 50th percentile and jumped to the 90th percentile at the 2-month check-up.
RESULTS
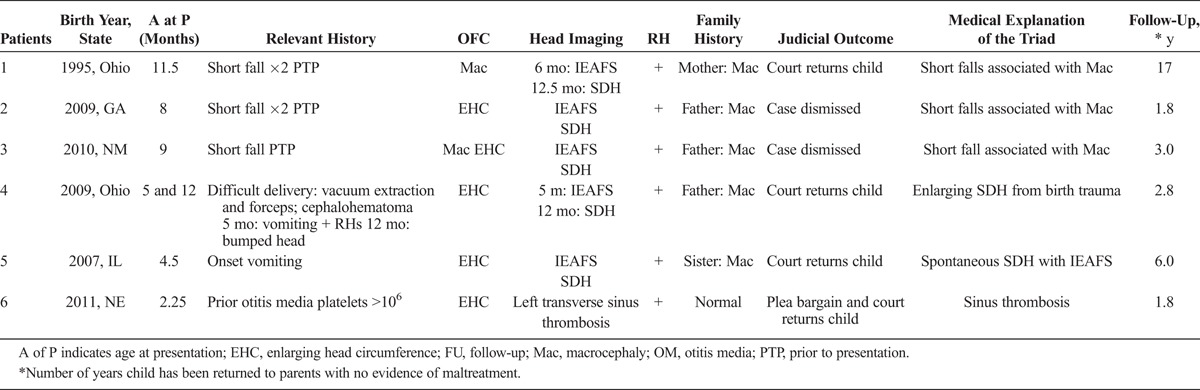
The results of these 6 cases are summarized in Table 1. Figure 1 is a graph showing each infant’s head circumference over time.1 Figures 2 to 5 show relevant head imaging studies. Figure 3C shows a photograph of patient 3 demonstrating his macrocephaly and frontal bossing.
TABLE 1.
Summary of 6 Cases
DISCUSSION
This series of 6 infants younger than 12 months with macrocephaly and/or enlarging head circumference presented with the triad were diagnosed as victims of child abuse by 1 of their parents, which the parents denied; were returned to their parents after legal proceedings in which alternative, medical explanations to child abuse were presented; and have thrived in the homes of their parents after returning. The enlarging head circumference suggests a chronic process. That these infants have shown no evidence of child maltreatment on returning home to their parents strongly suggests that these are safe homes, that they were unlikely abused, and that the most likely explanation for the triad was the alternative medical explanation.
Mimics of SBS/AHT
The alternative medical explanations of the 6 infants include the following:
3 infants with short fall(s) associated with macrocephaly
1 infant with spontaneous SDH and IEAFS
1 infant with a birth-related SDH that enlarged
1 infant with a transverse sinus thrombosis
The concept that shaking alone can cause the triad has been challenged.2–7 Caregivers can abuse an infant and cause the triad, but it is unlikely from shaking alone. Biomechanical studies indicate that head impact, even from short falls of 2 feet, produces physical forces far greater than from shaking alone, and such an event can produce sufficient physical forces that can lead to the triad.6–8 It has been suggested that the RHs in SBS are from the shaking and resultant traction on the retina. However, a more likely explanation for the RHs in the triad is the increased retinal vein pressure associated with the increased ICP, which leads to extrusion of blood in the retina and the RHs.9,10 Thus, RHs can be caused by any process that results in increased ICP, and this pathogenesis of RHs fits with the observations of Geddes et al,11–13 who found that the neuropathology findings of most cases of SBS were those of hypoxia and not those of trauma.
Because clinicians often reflexively diagnose SBS/AHT in a young infant who presents with the triad, mimics of SBS/AHT are often overlooked. Common mimics of SBS/AHT were noted in the 6 infants of this series and include the following:
short falls
macrocephaly associated with IEAFS
CVT (cortical vein thrombosis)
birth trauma–related SDH with rebleeding
multifactorial causation
Head imaging studies of the infant with the triad can sometimes be helpful in establishing an etiologic diagnosis, but it has been emphasized there are no diagnostic head imaging findings for SBS/AHT.14
1. Short falls
Short falls have clearly been documented to cause the triad as they can lead to increased ICP and thus cause not only an SDH and neurologic dysfunction, but also RHs.15–19 As previously noted, biomechanical studies have clearly shown that the traumatic deceleration forces produced by a short fall are dramatically higher than those produced by manual shaking.6–8 Neck injuries are conspicuously absent from alleged instances of manual shaking, whereas neck injuries would not necessarily be prevalent in short-fall injuries.20,21 Furthermore, the link between RHs and SBS has been called into question.10,16–18
2. Macrocephaly associated with IEAFS
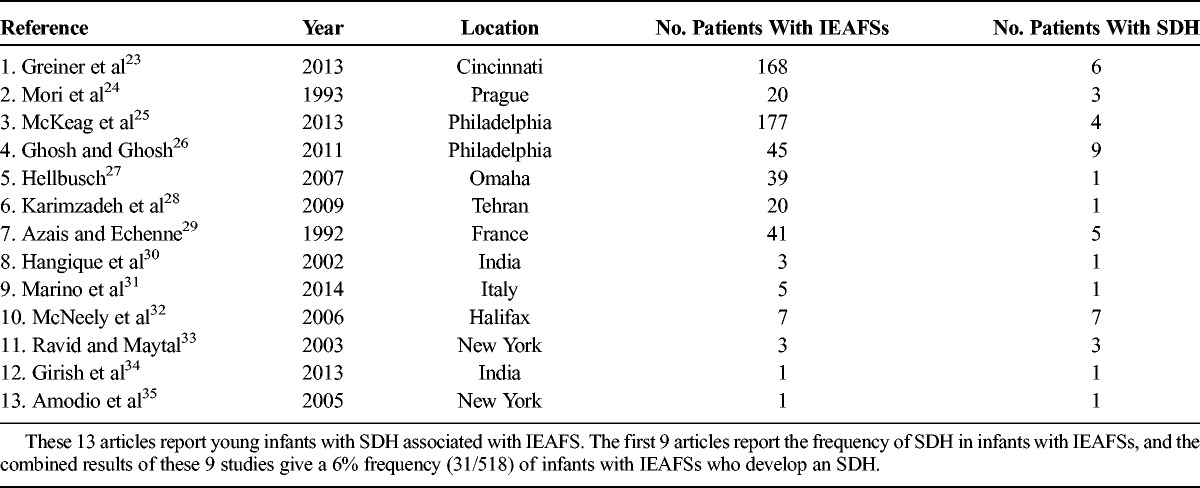
Many instances of familial macrocephaly are related to IEAFS (also known as benign subdural hygromas).22 Young infants with IEAFS are at greater risk for incurring a spontaneous SDH or an SDH with minimal trauma as summarized in the reported cases listed in Table 2.23–35 The first 9 articles report frequency of SDH in infants with IEAFS, and the combined results of these 9 studies give a 6% frequency (30/513) of infants with IEAFSs who develop an SDH. Thus, this is not an insignificant risk. In Vinchon and colleagues’36 series of 16 infants with spontaneous SDH without a history of trauma, 12 had macrocrania, and 7 of the 12 had IEAFSs. Patient 5 had a spontaneous SDH associated with IEAFS.
TABLE 2.
Articles Reporting SDH Associated With IEAFSs
Four cases from the present series have familial macrocephaly. The fathers of both patients 3 and 4 have macrocephaly and interestingly have doctorate degrees in physics. The father of patient 2 also has a large head, and the sister of patient 5 has a large head.
The interrelationships between cerebrospinal fluid (CSF) volume, ICP, and cortical vessels is complex and has been eruditely reviewed by Leestma.37 Infants with IEAFSs have a greater risk for developing a spontaneous SDH or an SDH with minimal trauma, and the pathogenesis of the SDH is likely multifactorial in origin. First, these infants have a greater head mass, so that any traumatic impact, however small, will result in a slightly greater force than if the infant had a normal head mass.2 Second, the theoretical model of IEAFS developed by Papasian and Frim38 shows that the cortical veins in infants with IEAFSs will incur venous stretch injury at lower forces than those in infants with normal extra-axial fluid spaces. Third, the volume-pressure dynamics of CSF in infants with IEAFSs predispose them to increased ICP that could lead to neurologic dysfunction and RHs. Shapiro and Marmarou39 have shown the relationship between the volume of CSF and the ICP in infants. Small increases in CSF volume in infants can have significant increases in the ICP. Instillation of 2 mL of saline solution raised the ICP by 15 mm Hg, and instillation of 4 mL increased the ICP by 30 mm Hg. Thus, as infants with IEAFSs increase their CSF volume above normal, they may also pathologically increase the ICP to the point that it causes both neurologic dysfunction and RHs. Muller and Deck40 have demonstrated that increased ICP can lead to increased retinal vein pressure with the development of RHs. An infant with IEAFS who develops increased ICP will often develop neurologic dysfunction (seizures, ALTE, apnea) and be taken to an emergency room. Evaluation will often reveal RHs as discussed above, and because the IEAFS can be confused for an SDH or the infant has bleeding into the IEAFS, this infant has the triad, and child abuse will often be diagnosed in such an infant. When such an infant with IEAFS presents with neurologic abnormalities and RHs, the radiographic appearance of the material in the extra-axial compartment can be that of CSF, proteinaceous material, or blood. The nature of the material is not helpful in establishing causation. It is important to consider a broad differential diagnosis in such an infant that includes the following: spontaneous SDH, trauma (trivial, accidental, nonaccidental), infection, metabolic disorders (eg, glutaric acidemia type 1), bone dysplastic disorders (eg, osteogenesis imperfecta), venous thrombosis, and coagulopathy.14
3. Cortical vein/sinus thrombosis
Cortical vein or sinus thrombosis has been shown to sometimes cause the triad as illustrated in patient 6 in which the head MRI is clearly different than the typical head MRI seen in SBS.14,41
4. Birth trauma with rebleeding
Approximately 20% to 50% of all deliveries result in a small SDH that usually resolves without clinical sequelae.42–44 However, it is thought that a small portion of these infants who have a small SDH might rebleed, especially if there is minimal head trauma such as a short fall in infancy.2,45–47 Noteworthy, the finding of a cephalohematoma at birth is associated with an underlying SDH, and in patient 4, there was a cephalohematoma as well as a difficult delivery. It is highly likely therefore that this infant had a subclinical SDH at birth that later rebled.48
5. Multiple factors explanation/multifactorial causation of the triad
It is likely that some young infants develop the triad because of several interacting factors. For example, infants who have macrocephaly related to IEAFS who incur a short fall are especially liable to develop an SDH, as illustrated in patients 1, 2, and 3. The various cases have different elements of evidence demonstrating the erroneous diagnosis of SBS. In patient 2, psychology evaluations deemed the parents to be of low risk to commit child abuse. In both patients 1 and 3, the infants had large head circumferences at birth in which imaging studies demonstrated IEAFS. Prior studies have indicated IEAFS is a predisposing factor to the developmental of SDH in young infants with minimal trauma or spontaneous events. Patients 3 and 4 experienced a difficult birth delivery with subsequent enlarging head circumference, which is a predisposing factor for developing the triad as the small SDH might enlarge over time and present later with neurologic signs and symptoms.
In patient 6, the fussiness and spitting noted at 4.5 weeks of age that persisted over the next month may also be related to the evolving increased ICP. The chronic blood fluid under large pressure in this case is also consistent with a longstanding process.
Imaging studies report there was a sinus thrombosis, which is a known mimic of SBS. Cerebral sinovenous thrombosis is associated with antecedent otitis media, which she had, and is also associated with thrombocytosis, and she had a markedly elevated platelet count of 1,078,000, which clearly places her at increased risk for a thrombosis.
Importance of OFC Measurements in the Differential Diagnosis of the Triad
Clinicians involved in the evaluation and management of infants with the triad in which child abuse is alleged should obtain all recorded OFC measurements including the OFC at birth, at well-child visits, and at any other health care visit where an OFC might be obtained. The finding of macrocephaly or an enlarging OFC raises the strong possibility of a medical diagnosis other than child abuse, as illustrated in these 6 cases. Measurement of the OFCs of the parents is also indicated as it might reveal a familial macrocephaly if one of the parents has a large OFC.22
Significance of Findings as They Relate to Our Judicial System
While the American judicial system is as fair as any in the world, it still has an inherent shortcoming—a verdict or outcome of any legal proceeding in a child abuse allegation case does not necessarily reveal the ultimate truth of the case, but rather which side presented the most compelling arguments. This shortcoming emanates from several weaknesses and operational processes of our judicial system, which, on the balance, favor the prosecution and include the following:
coerced confessions
concealment of exculpatory evidence by the prosecution
plea bargaining
greater financial position of the prosecution that allows for greater number and depth of expert witnesses
There are several previous experiences that provide similar observations to ours in which contested cases between a defendant and the prosecution have ultimately demonstrated that the allegation of wrongdoing by the prosecution was likely wrong:
Piatt49 presented a case identical to ours in which a 6-week-old male infant with macrocephaly from IEAFS developed the triad after striking his head on a carpeted floor. There was an investigation for possible child abuse, and the infant remained with his parents. Follow-up at 44 months of age showed the infant was thriving.
Paterson and Monk50 presented a series of 85 infants with multiple unexplained fractures in which child abuse was alleged by the child advocacy team and prosecution, but in which Paterson and Monk diagnosed temporary brittle bone disease and thought child abuse was highly unlikely. As a result of Paterson and Monk’s testimony in 63 of these cases, these infants were returned to the parents, and Paterson and Monk were able to obtain follow-up status on 61 of these infants. All 61 infants were doing well without any evidence of injuries suggestive of child abuse. The mean follow-up period was 6.9 years (range, 1–17 years).
The most extensive, highly publicized, and compelling example of our judicial court system reaching the wrong decision is in rape cases in which the alleged and convicted perpetrator who has often been incarcerated for many years is exonerated by DNA testing years after the trial.51 However, there is now a record number of exonerations in the United States for other crimes including murder in which false confessions and plea bargaining led to a wrongful conviction.52
CONCLUSIONS
The results of this study suggest 3 important conclusions:
There are mimics of SBS associated with either absolutely large OFCs (OFC >2 SDs) or enlarging OFCs over time and that careful attention to the OFC graph might suggest a medical explanation for the triad.
There are many different causes of the triad and several predisposing factors to developing the triad. In some instances, there may be more than 1 predisposing factor that leads to the triad, such as seen in some of the cases presented in this series.
The cases of the 6 infants described herein, alleged to have SBS and abused by their parents and who were returned to their parents without incident, suggest that child abuse was not the explanation of the triad in these instances and that the medical explanations provided are likely the explanations for the triad. We do not believe that shaking alone can cause the triad and that most cases of alleged SBS that have no evidence of impact are likely caused by mimics of SBS that have not been correctly diagnosed.
Forensic pathologists who perform autopsies on children with alleged SBS and child abuse experts who perform forensic evaluations on living infants with alleged SBS might find our observations important in their approach to the infant with alleged SBS. In the infant who presents with the triad, child abuse and the medical mimics and predisposing factors to developing the triad should be considered in the differential diagnosis.
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1. Center for Disease Control and Prevention Head circumference-for-age charts. May 30, 2000. Available at: http://www.cdc.gov/growthcharts/percentile_data_files.htm Accessed January 4, 2013.
- 2. Miller R, Miller ME. Overrepresentation of males in traumatic brain injury of infancy and in infants with macrocephaly: further evidence that questions the existence of shaken baby syndrome. Am J Forensic Med Pathol. 2010; 31: 165– 173. [DOI] [PubMed] [Google Scholar]
- 3. Gabaeff S. Challenging the pathophysiologic connection between subdural hematoma, retinal hemorrhage and shaken baby syndrome. West J Emerg Med. 2011; 12: 144– 158. [PMC free article] [PubMed] [Google Scholar]
- 4. Squier W. Shaken baby syndrome: the quest for evidence. Dev Med Child Neurol. 2008; 50: 10– 14. [DOI] [PubMed] [Google Scholar]
- 5. Plunkett J. Shaken baby syndrome and the death of Matthew Eappen: a forensic pathologist’s response. Am J Forensic Med Pathol. 1999; 20: 17– 21. [DOI] [PubMed] [Google Scholar]
- 6. Duhaime A, Gennarelli T, Thibault L, et al. The shaken baby syndrome. A clinical, pathological and biomechanical study. J Neurosurg. 1987; 66: 409– 415. [DOI] [PubMed] [Google Scholar]
- 7. Uscinski R. Shaken baby syndrome: fundamental questions. Br J Neurosurg. 2002; 16: 217– 219. [DOI] [PubMed] [Google Scholar]
- 8. Lloyd J, Willey EN, Galaznik JG, et al. Biomechanical evaluation of head kinematics during infant shaking versus pediatric activities of daily living. J Forensic Biomech. 2011; 2: 1– 8. [Google Scholar]
- 9. Gardner H. Scientific correspondence. Neuropathol Appl Neurobiol. 2004; 30: 192. [DOI] [PubMed] [Google Scholar]
- 10. Lantz P, Stanton CA. Postmortem detection and evaluation of retinal hemorrhages. Presented at the 58th Annual Scientific Meeting of the American Academy of Forensic Sciences; February 20–25, 2006; Seattle, WA. Abstract G1 04. [Google Scholar]
- 11. Geddes JF, Tasker RC, Hackshaw AK, et al. Dural hemorrhage in non-traumatic infant deaths: does it explain the bleeding in “shaken baby syndrome”. Neuropathol Appl Neurobiol. 2003; 29: 14– 22. [DOI] [PubMed] [Google Scholar]
- 12. Geddes JF, Hackshaw AK, Vowles GH, et al. Neuropathlogy of inflicted head injury in children 1. Patterns of brain damage. Brain. 2001; 124: 1290– 1298. [DOI] [PubMed] [Google Scholar]
- 13. Geddes JF, Vowles GH, Hackshaw AK, et al. Neuropathology of inflicted head injury in children 2. Microscopic brain injury in infants. Brain. 2001; 124: 1299– 1306. [DOI] [PubMed] [Google Scholar]
- 14. Barnes P. Imaging of Nonaccidental injury and the mimics: issues and controversies in the era of evidence-based medicine. Radiol Clin North Am. 2011; 49: 205– 229. [DOI] [PubMed] [Google Scholar]
- 15. Plunkett J. Fatal pediatric head injuries caused by short distance falls. Am J Forensic Med Pathol. 2001; 22: 1– 12. [DOI] [PubMed] [Google Scholar]
- 16. Gardner H. A witnessed short fall mimicking presumed shaken baby syndrome (inflicted childhood neurotrauma). Pediatr Neurosurg. 2007; 43: 433– 435. [DOI] [PubMed] [Google Scholar]
- 17. Lantz P, Sinai S, Stanton C, et al. Perimacular retinal folds from childhood head trauma: case report with critical appraisal and review of the literature. Br Med J. 2004; 328: 754– 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lueder GT, Turner JW, Paschall R. Perimacular retinal folds simulating nonaccidental injury in an infant. Arch Ophthalmol. 2006; 124: 1782– 1783. [DOI] [PubMed] [Google Scholar]
- 19. Aoki N, Masuzawa H. Infantile acute subdural hematoma: clinical review of 26 cases. J Neurosurg. 1984; 61: 273– 280. [DOI] [PubMed] [Google Scholar]
- 20. Bandak F. Shaken baby syndrome: a biomechanics analysis of injury mechanisms. Forensic Sci Int. 2005; 151: 71– 79. [DOI] [PubMed] [Google Scholar]
- 21. Bandak FA. Response letter to the editor. Forensic Sci Int. 2006; 164: 282– 283. [Google Scholar]
- 22. Alvarez LA, Maytal J, Shlomo S. Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics. 1986; 77: 901– 907. [PubMed] [Google Scholar]
- 23. Greiner M, Richards T, Care M, et al. Prevalence of subdural collections (SDC) in children with macrocrania. AJNR Am J Neuroradiol. 2013; 34: 2373– 2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori K, Sakamoto T, Mishimura K, et al. Subarachnoid fluid collection in infants complicated by subdural hematoma. Childs Nerv Syst. 1993; 9: 282– 284. [DOI] [PubMed] [Google Scholar]
- 25. McKeag H, Christian CW, Rubin D, et al. Subdural hemorrhage in pediatric patients with enlargement of the subarachnoid spaces. J Neurosurg Pediatr. 2013;11:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghosh PS, Ghosh D. Subdural hematoma in infants without accidental or nonaccidental injury: benign external hydrocephalus, a risk factor. Clin Pediatr. 2011; 50: 897– 903. [DOI] [PubMed] [Google Scholar]
- 27. Hellbusch L. Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J Neurosurg. 2007; 107: 119– 117. [DOI] [PubMed] [Google Scholar]
- 28. Karimzadeh P, Seyed H, Shariatmadari F. Benign external hydrocephalus and its relation to familial megalencephaly: an analysis of 20 cases. J Pedaitr Neurol. 2009; 7: 157– 163. [Google Scholar]
- 29. Azais M, Echenne B. Idiopathic pericerebral effusions of infancy (external hydrocephalus). Ann Pediatr (Paris). 1992; 39: 550– 558. [PubMed] [Google Scholar]
- 30. Hangique S, Das R, Barua N, et al. External hydrocephalus in children. Ind J Radiol Imaging. 2002; 12: 197– 200. [Google Scholar]
- 31. Marino M, Morabito R, Vinci S, et al. Benign external hydrocephalus in infants: a single centre experience and literature review. Neuroradiol J. 2014; 27: 245– 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McNeely PD, Atkinson JD, Saigal G, et al. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. Am J Neuroradiol. 2006; 27: 1725– 1728. [PMC free article] [PubMed] [Google Scholar]
- 33. Ravid S, Maytal J. External hydrocephalus: a probable cause for subdural hematoma in infancy. Pediatr Neurol. 2003; 28: 139– 141. [DOI] [PubMed] [Google Scholar]
- 34. Girish M, Dandge VR, Mujawar N, et al. Benign enlargement of subarachnoid space with subdural hematoma: a not so benign complication. J Pediatr Neurol. 2013; 11: 63– 65. [Google Scholar]
- 35. Amodio J, Spektor V, Pramanik B, et al. Spontaneous development of bilateral subdural hematomas in infant with benign infantile hydrocephalus. Pediatr Radiol. 2005; 35: 1113– 1117. [DOI] [PubMed] [Google Scholar]
- 36. Vinchon M, Delestret I, Defoort-Dhellemmes S, et al. Subdural hematoma in infants: can it occur spontaneously?: Data from a prospective series and critical review. Child Nerv Syst. 2010; 26: 1195– 1205. [DOI] [PubMed] [Google Scholar]
- 37. Leestma J. Forensic aspects of intracrania equilibria. In: Leestma J, ed. Forensic Neuropathology. 3rd ed Chapter 3 Boca Raton, FL: CRC Press; 2014: 364– 414. [Google Scholar]
- 38. Papasian NC, Frim DM. A theoretical model of benign external hydrocephalus that predicts a predisposition towards extra-axial hemorrhage after minor head trauma. Pediatr Neurosurg. 2000; 33: 188– 193. [DOI] [PubMed] [Google Scholar]
- 39. Shapiro K, Marmarou A. Mechanism and treatment of intracranial hypertension in the head injured child. In: K Shapiro, ed. Pediatric Head Trauma. New York City, NY: Futura Publishing Co; 1983: 45– 69. [Google Scholar]
- 40. Muller PJ, Deck JHN. Intraocular and optic nerve hemorrhages in cases of sudden intracranial hypertension. Neurosurgery. 1974; 41: 160– 166. [DOI] [PubMed] [Google Scholar]
- 41. Krasnokutsky M. Cerebral venous thrombosis: a potential mimic of primary traumatic brain injury in infants. Am J Radiol. 2011; 197: W503– W507. [DOI] [PubMed] [Google Scholar]
- 42. Whitby EH, Griths PD, Rutter S, et al. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004; 363: 846– 851. [DOI] [PubMed] [Google Scholar]
- 43. Looney CB, Smith JK, Merck LH, et al. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007; 242: 535– 541. [DOI] [PubMed] [Google Scholar]
- 44. Rooks VJ, Eaton JP, Ruess L, et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. Am J Neuroradiol. 2008; 29: 1082– 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sherwood D. Chronic subdural hematoma in infants. Am J Dis Child. 1930; 39: 980– 1021. [Google Scholar]
- 46. Ito H, Komai T, Yamamaoto S. Fibrinolytic enzyme in the lining walls of chronic subdural hematoma. J Neurosurg. 1978; 48: 197– 200. [DOI] [PubMed] [Google Scholar]
- 47. Ito H, Komai K, Yamamoto S. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976; 45: 26– 31. [DOI] [PubMed] [Google Scholar]
- 48. Chamnanvanakij S, Rollins N, Perlman J. Subdural hematoma in term infants. Pediatr Neurol. 2002; 26: 301– 304. [DOI] [PubMed] [Google Scholar]
- 49. Piatt JH. A pitfall in the diagnosis of child abuse: external hydrocephalus, subdural hematoma, and retinal hemorrhages. Neurosurg Focus. 1999; 7 (4): E5. [DOI] [PubMed] [Google Scholar]
- 50. Paterson CR, Monk EA. Long-term follow-up of children thought to have temporary brittle bone disease. Pediatric Health, Medicine and Therapeutics. 2011; 2: 55– 58. [Google Scholar]
- 51. Gross SR, Shaffer M. Exonerations in the United States. 1989–2012. Report by the National Registry of Exonerations. Available at: https://www.law.umich.edu/special/exoneration/Documents/exonerations_us_1989_2012_full_report.pdf
- 52. Johnson K. USA Today. Exonerations hit record high in 2014. January 27, 2015. Available at: http://www.usatoday.com/story/news/nation/2015/01/27/exonerations-record-high/22358511/. Accessed on February 14, 2015.


