Abstract
The US National Cancer Institute estimates that cardiotoxicity (CTX) from target therapy refers mostly to four groups of drugs: epidermal growth factor receptor 2 inhibitors, angiogenic inhibitors, directed Abelson murine leukemia viral oncogene homolog inhibitors, and proteasome inhibitors. The main cardiotoxic side-effects related to antiepidermal growth factor receptor 2 therapy are left ventricular systolic dysfunction and heart failure. Angiogenesis inhibitors are associated with hypertension, left ventricular dysfunction/heart failure, myocardial ischemia, QT prolongation, and thrombosis. Moreover, other agents may be related to CTX induced by treatment. In this study, we review the guidelines for a practical approach for the management of CTX in patients under anticancer target therapy.
Keywords: angiogenesis inhibitors, HER2/epidermal growth factor receptor 2, tyrosine kinase inhibitors
Introduction
The US National Cancer Institute (NCI) estimates that at least 13.7 million cancer survivors were alive in the United States in 2012, and this number will approach 18 million by 2022.1
Modern oncologic treatments brought a strong reduction in the mortality rate among patients with cancer.2 However, the antineoplastic-related cardiotoxicity (CTX) is a major cause of morbidity and mortality in cancer survivors.3
In a US National Health and Nutrition Examination survey of 1807 cancer survivors followed for 7 years, 33% of survivors died of heart disease and 51% of cancer.1
In this review, we will discuss the management of cardiovascular side-effects of target therapy in oncology.
Foremost, it is necessary to know that any tyrosine kinase inhibitor (TKI) has potential CTX, and while cardiac events may or may not be likely, it is necessary for clinicians to know what to do before, during, and after treatment with these drugs.
CTX from target therapy refers mostly to four groups of drugs:
Epidermal growth factor receptor 2 (HER2/ErbB2) inhibitors.
Angiogenic inhibitors.
Directed Abelson murine leukemia viral oncogene homolog (ABL) inhibitors.
Proteasome inhibitors.
In addition to these, there are some miscellaneous agents that we will discuss individually.
Target therapy
Target therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for cell proliferation, tumor growth, and systemic spread.4 Progress in therapy and the increase in number of long-term survivors reveal the issue of cardiovascular side-effects of target therapy.
Target therapy may affect by ‘on-target’ or ‘off-target’ toxicities. On-target refers to excessive and adverse pharmacologic effects at the target of interest, shared by all agents that reliably inhibit a specific target. Off-target refers to adverse effects as a result of modulation of other targets.5 A schematic representation of the mechanisms of action of principal drugs used in target therapy are reported in Fig. 1.
Fig. 1.
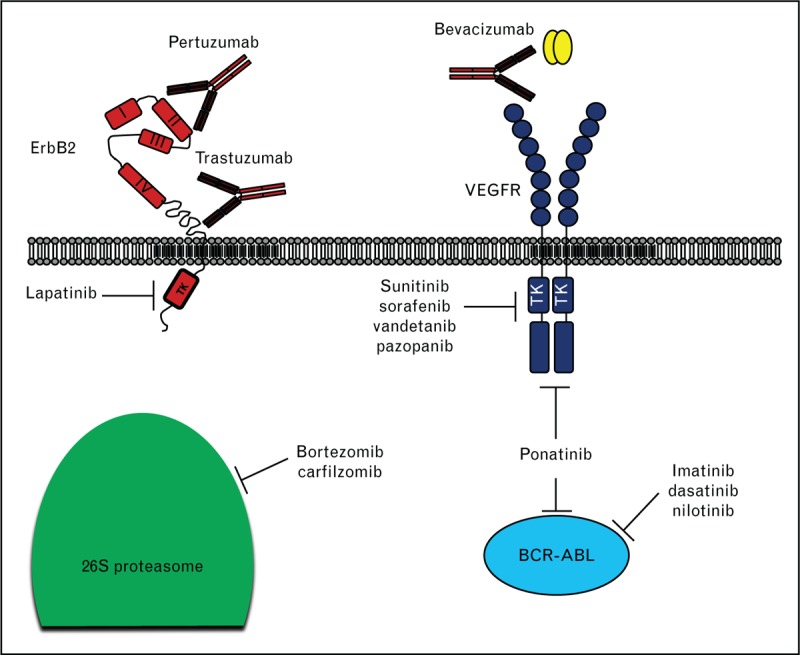
Schematic representation of mechanisms of action of target therapy. Target therapy may affect tumor growth inhibiting specific enzymatic activities (e.g. tyrosine kinase inhibitor and proteasome inhibitors), or binding specific receptors/ligands, thus inhibiting their biological functions (e.g. monoclonal antibodies trastuzumab, pertuzumab, and bevacizumab).
Cardiovascular side-effects of target therapy have become an important problem, for example, in patients treated with ponatinib, a multitarget TKI that presents a broad range of action, 11% developed arterial thrombosis and at least 5% arrhythmia.6 Moreover, sunitinib, a TKI of vascular endothelial growth factor, causes a high risk of congestive heart failure, hypertension (HTN), myocardial ischemia, and thromboembolism.7 Therefore, we retain the important collaboration between cardiologist and oncologists to evaluate and monitor the patients that receive target therapy at risk of CTX.
Prevention and diagnosis of cardiac adverse events
The Common Terminology Criteria for Adverse Events developed by NCI, provides a system for the consistent description and grading of cardiovascular adverse events observed during clinical trials of therapeutic agents.8 The criteria for cardiovascular adverse events have been revised and are shown in Table 1.8,9
Table 1.
The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE 4.03) grading severity of cardiac events associated with tyrosine kinase inhibitors9
| Cardiac event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Hypertension | Prehypertension | Stage 1 hypertension – SBP 140–159 mmHg or DBP 90–99 mmHg); medical intervention indicated; recurrent or persistent (>24 h); symptomatic increase by >20 mmHg (DBP) or to >140/90 mmHg if previously within normal limits; monotherapy indicated | Stage 2 hypertension – SBP >160 mmHg or DBP >100 mmHg; medical intervention indicated; more than one drug or more intensive therapy than previously used indicated | Life-threatening consequences (e.g. malignant hypertension, transient or permanent neurologic deficit, hypertensive crisis); urgent intervention indicated | Death |
| Heart failure | Asymptomatic with laboratory (e.g. brain natriuretic peptide) or cardiac imaging abnormalities | Symptoms with mild-to-moderate activity or exertion | Severe with symptoms at rest or with minimal activity or exertion; intervention indicated | Life-threatening consequences; urgent intervention indicated (e.g. continuous intravenous therapy or mechanical hemodynamic support | Death |
| QT prolongation | QTc 450–480 ms | QTc 481–500 ms | QTc >501 ms on at least two separate electrocardiograms | QTc >501 ms or >60 ms change from baseline and torsades de pointes, or polymorphic ventricular tachycardia, or signs or symptoms of serious arrhythmia | - |
QT is the duration of ventricular depolarization and repolarization. QTc, corrected QT interval.
Risk factors
A detailed clinical assessment is essential in identifying individuals at risk for cardiovascular side-effects. Cardiac evaluations of patients at particular risk for CTX should be carried out in conjunction with the oncologist, so that the choice of therapy may be optimized.
The patients should be evaluated for cardiovascular risk before undergoing treatment with antineoplastic drugs.10 Depending on the patient-specific risk factors, patients should be advised about lifestyle changes, and in some cases, their drug regimen may have to be changed. It is well known that physical exercise has a positive effect on cardiovascular reserve, risk factors and total mortality,11 and β-blockers, angiotensin-converting enzyme (ACE) inhibitors and Ca2+ antagonist are recommended for initial HTN therapy. Statins are recommended to maintain low-density lipoprotein cholesterol levels at less than 100 mg/ml. Antidiabetic therapy is recommended to maintain glycosylated hemoglobin levels at less than 7%.12 In addition to the above described risk factors, other factors that could contribute to cardiac stress should not be ignored. Risk factors should be modified or reduced by treating HTN, normalizing lipids, and encouraging weight reduction and smoking cessation.
Imaging
CTX is generally evaluated by assessing the left ventricular ejection fraction (LVEF). To date, the optimal method is echocardiography and the frequency of cardiac monitoring depends on the drug regimen. In the past, multiple gate acquisition was used more frequently, but this technique is now used only in select cases. We recommend performing a baseline echocardiogram before starting therapy with any drugs that have known or potential CTX.
A 2010 study13 demonstrated that for serial monitoring of ejection fraction in patients with breast cancer receiving adjuvant trastuzumab after treatment with anthracyclines, real-time three-dimensional transthoracic echocardiography yields measurements comparable with those of conventional multiple gate acquisition, using cardiac magnetic resonance as the gold standard. Moreover, noncontrast three-dimensional transthoracic echocardiography was the most reproducible technique for LVEF and LV volume measurement over 1 year of follow-up in patients undergoing cancer therapy.14
Diastolic indices lacked the predictive value for impaired systolic function and the routine assessment of diastolic flow characteristics does not offer additional information.15 A reduction in systolic mitral annular motion, as measured using tissue Doppler imaging, has been noted as early as 3 months following therapy and can predict the development of LV systolic dysfunction.16 However, other studies have not confirmed this finding17 and no clear link between tissue Doppler imaging diastolic abnormalities and systolic dysfunction has been demonstrated.
Recent studies have shown that global longitudinal strain (GLS) identifies LV dysfunction earlier than conventional echocardiographic measures in patients treated with chemotherapy.
A meta-analysis study suggested that GLS might have prognostic value for the development of CTX; however, this was on the basis of results from eight studies with fewer than 500 patients, most of which had only 1 year of follow-up data. Moreover, the definition of CTX was ambiguous, and the majority of patients had LVEF within the normal range.18
Another study [St. James Lifetime Cohort (SJLIFE) study] reported that the evaluation of GLS can provide evidence of cardiac dysfunction in almost one in three adult survivors with normal LVEF.19
These observation data are insufficient for justifying a change of practice to use GLS for the surveillance of these patients because the data are nonrandomized, and there is no evidence that the identification of subclinical dysfunction will change the outcomes of these patients.
However, Plana et al.20 propose the use of GLS in the algorithms of treatment initiation for all drugs potentially associated with LV dysfunction, and for the identification of toxicity and the initiation of cardioprotection.
Regarding MRI, Fallah-Rad et al.16 emphasized another important aspect of trastuzumab-related CTX. They showed that in trastuzumab cardiomyopathy there is evidence of subepicardial linear-delayed enhancement of the LV lateral wall, with a progressive decline in ejection fraction, despite the discontinuation of trastuzumab and the initiation of heart failure therapy. However, other researchers have not confirmed these data, and we believe that other diagnostic sequences, such as MRI, should be evaluated in prospective studies. MRI could be used in clinical practice to select cases or when it is impossible to acquire good images with echocardiography.
The management of epidermal growth factor receptor 2 inhibitor cardiotoxicity
Trastuzumab
Anti-ErbB2 CTX is represented mainly by LV systolic dysfunction and heart failure.
The main antihuman ErbB2 agent is trastuzumab, a humanized monoclonal antibody against the extracellular domain of ErbB2. Trastuzumab treatment is an integral part of standard treatments for breast cancer with HER2 overexpression, and large-scale clinical studies have shown that up to 7 or 28% of patients suffer from cardiac dysfunction when trastuzumab is used in monotherapy, or in combination with anthracyclines plus cyclophosphamide.21
Before starting trastuzumab treatment, a careful evaluation of the patient's medical history, baseline ejection fraction, and correction of risk factors is needed.22
Patients treated with trastuzumab are at a higher risk of developing cardiovascular complications, particularly if they have a history with prior/concomitant anthracyclines, are more than 50 years old, have previous cardiac disease (ejection fraction <55%), have a higher BMI, HTN, and abnormal renal function. Such patients might require interventions, such as cardioprotective agents [e.g. ACE inhibitors, angiotensin receptor blockers (ARBs), and β-blockers].3,23,24
Patients who are not eligible to start trastuzumab treatment for low ejection fraction should begin ACE inhibitors or ARB and β-blockers, and should be referred to a cardiologist25,26 (Table 2). A repeated assessment of cardiac function should occur every 3 months.
Table 2.
The prevention, monitoring, and management of cardiac events in patients undergoing cytotoxic chemotherapy22,25
| Treatment phase | Patient profile | Management strategy |
| Before trastuzumab-based therapy | A. No cardiac history of risk factors with normal EF | Treat and monitor EF every 3 months |
| B. Cardiac history and/or risk factors with normal EF | Treat. Ask of symptoms and perform PE before each cycle | |
| C. Decreased EF | Treat low EF (ACE-I or ARB, BB) and remeasure | |
| Individual decisions about initiating trastuzumab | ||
| During trastuzumab-based therapy | First decrease in EF | Trastuzumab holiday for 1 month |
| A. Treat HF and remeasure | ||
| 1. Return to baseline. Restart trastuzumab | ||
| 2. If EF remains low: intensify HF treatment and remeasure | ||
| 3. If EF remains low: individual decisions | ||
| Second decrease in EF | A. Stop trastuzumab | |
| B. If trastuzumab only option: ‘holiday’ and maximize HF therapy | ||
| Completion of trastuzumab-based therapy | No change in EF and no symptoms during treatment | If you have already used anthracyclines is necessary to monitor LVEF at the end of treatment and after 1.2 and 5 years if doxorubicin <200 mg/m2. More strict monitoring if dosage > 200mg/m2. In the case of only trastuzumab, we advice, anyway, follow-up, considering the last results of real-world retrospective studies |
| EF decreased or symptoms of heart failure during therapy with trastuzumab | Continue HF treatment. Monitor according to clinical practice for HF. The duration of therapy for HF is variable, if previous anthracyclines may be required for life |
The management of cardiac dysfunction before trastuzumab, a major integration to Suter's algorithm (modified from Carver25), is indicated in bold.
The ejection fraction is considered to be reduced when it declines according to Suter's limits (EF < 44, or EF 45–49 and >10 from baseline26). ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β-blocker; EF, ejection fraction; HF, heart failure; PE, physical examination.
Upon initiating trastuzumab, if a patient's ejection fraction declines according to the Suter limits26 (ejection fraction ≤44, or ejection fraction 45–49 and ≥10 from baseline), trastuzumab should be continued for 4 weeks and routine treatment for heart failure should be initiated.
The target dose of heart failure drugs should be achieved by increasing the dose every 1–2 weeks while monitoring renal function and electrolytes, weekly or every 2 weeks, and trying to reach the target dose within 4 weeks. After dose titration, the ejection fraction should be measured and, if it returns to baseline, heart failure medication should be continued, and trastuzumab can be restarted.12 The ejection fraction values of most patients return to baseline within 1–2 months. In the case of persistently low ejection fraction values or in symptomatic patients, aldosterone inhibitors, an ARB, and digoxin should be administered.12 If the ejection fraction values returns to normal, trastuzumab treatment may be restarted. If the ejection fraction values remain low, individual decisions are made based on the patient's clinical conditions and prognosis.
If the patient is receiving trastuzumab, and the ejection fraction declines a second time while receiving stable heart failure treatment, the permanent discontinuation of trastuzumab is recommended.
After the completion of therapy, without changes in the ejection fraction and no symptoms during treatment, if the patient has already been treated with anthracyclines, it is necessary to monitor the LVEF at the end of treatment and after 1, 2, and 5 years, if the doxorubicin dosage is less than 200 mg/m2. Stricter monitoring is necessary if the dosage is higher than 200 mg/m2. In cases of therapy with only trastuzumab, we advise follow-up in consideration of the latest results from real-world retrospective studies.27,28
If after the end of trastuzumab therapy, the ejection fraction is decreased or the patient has symptoms or heart failure during therapy, it is necessary to continue heart failure treatment and to monitor the patient according to the guidelines for heart failure.29 The duration of therapy for heart failure is variable, and if anthracyclines were required previously, heart failure therapy may be required for life.
In metastatic breast cancer, trastuzumab treatment has imminent life-extending potential if clinicians use it more aggressively with considerations that the benefits of therapy may outweigh the risks of cardiac dysfunction. According to the Carver25 protocol, patients with asymptomatic heart failure may continue to receive trastuzumab unless their ejection fraction has decreased more than 20% or to less than 40%, or their ejection fraction is less than 30%. In these cases, trastuzumab is administered for at least one cycle and is titrated to the maximal-tolerated doses of heart failure medical therapy, and the ejection fraction value is remeasured. If the ejection fraction improves to more than 44%, trastuzumab may be resumed. In patients with symptomatic heart failure, or ejection fraction values less than 30%, trastuzumab should be discontinued permanently.
As we have already discussed in the imaging section, the study of GLS by two-dimensional speckle tracking could be an interesting approach in the identification of early cardiac damage.
A reduction of GLS more than 15% from baseline is likely abnormal.30 The use of GLS could be an effective parameter for identifying systolic dysfunction and responses to cardioprotection.31
As oncologists know, the reduction and discontinuation of treatment for only 1 month compromises the efficacy of trastuzumab treatment.32 If randomized prospective studies will confirm the utility of reducing GLS to identify early myocardial damage predictive of CTX, we could use this technique in routine clinical practice to protect the heart without affecting trastuzumab therapy.
Lapatinib, pertuzumab, and ado-trastuzumab emtansine
The CTX of the new ErbB2 inhibitors, ado-trastuzumab emtansine, pertuzumab, and lapatinib, is not superior or additive to that of trastuzumab alone.33–35 For this reason, we advise similar management to that of trastuzumab, with consideration of the fact that these drugs are used, at the moment, only in the metastatic settings.
The management of angiogenic inhibitor cardiotoxicity
The most important cardiovascular side-effects of the angiogenesis inhibitors are HTN, coronary artery disease, LV dysfunction/heart failure, QT prolongation, and thrombosis.
Hypertension
The most common cardiovascular risk factor for cardiac events with the angiogenic inhibitor therapy is HTN. Other risk factors include: age (men >55 years and women >65 years), hyperlipidemia, smoking, diabetes mellitus, a family history of early heart disease (men< 55 years and women <65 years), obesity, a sedentary lifestyle, preexisting HTN, and the Hemolysis Elevated Liver enzymes and Low Platelet count (HELLP) syndrome.36 The HELLP syndrome is a variant of preeclampsia. ‘HELLP’ is an abbreviation of the three main features of the syndrome: hemolysis, elevated liver enzymes, and a low platelet count. HELLP syndrome occurs in 4–12% of patients with preeclampsia or eclampsia and seems to be linked to the dysregulation of VEGF signaling.
Therefore, the evaluation and treatment of HTN is a practical starting point for interventions for CTX induced by angiogenic inhibitors.37,38
The Cardiovascular Toxicities Panel of the NCI has defined grade 1 toxicity as pre-HTN (SBP 120–139 mmHg or DBP 80–89 mmHg). Patients with pre-HTN should begin antihypertensive therapy, which should eventually be reinforced with other agents 3–7 days before starting the angiogenic inhibitor therapy38–40 (Fig. 2).
Fig. 2.
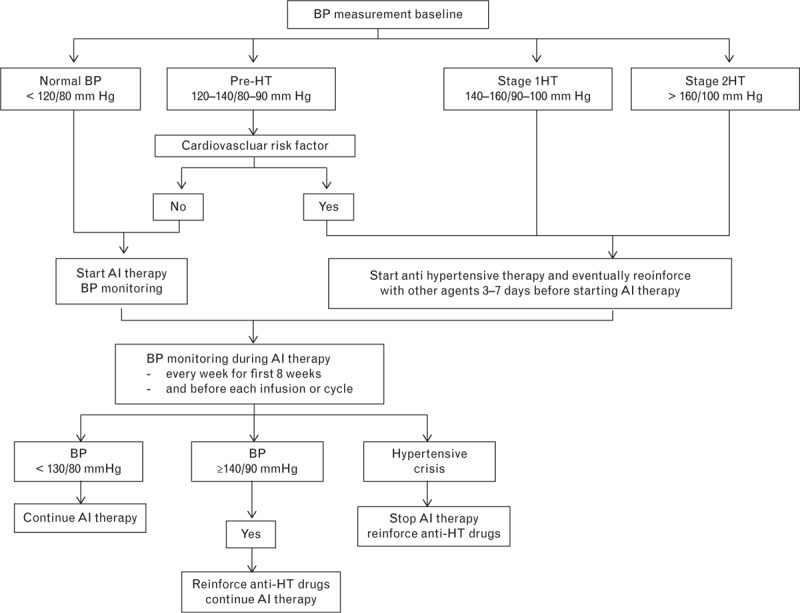
The management of hypertension induced by angiogenic inhibitors. AI, angiogenesis inhibitor; CCB, calcium channel blocker; HT, hypertension. Modified from Ederhy et al.40
During angiogenic inhibitor therapy, blood pressure (BP) monitoring should be performed every week for the first 8 weeks and before each new infusion cycle. If the BP stays less than 130/80 mmHg, angiogenic inhibitor therapy can be continued, but if BP increases to at least 140/90 mmHg, it is necessary to reinforce the regimen with antihypertensive drugs. In cases of hypertensive crisis, it is necessary to stop angiogenic inhibitors and administer antihypertensive drugs.
Myocardial ischemia
The recommendations for the management of myocardial ischemia include:
Cardiology consultation in patients with asymptomatic ECG changes and in those with symptoms suggesting myocardial ischemia.
Suspending angiogenic inhibitor therapy in case of documented myocardial ischemia. A collaborative decision should then be made as to whether more advanced cardiac testing (e.g. stress testing and coronary angiography) is needed and whether the benefits of resuming therapy with aggressive supportive care outweigh the risks.
Myocardial infarction during antiangiogenic inhibitor therapy could indicate the permanent discontinuation of that therapy.40
Left ventricular dysfunction and heart failure
In the case of asymptomatic LV dysfunction, angiogenic inhibitors should be continued in cases of mild (ejection fraction decrease >15%, with ejection fraction >50%) or moderate (ejection fraction 50–40%) dysfunction, while monitoring for HTN. Only in severe (ejection fraction <40%) LV dysfunction and in symptomatic patients is it recommended to stop anticancer therapy, discuss alternative drugs, and treat the LV dysfunction (Fig. 3).41
Fig. 3.
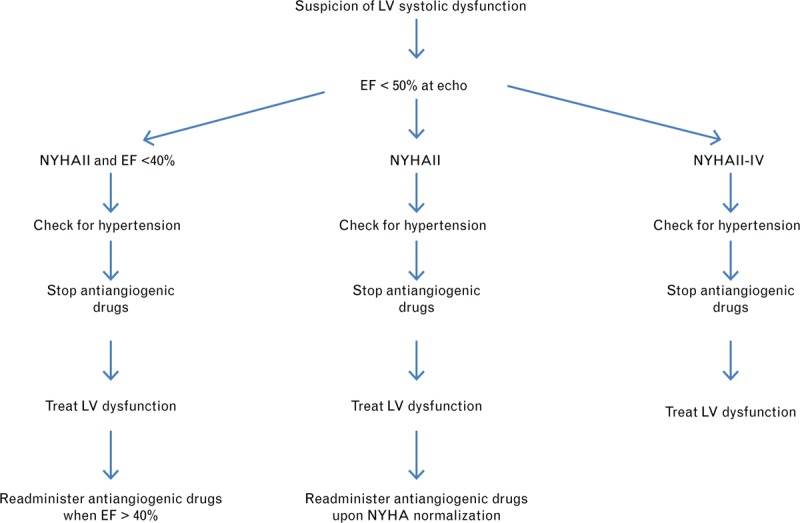
Considerations for the management of left ventricular dysfunction and heart failure induced by antiangiogenic therapies.39–41 Modified from Ederhy et al.40 and Suter and Ewer.41
Antiangiogenic drugs should then be resumed upon ejection fraction improvement and the normalization of symptoms, but the issue of reversibility of LV dysfunction and the opportunity for readministering antiangiogenic inhibitors after improvement to the New York Heart Association III–IV criteria remain unclear.42,43
QT interval prolongation
The QT interval recorded on an ECG reflects the total duration of ventricular activation and recovery.44 Several correction formulae have been developed to improve the accuracy of QT measurement with corrected QT (QTc) values.44–49
Bazett's correction is frequently used in clinical practice and in the medical literature. However, Bazett's correction overcorrects in cases of elevated heart rates and undercorrects in cases with heart rates below 60 beats/min (bpm) and, hence, is not an ideal correction. Fridericia's correction is more accurate than Bazett's correction in study participants with such altered heart rates. The NCI classification of five grades of QT prolongation associated with TKIs is presented in Table 1.
It is difficult to evaluate the risk of developing life-threatening arrhythmias from QTc prolongation.50,51 There is no correlation between the prolonged QTc interval and the incidence of torsades de pointes and sudden death. Additionally, the risk of potentially fatal ventricular tachycardia is small.50
There are several factors that can influence the prolongation of QT in cancer patients treated with angiogenic inhibitors:
Drugs: vandetanib, sunitinib, pazopanib (a higher incidence with vandetanib has been reported), and vemurafenib.
Coexisting conditions (e.g. noncardiac: hypothyroidism and congenital long QT, and cardiac: LV dysfunction and cardiac ischemia).
Concomitant medications: antidepressants, antiemetics, antibiotics, antipsychotics, antifungals, antihistamines, and methadone.
Cancer therapy-related factors: nausea and emesis, diarrhea, diuresis, and poor oral intake can result in dehydration/electrolyte imbalances with subsequent hypokalemia, hypomagnesemia, hypocalcemia, renal insufficiency, hepatic dysfunction, and poorly controlled diabetes.
For all of the above reasons, the following must be optimized to improve patient care:
Data collection, using the ‘tangent’ method for QT measurement and the Fridericia formula for heart rate correction.
The identification of risk factors associated with the repletion of electrolyte disturbances, particularly K+ and Mg2+, and of concomitant QT-prolonging medications.
Cardiac monitoring, using practice guidelines for high-risk biological therapy and referring to Food and Drug Administration guidelines for specific agents.
A general algorithm for QT monitoring during antineoplastic therapy is shown in Fig. 4, but the management could be different for each drug. It depends on the drug-specific pharmacokinetics and pharmacodynamics. Thus, it is necessary to develop a specific algorithm for each drug.
Fig. 4.
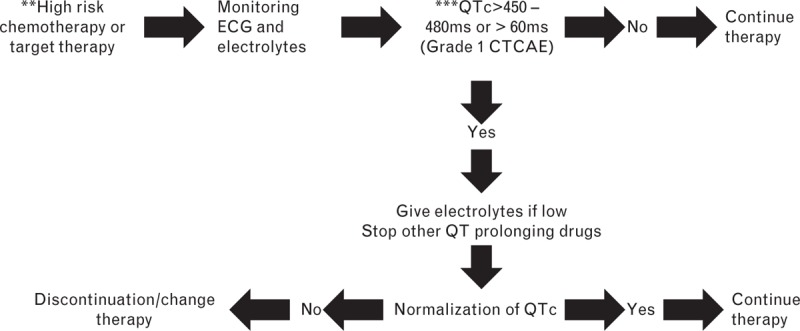
A general algorithm for QT monitoring during antineoplastic therapy.
Vandetanib, sunitinib, and pazopanib can prolong the QT interval, and torsades de pointes and sudden death have been reported with these drugs.52–54 The highest incidence of these abnormalities has been seen with vandetanib. The package leaflet indicates that vandetanib should not be administered in patients with hypocalcemia, hypokalemia, hypomagnesemia, or long QT syndrome.
ECGs should be obtained at baseline, at 2–4 weeks, and 8–12 weeks after starting treatment and every 3 months thereafter.37
Vascular toxicity (arterial venous thrombosis/hemorrhage)
It has been shown that angiogenesis inhibitors, such as bevacizumab, sorafenib, and sunitinib, may cause arterial and venous thromboembolism. In a systematic review, Faraque et al.55 demonstrated that the risk of any grade thrombotic or thromboembolic events was significantly higher with these drugs.
The Bevacizumab Expanded Access trial and Bevacizumab Regimens’ I Investigation of Treatment Effect studies found an arterial thromboembolic event incidence of 3.3%–6.1% in treated patients,56 especially as myocardial infarctions or cerebrovascular damage.57–59 Venous thromboembolism events are otherwise not correlated with bevacizumab treatment in the literature.
Choueiri et al.59 found an arterial thromboembolic event incidence of 1.3–1.8% during both sunitinib and sorafenib therapy. Moreover, 1% of patients treated with sunitinib were reported to have venous thromboembolic events.60
However, antithrombotic and antithromboembolic therapies remain difficult to manage in patients treated with antiangiogenic agents. Elice and Rodeghiero61 suggest the administration of venous or arterial thromboprophylaxis, particularly in patients at the highest risk for thrombosis or for those treated with a combination of surgery and chemotherapy.
The overall incidence of severe hemorrhagic events with bevacizumab was 2.8% with higher risks seen in patients with renal cell carcinoma, nonsmall cell lung cancer (NSCLC), and colorectal cancer.62 Similarly, the incidence of hemorrhage for sorafenib and sunitinib was 16.7%.63
Data regarding the value of aspirin prophylaxis for arterial thromboembolic events for patients on bevacizumab are conflicting. Scappaticci et al.64 reported that in both bevacizumab-treated and control patients, aspirin use was associated with an 1.3-fold increase in grades 3 and 4 bleeding events. Instead, Tebbutt et al.65 reported slightly more grade 3 and 4 bleeding events among aspirin users on bevacizumab (3.7%) than controls (1.8%).
Abelson murine leukemia viral oncogene homolog inhibitors
Ponatinib
Ponatinib is a multitarget TKI that inhibits ABL and blocks angiogenesis by inhibiting the actions of VEGF and other growth factors (e.g. platelet-derived growth factor). Ponatinib received approval for treatment of a variety of tumors, including renal cell carcinoma, hepatocellular cancer, gastrointestinal stromal tumors (GISTs), thyroid cancer, pancreatic neuroendocrine tumors, soft tissue sarcomas, refractory chronic myelogenous leukemia (CML), and refractory metastatic colorectal cancer. Higher rates of arterial thromboembolic events are reported with ponatinib. Among patients receiving ponatinib therapy for refractory CML, 11% developed arterial thrombosis of any grade; 8% had serious arterial thrombosis.66 The risk of serious thromboembolic events may be higher. Approximately 24% of patients in the phase 2 clinical trial and approximately 48% of patients in the phase 1 clinical trial experienced serious adverse vascular events, including fatal and life-threatening heart attack, stroke, loss of blood flow to the extremities resulting in tissue death, and severe narrowing of blood vessels in the extremities, heart, and brain, requiring urgent surgical procedures to restore blood flow.66
Given the high rate of thromboembolic events in patients treated with ponatinib, clinicians should carefully consider whether the benefits of treatment are likely to exceed the risks, particularly for patients with one or more cardiac risk factor.66
Imatinib
Imatinib, a small molecule inhibitor of the breakpoint cluster region-ABL, tyrosine protein kinase kit, the platelet-derived growth factor receptor, and the nonreceptor tyrosine kinase sarcoma (SRC) family of TKIs, are used in the treatment of Philadelphia chromosome-positive CML (Ph + CML) and GISTs. In an early report of patients treated for Ph + CML, imatinib was associated with the development of severe heart failure,67 prompting the manufacturer to revise the drug labeling to include warnings about possible heart failure.
Laboratory studies indicate that adverse cardiac events in patients receiving imatinib are likely mediated by the inhibition of ABL.67,68
Despite the biologic rationale for potential CTX in patients receiving imatinib, subsequent publications indicate a low incidence of clinically significant heart failure in CML clinical trial settings (no more than 1%-2%69); and an increased risk for heart failure or LV dysfunction has not been observed in patients receiving imatinib for the treatment of GIST.70–72
However, obtaining a baseline assessment of LVEF in all patients receiving imatinib (particularly for GIST where it is not even clear that there is a risk of CTX) is not supported by compelling data. Nonetheless, patients receiving imatinib should be monitored for signs and symptoms of heart failure, and physicians should have a low threshold for the formal assessment of LV dysfunction.
Guidelines for the management of imatinib toxicity from the National Comprehensive Cancer Network suggest that only patients with cardiac disease or risk factors for heart failure who are receiving imatinib need to be monitored carefully and that any patient with signs or symptoms consistent with heart failure should be evaluated and treated.73
Dasatinib and nilotinib
Dasatinib and nilotinib are two second-generation multitargeted TKIs that are used for the treatment of Ph + CML. Both dasatinib and nilotinib have been associated with QT prolongation.44 Abnormalities in potassium and magnesium levels must be corrected prior to drug initiation; other drugs that may affect the QTc interval should be avoided; caution should be used in patients at risk for QT interval prolongation, and serial ECGs should be performed.
Although a definite causal relationship has not been established, dasatinib has also been associated with chest pain, pericardial effusion, pulmonary HTN, ventricular dysfunction, and heart failure.74 The US prescribing information states that 1.6% of 258 patients taking dasatinib developed cardiomyopathy, heart failure, diastolic dysfunction, fatal myocardial infarction, and/or LV dysfunction.75
Proteasome inhibitors
Bortezomib and carfilzomib are proteasome inhibitors of the first and second generation, respectively. They are used for the treatment of multiple myeloma.
In clinical trials of carfilzomib, new onset or worsening of preexisting heart failure with decreased LV function or myocardial ischemia has been reported in approximately 7% of patients, and deaths because of cardiac arrest have occurred within 1 day of drug administration.76 In addition, pulmonary arterial HTN has been reported in 2% of patients treated with carfilzomib.
In a phase II trial of 266 patients treated with carfilzomib monotherapy for relapsed myeloma, 10 experienced heart failure (3.8%), four (1.5%) had a cardiac arrest, and two (0.8%) had a myocardial infarction during the study.77 The risk did not appear to be cumulative, at least through 12 cycles of therapy. However, the magnitude of the attributable risk, risk factors, and the natural history, including the reversibility of carfilzomib-related CTX, remain incompletely characterized. The recommended dose modification based on CTX is available in the US prescription information.
CTX might represent a class effect, as heart failure events (acute pulmonary edema, cardiac failure, and cardiogenic shock) have also been described in patients treated with bortezomib. However, causality remains unclear. In a phase III trial comparing bortezomib versus dexamethasone for relapsed myeloma, the incidence of treatment-emergent cardiac disorders during treatment with bortezomib or dexamethasone was 15 and 13%, respectively; seven patients receiving bortezomib (2%) and eight patients receiving dexamethasone (2%) developed heart failure during the study.78 Similarly to the outcomes of carfilzomib therapy, cardiac dysfunction does not appear to be cumulative in patients treated with bortezomib.79
Abnormalities appear to be largely reversible with prompt therapy cessation and the initiation of traditional heart failure treatment.80
For these reasons, we recommend a baseline assessment of the LVEF before starting proteasome inhibitors.
Miscellaneous agents
Crizotinib and ceritinib
Crizotinib and ceritinib are orally active inhibitors of the anaplastic lymphoma kinase. They are both approved for the treatment of advanced or metastatic NSCLC. Sinus bradycardia is common in patients receiving these agents and can be profound, although it is generally asymptomatic and not associated with other events, such as other arrhythmias.
In two trials evaluating the efficacy of crizotinib for advanced NSCLC, bradycardia was reported in only 12 of 240 patients who were assessable for treatment-related CTX, and all cases were mild (grade 1 or 2) in severity.81
In another report of 42 patients receiving treatment with crizotinib for advanced NSCLC, there was an average bpm decrease of 26 among all patients; 69% had at least one episode of sinus bradycardia (heart rate <60 bpm).82 Profound sinus bradycardia (heart rate <50 bpm) developed in 13 patients (31%). None of the patients who developed bradycardia during treatment was symptomatic or had ECG changes such as QTc interval prolongation.
In addition to bradycardia, QTc interval prolongation has been observed with both drugs, although it is uncommon. Three percentage of 255 patients treated with ceritinib experienced a QTc interval increase over baseline of 60 ms; in a larger population of 304 patients treated with the drug, only one (<1%) developed a QTc interval of more than 500 ms.83 The US prescribing information for crizotinib and ceritinib recommends avoiding both drugs in patients with congenital long QT syndrome and patients with heart failure, bradyarrhythmias, electrolyte abnormalities, or those who are taking other medications known to prolong the QTc interval, in addition to performing periodic monitoring with ECGs and assessments of the serum electrolyte levels. Treatment interruption and dose reduction is advised if the QTc interval exceeds more than 500 ms during treatment, with permanent discontinuation if it recurs or is accompanied by arrhythmia, heart failure, hypotension, shock, syncope, or torsades de pointes.
Practice is variable regarding cardiac monitoring during therapy; however, some clinicians perform a baseline ECG for patients starting crizotinib or ceritinib only if they have a known history of heart failure or cardiac arrhythmia issues, and perform ECGs during therapy if bradycardia (symptomatic or not) develops or if the patient is started on another drug with known QTc prolongation side-effects.
Vemurafenib
Vemurafenib, an orally available inhibitor of some mutated forms of homolog B of rapidly accelerated fibrosarcoma kinase (BRAF), is approved for the treatment of metastatic melanoma with a V600E BRAF mutation. Vemurafenib has been associated with the prolongation of the QTc interval. Furthermore, ECG and electrolyte monitoring are recommended before treatment and after dose modification. For patients starting therapy with vemurafenib, ECGs are recommended at day 15, monthly during the first 3 months of treatment, and every 3 months thereafter, and more often as clinically indicated. If the QTc interval exceeds 500 ms, treatment should be temporarily interrupted, and electrolyte abnormalities should be evaluated and corrected.84
Trametinib
Trametinib is an orally active inhibitor of the mitogen-activated protein kinase enzymes (MAPK1 and MAPK2), and it is approved for the treatment of metastatic melanoma with a specific BRAF mutation. In clinical trials of trametinib in patients with metastatic melanoma, LV dysfunction has been observed in up to 11% of treated patients.85
The US prescribing information recommends the following86:
Assess LVEF before initiating therapy, 1 month after treatment initiation, and at 2 to 3-month intervals during treatment.
Withhold treatment if the absolute LVEF decreases by 10% from the pretreatment values to less than the institutional lower limit of normal.
Permanently discontinue trametinib for symptomatic heart failure, any absolute decrease in LVEF of more than 20% from baseline that is below the lower institutional limit of normal, and persistent LVEF reductions of at least 10% from baseline that does not resolve within 4 weeks.
Conclusion
Cancer patients receiving chemotherapy have an increased risk of developing cardiovascular complications, and the risk is even greater if there is a known history of heart disease. Anthracycline and anthracycline-like agents and agent targeting the human ErbB2, such as trastuzumab, are the most frequently known anticancer agents associated with CTX.87
In addition to trastuzumab, a wide range of other biological agents has been associated with CTX. To date, there are more than 600 TKIs in development for cancer treatment. Each of these drugs is potentially cardiotoxic through their ‘on-target’ or ‘off-target’ effects on cardiac tyrosine kinase. Moreover, the specificity profile of these drugs and the biology of most TKIs are usually not known, and thus each new drug will have its own unpredictable impact on the risk of CTX.
For all patients receiving potential cardiotoxic therapy, primary prevention to reduce cardiovascular risk may be achieved by measures ‘that rest on common sense.’83 The management of preexisting comorbidities (HTN, LV dysfunction, arrhythmias, and metabolic disorder) should be optimized and a healthy lifestyle should be encouraged both before and after initiating cancer therapy.
In the absence of specific cardiac monitoring guidelines for potentially cardiotoxic agents, the evaluation and monitoring of LVEF must be considered. The toxicity profile, patient, and disease characteristics should be considered when making this decision. When starting an agent that may cause or worsen HTN, serial BP monitoring should be performed and maintained during therapy with that particular drug. A proposed algorithm regarding interventions for treatment-related HTN is provided in Fig. 2.
Outcomes may be improved when cancer survivors who have been treated with potentially cardiotoxic drugs are referred to centers with expertise in long-term surveillance and risk-based medical care. Secondary prevention of CTX after treatment currently depends on clinical observations as research continues to identify reliable measures of subclinical disease.
References
- 1.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014; 64:938–945. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011; 377:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truong J, Yan AT, Cramarossa G, Chan KK. Chemotherapy-induced cardiotoxicity: detection, prevention, and management. Can J Cardiol 2014; 30:869–878. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438:967–974. [DOI] [PubMed] [Google Scholar]
- 5.Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin 2013; 63:249–279. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration (FDA)-approved manufacturer's package insert. [[Accessed 3 January 2013]]. available online at http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=807f988e-117b-4497-934d-73aa78baac71. [Google Scholar]
- 7.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007; 370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hossain A, Chen A, Ivy P, et al. The importance of clinical grading of heart failure and other cardiac toxicities during chemotherapy: updating the common terminology criteria for clinical trial reporting. Heart Fail Clin 2011; 7:373–384. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Common Terminology Criteria for Adverse Events v 4.0. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010 – 06–14_QuickReference_8.5x11.pdf [Accessed 13 May 2013] [Google Scholar]
- 10.Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007; 50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 11.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol 2008; 24:3958–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackey JR, Clemons M, Côté MA, et al. Cardiac management during adjuvant trastuzumab therapy: recommendations of the Canadian Trastuzumab Working Group. Curr Oncol 2008; 15:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol 2010; 28:3429–3436. [DOI] [PubMed] [Google Scholar]
- 14.Thavendiranathan P, Grant AD, Negishi T, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013; 61:77–84. [DOI] [PubMed] [Google Scholar]
- 15.Dorup I, Levitt G, Sullivan I, Sorensen K. Prospective longitudinal assessment of late anthracycline cardiotoxicity after childhood cancer: the role of diastolic function. Heart 2004; 90:1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. Am Coll Cardiol 2011; 57:2263–2270. [DOI] [PubMed] [Google Scholar]
- 17.Ong DS, Scherrer-Crosbie M, Coelho-Filho O, et al. Imaging methods for detection of chemotherapy-associated cardiotoxicity and dysfunction. Expert Rev Cardiovasc Ther 2014; 12:487–497. [DOI] [PubMed] [Google Scholar]
- 18.Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014; 63:2751–2768. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol 2015; 65:2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014; 27:911–939. [DOI] [PubMed] [Google Scholar]
- 21.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009; 53:2231–2247. [DOI] [PubMed] [Google Scholar]
- 22.Tocchetti CG, Ragone G, Coppola C, et al. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. Eur J Heart Fail 2012; 14:130–137. [DOI] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 24.Cardinale D, Bacchiani G, Beggiato M, et al. Strategies to prevent and treat cardiovascular risk in cancer patients. Semin Oncol 2013; 40:186–198. [DOI] [PubMed] [Google Scholar]
- 25.Carver JR. Management of trastuzumab-related cardiac dysfunction. Prog Cardiovasc Dis 2010; 53:130–139. [DOI] [PubMed] [Google Scholar]
- 26.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J Clin Oncol 2007; 25:3859–3865. [DOI] [PubMed] [Google Scholar]
- 27.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012; 104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol 2012; 60:2504–2512. [DOI] [PubMed] [Google Scholar]
- 29.Heart Failure Society of America Management of asymptomatic patients with reduced left ventricular ejection fraction. J Card Fail 2006; 12:e26–e28. [DOI] [PubMed] [Google Scholar]
- 30.Negishi K, Negishi T, Hare JL, et al. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013; 26:493–498. [DOI] [PubMed] [Google Scholar]
- 31.Negishi K, Negishi T, Haluska BA, et al. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 2014; 15:324–431. [DOI] [PubMed] [Google Scholar]
- 32.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012; 4:CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krop IE, Suter TM, Dang CT, et al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol 2015; 33:1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012; 366:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez EA, Koehler M, Byrne J, et al. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc 2008; 83:679–686. [DOI] [PubMed] [Google Scholar]
- 36.Khakoo AY, Liu PP, Force T, et al. Cardiotoxicity due to cancer therapy. Tex Heart Inst J 2011; 38:253–256. [PMC free article] [PubMed] [Google Scholar]
- 37.Steingart RM, Bakris GL, Chen HX, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J 2012; 163:156–163. [DOI] [PubMed] [Google Scholar]
- 38.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance and management of blood pressure in patients receiving vascular endothelial growth factor pathway inhibitors. J Natl Cancer Inst 2010; 102:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tocchetti CG, Gallucci G, Coppola C, et al. The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. Eur J Heart Fail 2013; 15:482–489. [DOI] [PubMed] [Google Scholar]
- 40.Ederhy S, Izzedine H, Massard C, et al. Cardiac side effects of molecular targeted therapies: towards a better dialogue between oncologists and cardiologists. Crit Rev Oncol Hematol 2011; 80:369–379. [DOI] [PubMed] [Google Scholar]
- 41.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013; 34:1102–1111. [DOI] [PubMed] [Google Scholar]
- 42.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 2008; 19:1613–1618. [DOI] [PubMed] [Google Scholar]
- 43.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008; 26:5204–5212. [DOI] [PubMed] [Google Scholar]
- 44.Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 2007; 25:3362–3371. [DOI] [PubMed] [Google Scholar]
- 45.Taran LM, Szilagyi N. The duration of the electrical systole (Q-T) in acute rheumatic carditis in children. Am Heart J 1947; 33:14–26. [DOI] [PubMed] [Google Scholar]
- 46.Fridericia LS. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann Noninvasive Electrocardiol 2003; 8:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992; 70:797–801. [DOI] [PubMed] [Google Scholar]
- 48.Desai M, Li L, Desta Z, et al. Variability of heart rate correction methods for the QT interval. Br J Clin Pharmacol 2003; 55:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Available at: http://www.fda.gov/downloads/Regulatory Information/Guidances/ucm129357.pdf [Accessed 13 May 2013] [Google Scholar]
- 50.Brell JM. Prolonged QTc interval in cancer therapeutic drug development: defining arrhythmic risk in malignancy. Prog Cardiovasc Dis 2010; 53:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson ME, Al-Khatib SM, Roden DM, et al. Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am Heart J 2002; 144:769–781. [DOI] [PubMed] [Google Scholar]
- 52.Sutent (sunitinib malate) capsules [package, insert]. 2008; New York, NY:Pfizer Labs, 6. [Google Scholar]
- 53.Votrient (pazopanib) tablets Highlights of prescribing information. Triangle Park, NC:GlaxoSmithKline Research; 2010. [Google Scholar]
- 54.Caprelsa (vandetanib) tablets Highlights of prescribing informations. Wilmington, DE:Astra Zeneca; 2011. [Google Scholar]
- 55.Faraque LI, Lin M, Battistella M, et al. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factors inhibitors for the treatment of cancer. PLOS One 2014; 9:e101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol 2010; 49:287–297. [DOI] [PubMed] [Google Scholar]
- 57.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009; 20:1842–1847. [DOI] [PubMed] [Google Scholar]
- 58.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008; 300:2277–2285. [DOI] [PubMed] [Google Scholar]
- 59.Choueiri TK, Schutz FA, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 2010; 28:2280–2285. [DOI] [PubMed] [Google Scholar]
- 60.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006; 368:1329–1338. [DOI] [PubMed] [Google Scholar]
- 61.Elice F, Rodeghiero F. Side effects of antiangiogenic drugs. Thromb Res 2012; 129 (Suppl 1):S50–S53. [DOI] [PubMed] [Google Scholar]
- 62.Hang XF, Xu WS, Wang JX, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2011; 67:613–623. [DOI] [PubMed] [Google Scholar]
- 63.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol 2009; 10:967–974. [DOI] [PubMed] [Google Scholar]
- 64.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. Nat Cancer Inst 2007; 99:1232–1239. [DOI] [PubMed] [Google Scholar]
- 65.Tebbutt NC, Murphy F, Zannino D, et al. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol 2011; 22:1834–1838. [DOI] [PubMed] [Google Scholar]
- 66.US Food and Drug Administration (FDA)-approved manufacturer's package insert. [[Accessed 3 January 2013]]. available online at http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=807f988e-117b-4497-934d-73aa78baac71. [Google Scholar]
- 67.Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 2006; 12:908–916. [DOI] [PubMed] [Google Scholar]
- 68.Fernández A, Sanguino A, Peng Z, et al. An anticancer C-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. J Clin Invest 2007; 117:4044–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood 2007; 110:1233–1237. [DOI] [PubMed] [Google Scholar]
- 70.Verweij J, Casali PG, Kotasek D, et al. Imatinib does not induce cardiac left ventricular failure in gastrointestinal stromal tumours patients: analysis of EORTC-ISG-AGITG study 62005. Eur J Cancer 2007; 43:974–978. [DOI] [PubMed] [Google Scholar]
- 71.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009; 373:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trent JC, Patel SS, Zhang J, et al. Rare incidence of congestive heart failure in gastrointestinal stromal tumor and other sarcoma patients receiving imatinib mesylate. Cancer 2010; 116:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Comprehensive Cancer Network (NCCN). NCCN Clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [Accessed 1 April 2014] [Google Scholar]
- 74. http://www.sprycel.com/. November 2015. [Google Scholar]
- 75. [[Accessed 22 November 2010]]. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=32204&CFID=11456208&CFTOKEN=1f74eda9f81e615f-6771DE68–92AA-956A-3738E4B0F5A05DA7&jsessionid=ca30878ec315457e3b38. [Google Scholar]
- 76. [[Accessed 13 August 2012]]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202714lbl.pdf?et_cid=29661884&et_rid=463638624&linkid=http%3a%2f%2fwww.accessdata.fda.gov%2fdrugsatfda_docs%2flabel%2f2012%2f202714lbl.pdf. [Google Scholar]
- 77.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012; 120:2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352:2487–2498. [DOI] [PubMed] [Google Scholar]
- 79.Berenson JR, Jagannath S, Barlogie B, et al. Safety of prolonged therapy with bortezomib in relapsed or refractory multiple myeloma. Cancer 2005; 104:2141–2148. [DOI] [PubMed] [Google Scholar]
- 80.Grandin EW, Ky B, Cornell RF, et al. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail 2015; 21:138–144. [DOI] [PubMed] [Google Scholar]
- 81.US FDA approved manufacturer's labeling for crizotinib. [[Accessed 10 September 2013]]. available online at http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2a51b0de-47d6–455e-a94c-d2c737b04ff7. [Google Scholar]
- 82.Ou SH, Tong WP, Azada M, et al. Heart rate decrease during crizotinib treatment and potential correlation to clinical response. Cancer 2013; 119:1969–1975. [DOI] [PubMed] [Google Scholar]
- 83. [[Accessed 29 April 2014]]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205755lbl.pdf?et_cid=33681002&et_rid=585254827&linkid=http%3a%2f%2fwww.accessdata.fda.gov%2fdrugsatfda_docs%2flabel%2f2014%2f205755lbl.pdf. [Google Scholar]
- 84. http://www.ema.europa.eu/docs/it_IT/document_library/EPAR_-Product_Information/human/002409/WC500124317.pdf. November 2015. [Google Scholar]
- 85.Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013; 31:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204114s000lbl.pdf. November 2015. [Google Scholar]
- 87.Maurea N, Coppola C, Ragone G, et al. Women survive breast cancer but fall victim to heart failure: the shadows and lights of targeted therapy. J Cardiovasc Med (Hagerstown) 2010; 11:861–868.doi: 10.2459/JCM.0b013e328336b4c1. [DOI] [PubMed] [Google Scholar]


