Abstract
Purpose of review
Perioperative opioid-based pain management of patients suffering from obstructive sleep apnea (OSA) may present challenges because of concerns over severe ventilatory compromise. The interaction between intermittent hypoxia, sleep fragmentation, pain, and opioid responses in OSA, is complex and warrants a special focus of perioperative outcomes research.
Recent findings
Life-threatening opioid-related respiratory events are rare. Epidemiologic evidence suggests that OSA together with other serious renal and heart disease, is among those conditions predisposing patients for opioid-induced ventilatory impairment (OIVI) in the postoperative period. Both intermittent hypoxia and sleep fragmentation, two distinct components of OSA, enhance pain. Intermittent hypoxia may also potentiate opioid analgesic effects. Activation of major inflammatory pathways may be responsible for the effects of sleep disruption and intermittent hypoxia on pain and opioid analgesia. Recent experimental evidence supports that these, seemingly contrasting, phenotypes of pain-increasing and opioid-enhancing effects of intermittent hypoxia, are not mutually exclusive. Although the effect of intermittent hypoxia on OIVI has not been elucidated, opioids worsen postoperative sleep-disordered breathing in OSA patients. A subset of these patients, characterized by decreased chemoreflex responsiveness and high arousal thresholds, might be at higher risk for OIVI.
Summary
OSA may complicate opioid-based perioperative management of pain by altering both pain processing and sensitivity to opioid effect.
Keywords: obstructive sleep apnea, opioids, pain, sleep-disordered breathing
INTRODUCTION
Opioids are widely used to manage postoperative pain. In patients with obstructive sleep apnea (OSA), clinicians may hesitate to use opioids liberally for managing postoperative pain because of concerns about respiratory depression. Important components of OSA like sleep fragmentation and intermittent hypoxia may enhance pain behavior and also increase sensitivity to opioid analgesia [1▪▪]. The purpose of this review is to examine the various aspects of OSA with regards to opioid analgesic therapy. Are patients with OSA more sensitive to the effects of opioids? Are they more vulnerable to the respiratory depressant effects of opioids compared with patients who do not suffer from OSA? Do patients with sleep fragmentation and intermittent hypoxia because of their OSA status demonstrate enhanced pain behavior? Do they require higher or lower doses of opioids to treat postoperative pain compared with non-OSA patients? Is there a physiological phenotype of OSA that is associated with increased susceptibility to opioid-induced ventilatory impairment (OIVI)?
Box 1.
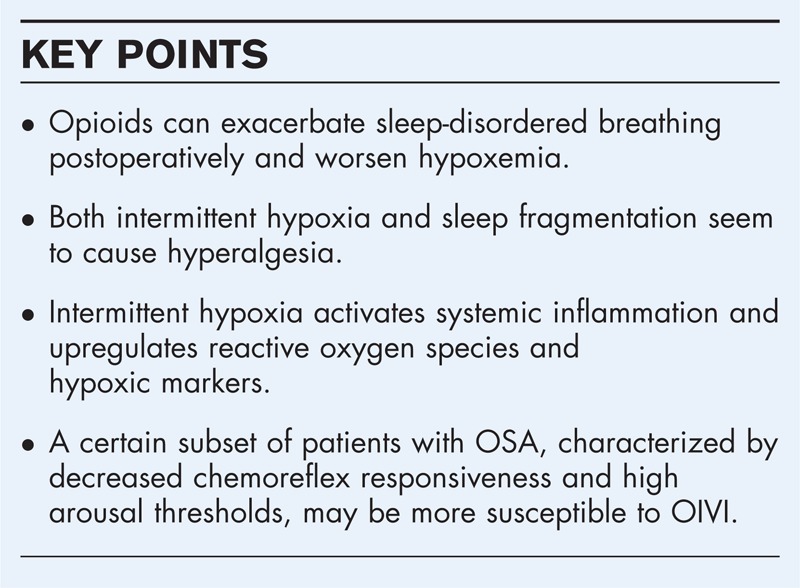
no caption available
REVIEW
OSA is characterized by repetitive partial or complete collapse of the airway during sleep, which could lead to hypoxemia and/or hypercapnia with associated clinical signs of daytime sleepiness, loud snoring, witnessed breathing interruptions, or awakenings because of gasping or choking in the presence of at least five obstructive respiratory events per hour of sleep [2]. A complex interaction between physiological traits of OSA, pharmacologic effect of opioids, and pain processing may pose clinical challenges to the postoperative analgesic management of this patient population. Patients suffering from sleep-disordered breathing may present with increased sensitivity both to the central (i.e., sedation, diminished central respiratory drive) and peripheral (i.e., increased airway collapsibility) effects of opioids, whereas certain pathophysiological characteristics of OSA like sleep disruption, intermittent hypoxemia, and systemic inflammation also have the potential to influence pain behavior and/or sensitivity to opioids [1▪▪,3]. A better understanding of these relationships is clinically important because it directly pertains to our ability to prevent possible opioid-related respiratory and enhance perioperative safety in this patient population.
OPIOID-INDUCED VENTILATORY IMPAIRMENT
Ventilatory depression is a potentially dangerous complication of opioid therapy. There is a delicate balance between achieving adequate analgesia and causing potentially severe respiratory depression. The incidence of OIVI in the perioperative period is highly variable depending on which physiologic, biochemical, or clinical variable is used to define respiratory depression. A large retrospective analysis of postoperative pain management that included 165 articles and nearly 20 000 surgical patients, reported an incidence of OIVI between 0.1 and 37%, depending on the indicator used to detect respiratory events (i.e., therapeutic intervention with naloxone versus oxyhemoglobin desaturation using pulse oximetry) [4].
In most cases, OIVI occurs without obvious overdose of opioids [5]. Drug-related factors, such as the type of opioids and routes of administration, and/or patient-related parameters including comorbidities and individual pharmacokinetic and pharmacodynamic variability in drug response, characterize the majority of opioid-related respiratory adverse events. Thus, common conditions that could precipitate OIVI after receiving therapeutic doses of opioids include: underlying diseases like renal failure, polymorphisms of genes that are involved in drug metabolism (e.g., CYP2D6 gene polymorphism), acquired increased sensitivity to opioids, and pharmacokinetic and pharmacodynamic interactions with other respiratory depressant agents (e.g., combining opioids and sedatives) [6]. Other patient populations who are potentially at high risk for OIVI are the elderly, morbidly obese, and patients who suffer from sleep-disordered breathing and neuromuscular conditions. Preoperative evaluation should include adequate characterization of these comorbid or high-risk conditions, so as to devise a safety-enhancing anesthetic plan.
Although critical events related to OIVI are rare, the incidence of hypoxemia associated with opioid-based analgesia is high, ranging from 12.5 to 20% [7–9]. A recent prospective observational study reveals that desaturations are frequent, and in some cases, persistent, in the first 48 h postoperatively in a general surgical population. In the first 48 h after noncardiac surgery, 21% of patients spent on average 10 min/h or more with oxygen saturation (SpO2) <90% and 11% experienced at least one episode of SpO2 <90% lasting 6 h or more [10]. The proportion of patients with sleep apnea was not reported in these studies. However, patients with pre-existing OSA may be more susceptible to the respiratory depressant effects of opioids. In a recent retrospective analysis of 87 000 surgical patients, Ramachandran and colleagues reported 32 life-threatening postoperative respiratory events that were preceded by deep sedation or unresponsiveness during opioid-based analgesic therapy [11]. In this cohort, OSA was diagnosed in one-third of patients and in 50% of patients who died. A recent American Society of Anesthesiologists’ closed claims analysis examined 341 deaths or ischemic brain injury in the context of receiving opioid analgesia in the immediate postoperative period. Forty percent of these cases had an established diagnosis of, or were at high risk for OSA [12▪], and acute renal and congestive heart failure were also highly prevalent, implicating impaired opioid clearance and global organ dysfunction as a precipitating condition for OIVI.
In an observational cohort of noncardiac surgery patients, the severity of postoperative sleep-disordered breathing in patients suffering from OSA was significantly associated with the total opioid dose administered for postoperative analgesia. Interestingly, preoperative apnea/hypopnea index, patient age, and 72-h opioid dose were all significant predictors of increased postoperative apnea/hypopnea index compared with preoperative baseline, in both OSA and non-OSA patients [13▪▪], suggesting that surgical patients suffering from severe OSA and the elderly might be more vulnerable to OIVI.
APNEA MECHANISMS AND OPIOID EFFECTS IN OBSTRUCTIVE SLEEP APNEA
OSA is a disorder of ventilatory control [14,15]. As a consequence, OSA severity (as measured by the frequency of apnea/hypopnea events during sleep) is largely determined by the type and effectiveness of compensatory mechanisms that are engaged in response to airway obstruction, rather than by the narrowing of the pharyngeal airway, per se[16].
Pharyngeal dilator muscles receive input from at least three different types of sources: central respiratory drive (i.e., rising arterial partial pressure of carbon dioxide (PaCO2) and declining arterial partial pressure of oxygen (PaO2)), local negative airway pressure during inspiration (negative pressure reflex), and wakefulness drive [15,17]. In OSA patients, the anatomically compromised airway [18], compounded with a physiologically diminished pharyngeal dilator activity during sleep, undergoes repetitive, partial, or complete occlusion as a result of the negative inspiratory pressure exerted by the diaphragm [19,20]. Airway obstruction is followed by a gradual rise in the contracting force of pharyngeal dilators as a result of both the negative airway pressure and the rising chemical respiratory drive (i.e., because of rising PaCO2 and declining PaO2) with the essential endpoint to restore airway patency. When the rising chemical drive reaches a certain level that can effectively recruit pharyngeal dilators and open the airway (effective recruitment threshold) [21,22], airway patency is restored. Although arousal from sleep (cortical arousal) also assists toward that end, by reinstating wakefulness drive [15,22–24], cortical arousals can further destabilize ventilatory control and promote apneas through the enhancement of postobstruction hyperventilation [25]. Depending on the operation and interaction between all the above features of ventilatory control, including chemoreflex sensitivity and arousal threshold, prediction of the exact pattern and frequency of airway obstruction in individual OSA patients becomes a difficult task, which may further complicate effective management of the disease [22,26,27].
Therapies like the administration of oxygen [28] and sedatives [29,30] may stabilize ventilatory control and benefit OSA patients with increased chemosensory sensitivity and low arousal thresholds, whereas the same therapeutic measures could prolong the duration of airway obstruction, potentially leading to severe hypoxemia, in patients with decreased ventilatory responses to hypoxia/hypercapnia and high arousal thresholds [31,32▪]. Although this latter group of patients represents a minority among OSA populations [32▪], they might be at a greater risk for opioid-related respiratory events in the postoperative period because they rely heavily on arousal to restore adequate airflow and oxygenation. Opioids, by inhibiting chemical, behavioral, and motor control of respiration [17,33], could further raise arousal thresholds, prolong airway obstruction, and precipitate hypoxemia. The observation that fatal outcomes were more likely to occur during night-time in patients who were difficult to arouse [11], although not indicating a direct association with OSA, reinforces the belief that OSA patients with high arousal thresholds, longer obstructive events, and potentially larger arterial desaturations may demonstrate a lower reserve to withstand a serious respiratory event in the postoperative period than patients with a different disease phenotype.
Conversely, in the majority of OSA patients, where frequent obstructive episodes and mild-to-moderate hypoxemia prevail, the sedative effect of opioids may stabilize airway patency and breathing [34]. Interestingly, this contrast between the two main disease traits (i.e., low versus high arousal thresholds associated with the respective pattern of mild versus severe recurrent hypoxemia) agrees with the observed inconsistency between the high prevalence of OSA in surgical populations and the very low incidence of opioid-related critical respiratory events in postoperative patients, that is, lending support to the hypothesis that only a small subset of OSA patients are at increased risk for opioid-induced ventilatory compromise. It thus becomes mandatory that epidemiological as well as mechanism-based research efforts that are focusing on predicting risk for OSA (e.g., STOP-Bang questionnaire [35]) should also aim at identifying OSA patients who are at potentially higher risk for OIVI than others.
PAIN-RELATED BEHAVIOR, INTERMITTENT HYPOXIA, AND OPIOID EFFECTS
Experimental and clinical evidence suggest that two distinct pathophysiological components of OSA, namely sleep disruption and nocturnal intermittent hypoxemia, could enhance pain either acting directly or via activating complex inflammatory pathways. Sleep deprivation and/or sleep fragmentation enhance sensitivity to experimental pain and increase spontaneous pain complaints in volunteers, possibly by increasing the expression of hyperalgesic inflammatory mediators [36–39] or by acting on central pain-modulatory networks [40–42]. Conforming to these findings, patients suffering from insomnia [43] and temporomandibular joint disorder patients with primary insomnia [44] demonstrate hyperalgesia, whereas insomnia symptoms [45] and degraded sleep quality [46] predicted daily intensity [46] and chronicity [45] of pain in hospitalized burn patients.
Consistent with these observations, treatment of OSA with continuous positive airway pressure, that presumably restores sleep continuity and/or normal breathing, decreased the sensitivity to painful stimuli in adults [47]. However, in contrast with the main concept that sleep disruption enhances pain, temporomandibular joint disorder patients with OSA, a condition characterized by fragmented sleep, presented with hypoalgesia to experimental pain [44], suggesting that sleep fragmentation and recurrent nocturnal hypoxemia, both existing in OSA, may exert competing effects on pain. In an attempt to shed light on this issue, a recent retrospective analysis of the Cleveland Family Study (a longitudinal cohort designed to evaluate familial aggregation of OSA) showed that intermittent hypoxia significantly increased pain reporting from patients suffering from OSA, independently of the effects of sleep fragmentation and systemic inflammation [48▪]. More specifically, a decrease in the nocturnal nadir oxyhemoglobin saturation (SaO2) from 92 to 75% approximately doubled the odds for reporting pain in this population, suggesting that recurrent nocturnal hypoxemia in patients with OSA could enhance pain.
Hypoxemia and tissue hypoxia might also play a role in determining sensitivity to the effect of opioids. Children living at high altitude under conditions of chronic sustained hypoxia (i.e., resting oxyhemoglobin saturation of 92%) consumed 40% less fentanyl perioperatively compared with children living at sea level [49]. In the context of intermittent hypoxia, a retrospective cohort of 46 children undergoing adenotonsillectomy for OSA has shown that recurrent nocturnal hypoxemia was associated with lower opioid consumption in the perioperative period [50]. Consistent with the results of this analysis, a prospective study in a similar pediatric population (N = 22) demonstrated that children with a nocturnal nadir SaO2 <85% required half of the total dose of morphine required to treat postadenotonsillectomy pain in children with a nadir SaO2 ≥85% [3]. These findings were supported by independent experiments showing that intermittent hypoxia upregulated μ-opioid receptors in the developing rat [51,52] and hence, it might be responsible for an increased sensitivity to the analgesic and respiratory effects of opioids [53,54]. Although these findings were not confirmed by larger prospective assessments in children undergoing adenotonsillectomy [55,56], methodological differences in trial design do not facilitate direct comparisons between these investigations. On the other hand, a recent application of an experimental pain paradigm in adult volunteers suffering from OSA has shown that both nocturnal nadir SaO2 and insulin-like growth factor-binding protein 1, a serum marker of hypoxia [57], were significantly associated with increased sensitivity to the analgesic effect of remifentanil, a short-acting opioid [58▪]. Along these lines, a recent retrospective analysis of 218 obese adults with OSA, who underwent bariatric surgery, demonstrated that the percentage of total sleep time spent at SaO2 <90% was inversely associated with total postoperative opioid consumption (i.e., a 5% absolute increase in the former would relatively decrease median opioid consumption by 16%) [59▪]. Table 1 summarizes the experimental and clinical evidence supporting the association between OSA and/or intermittent hypoxia with pain and sensitivity with the opioid analgesic effect.
Table 1.
Literature evidence for the associations between intermittent hypoxia and pain perception and/or opioid analgesic effect
| Investigations | Exposure | Outcome | |
| Pain perception | Opioid analgesic effect | ||
| Experimental | |||
| Smith 2009 [44] | OSA | Decreased | – |
| Khalid 2011 [47] | OSA | Increased | – |
| Doufas 2013 [58■] | Nadir SaO2 | – | Increased |
| Prospective | |||
| Brown 2006 [3] | Nadir SaO2 | – | Increased |
| Sadhasivam 2012 [55] | OSA | Increased | Decreased |
| Sanders 2006 [56] | RDI | – | Decreased |
| Retrospective | |||
| Brown 2004 [50] | Nadir SaO2 | Increased | |
| Doufas 2013 [48■] | Nadir SaO2 | Increased | – |
| Turan 2015 [59■] | Time at SaO2 <90% | – | Increased |
OSA, obstructive sleep apnea; RDI, respiratory disturbance index; SaO2, oxyhemoglobin saturation.
OSA is a chronic inflammatory state and intermittent hypoxia seems to be causally responsible (at least partially) for this outcome through a complex scheme of positive interactions between upregulation of hypoxia-inducible factor 1 alpha and increased production of reactive oxygen species by mitochondria [60,61]. The inflammatory products of these reactions, such as IL-6, IL-1β, and TNFα have been shown to be both hyperalgesic [62–64] and also potentiating the analgesic effect of opioids [58▪]. Although the molecular basis for the effect of intermittent hypoxia on opioid sensitivity is less clear, recent experimental evidence suggests that the two, seemingly contrasting, phenotypes of increased pain and enhanced opioid potency, as emerging consequences of intermittent hypoxia, are not mutually exclusive. More specifically, using an experimental pain paradigm in volunteers, Bruehl and colleagues [65] have recently demonstrated a significant inverse association between endogenous opioid function and morphine analgesic response; low endogenous opioid activity was partially responsible for the observed association between higher evoked pain sensitivity in the placebo condition and greater morphine analgesic response. This is an interesting finding, especially when combined with previous evidence showing decreased beta-endorphin-like (a major endogenous opioid peptide) activity in the cerebrospinal fluid of patients with OSA compared with controls [66].
OPIOIDS AND SLEEP
Opioids impair basic sleep–wake mechanisms [67] by inhibiting central cholinergic [68,69] and adenosinergic [70,71] transmission. These neurochemical effects result in inhibition of rapid eye movement (REM) sleep [72], overall sleep disruption, and decreased sleep consolidation [73], which in turn can promote sleepiness and hyperalgesia in humans [74]. These effects of opioids, by narrowing the safety margin between effective analgesia and ventilatory compromise, may have implications on the severity of OIVI.
Although the effect of opioids on sleep has not been formally assessed in surgical patients with OSA, continuous infusion of a short-acting opioid during sleep in volunteers with moderate OSA resulted in decreased REM and slow wave (restorative) sleep [75]. The obstructive episodes decreased, whereas central apneas increased during sleep, a finding, which may be related to the REM-suppressing effect of the opioid. In certain OSA patients who demonstrate REM-predominant apnea/hypopnea events, the obstructive events may recur with increased frequency and severity during an intense REM sleep rebound after the third postoperative night [76,77]; nonetheless, the clinical impact of this phenomenon is yet to be shown [78]. Characteristically, in a recent cohort of 58 noncardiac surgery patients who underwent preoperative polysomnography, Chung and colleagues have demonstrated severe sleep disturbances, including decreased slow wave and REM sleep in the first postoperative night, for both OSA (N = 38) and non-OSA (N = 20) patients, followed by a gradual recovery of normal sleep in the subsequent days. Although OSA status did not influence postoperative sleep disturbances, patients with OSA presented with increased apnea and more arterial desaturation on the third postoperative night [79▪]. This latter finding may be confounded by the weaning of supplemental oxygen on the third postoperative day, since the results of a recent closed claims analysis of fatal and life threatening opioid induced respiratory events found the first 24 h postoperatively represented the highest risk period for such morbidity [12▪].
CONCLUSION
Both intermittent hypoxia and sleep disruption enhance pain, and intermittent hypoxia may also potentiate opioid analgesic responses by activating major inflammatory pathways. Recent evidence shows these seemingly contrasting phenotypes of pain increasing and opioid-enhancing effects of intermittent hypoxia are not mutually exclusive. The determination of the various factors affecting pain and/or opioid analgesia in OSA patients would enhance our ability to predict opioid pharmacology and improve perioperative safety in this population. As a result of the immense variability in OSA phenotypes and pain/analgesia responses in humans, large prospective investigations for the effect of the various pathophysiological traits of OSA on postoperative pain and opioid analgesia are necessary.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Doufas AG. Obstructive sleep apnea, pain, and opioid analgesia in the postoperative patient. Curr Anesthesiol Rep 2014; 4:1–9. [Google Scholar]; The comprehensive review of the interactions between OSA, pain, and opioid analgesia in the postoperative setting including a discussion on strategies to mitigate opioid-induced ventilatory impairment.
- 2.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KA, Laferrière A, Lakheeram I, Moss IR. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology 2006; 105:665–669. [DOI] [PubMed] [Google Scholar]
- 4.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth 2004; 93:212–223. [DOI] [PubMed] [Google Scholar]
- 5.Overdyk F, Dahan A, Roozekrans M, et al. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag 2014; 4:317–325. [DOI] [PubMed] [Google Scholar]
- 6.Dahan A, Overdyk F, Smith T, et al. Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain physician 2013; 16:E85–E94. [PubMed] [Google Scholar]
- 7.Jayr C, Thomas H, Rey A, et al. Postoperative pulmonary complications. Epidural analgesia using bupivacaine and opioids versus parenteral opioids. Anesthesiology 1993; 78:666–676. [DOI] [PubMed] [Google Scholar]
- 8.Tsui SL, Lo RJ, Tong WN, et al. A clinical audit for postoperative pain control on 1443 surgical patients. Acta Anaesthesiol Sin 1995; 33:137–148. [PubMed] [Google Scholar]
- 9.Wheatley RG, Somerville ID, Sapsford DJ, Jones JG. Postoperative hypoxaemia: comparison of extradural, i.m. and patient-controlled opioid analgesia. Br J Anaesth 1990; 64:267–275. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015; 121:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran SK, Haider N, Saran KA, et al. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth 2011; 23:207–213. [DOI] [PubMed] [Google Scholar]
- 12▪.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015; 122:659–665. [DOI] [PubMed] [Google Scholar]; The authors examined 92 closed malpractice claims associated with respiratory depression over 10 years. Their findings that the majority of critical events happen within 24 h of surgery have implications in postoperative monitoring of high-risk patients.
- 13▪▪.Chung F, Liao P, Elsaid H, et al. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology 2014; 120:299–311. [DOI] [PubMed] [Google Scholar]; The prospective observational study is the largest cohort of polysomnographic studies performed on perioperative elective surgical patients. In 376 adults they found postoperative opioid dose to be associated with worsening sleep disordered breathing.
- 14.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010; 90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White DP, Younes MK. Obstructive sleep apnea. Compr Physiol 2012; 2:2541–2594. [DOI] [PubMed] [Google Scholar]
- 16.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 2003; 168:645–658. [DOI] [PubMed] [Google Scholar]
- 17.Koo CY, Eikermann M. Respiratory effects of opioids in perioperative medicine. Open Anesthesiol J 2011; 5 (Suppl 1-M6):23–34. [Google Scholar]
- 18.Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol (1985) 1997; 82:1319–1326. [DOI] [PubMed] [Google Scholar]
- 19.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 1996; 19:827–853. [DOI] [PubMed] [Google Scholar]
- 20.Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implication. J Appl Physiol (1985) 2014; 116:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younes M, Ostrowski M, Atkar R, et al. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol (1985) 2007; 103:1929–1941. [DOI] [PubMed] [Google Scholar]
- 22.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol 2008; 105:1389–1405. [DOI] [PubMed] [Google Scholar]
- 23.Loewen AH, Ostrowski M, Laprairie J, et al. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep 2011; 34:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younes M, Loewen AH, Ostrowski M, et al. Genioglossus activity available via nonarousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol (1985) 2012; 112:249–258. [DOI] [PubMed] [Google Scholar]
- 25.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004; 169:623–633. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Marshall NS, Duffin J, et al. Phenotyping interindividual variability in obstructive sleep apnoea response to temazepam using ventilatory chemoreflexes during wakefulness. J Sleep Res 2011; 20:526–532. [DOI] [PubMed] [Google Scholar]
- 27.Smith I, Lasserson TJ, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2013; 5:CD003002. [DOI] [PubMed] [Google Scholar]
- 28.Mokhlesi B, Tulaimat A, Parthasarathy S. Oxygen for obesity hypoventilation syndrome: a double-edged sword? Chest 2011; 139:975–977. [DOI] [PubMed] [Google Scholar]
- 29.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011; 120:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eikermann M, Fassbender P, Zaremba S, et al. Pentobarbital dose-dependently increases respiratory genioglossus muscle activity while impairing diaphragmatic function in anesthetized rats. Anesthesiology 2009; 110:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudgel DW, Hendricks C, Dadley A. Alteration in obstructive apnea pattern induced by changes in oxygen- and carbon-dioxide-inspired concentrations. Am Rev Respir Dis 1988; 138:16–19. [DOI] [PubMed] [Google Scholar]
- 32▪.Eckert DJ, Younes MK. Arousal from Sleep: Implications for Obstructive Sleep Apnea Pathogenesis and Treatment. J Appl Physiol (1985) 2014; 116:302–313. [DOI] [PubMed] [Google Scholar]; A review of the current pathophysiologic mechanism of cortical arousal in OSA, and the potential for high versus low arousal thresholds to restore normoxia or destabilize sleep. Describes the role of sedatives in specific subtypes of OSA patients to promote stable sleep.
- 33.Stuth EA, Stucke AG, Zuperku EJ. Effects of anesthetics, sedatives, and opioids on ventilatory control. Compr Physiol 2012; 2:2281–2367. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Somogyi AA, Yee BJ, et al. The effects of a single mild dose of morphine on chemoreflexes and breathing in obstructive sleep apnea. Respir Physiol Neurobiol 2013; 185:526–532. [DOI] [PubMed] [Google Scholar]
- 35.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821. [DOI] [PubMed] [Google Scholar]
- 36.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain 2009; 145:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haack M, Pollmächer T, Mullington JM. Diurnal and sleep-wake dependent variations of soluble TNF- and IL-2 receptors in healthy volunteers. Brain Behav Immun 2004; 18:361–367. [DOI] [PubMed] [Google Scholar]
- 38.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007; 30:1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med 2006; 166:1756–1762. [DOI] [PubMed] [Google Scholar]
- 40.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 2007; 30:494–505. [DOI] [PubMed] [Google Scholar]
- 41.Edwards RR, Grace E, Peterson S, et al. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain 2009; 13:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards RR, Quartana PJ, Allen RP, et al. Alterations in pain responses in treated and untreated patients with restless legs syndrome: associations with sleep disruption. Sleep Med 2011; 12:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haack M, Scott-Sutherland J, Santangelo G, et al. Pain sensitivity and modulation in primary insomnia. Eur J Pain 2012; 16:522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MT, Wickwire EM, Grace EG, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep 2009; 32:779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MT, Klick B, Kozachik S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain 2008; 138:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raymond I, Nielsen TA, Lavigne G, et al. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain 2001; 92:381–388. [DOI] [PubMed] [Google Scholar]
- 47.Khalid I, Roehrs TA, Hudgel DW, Roth T. Continuous positive airway pressure in severe obstructive sleep apnea reduces pain sensitivity. Sleep 2011; 34:1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Doufas AG, Tian L, Davies MF, Warby SC. Nocturnal intermittent hypoxia is independently associated with pain in subjects suffering from sleep-disordered breathing. Anesthesiology 2013; 119:1149–1162. [DOI] [PubMed] [Google Scholar]; The large cohort study of 634 individuals in the Cleveland Family Study, a study of the genetic link in OSA patients, found nocturnal desaturation had an independent association with increased pain, after adjusting for patient characteristics and sleep fragmentation.
- 49.Rabbitts JA, Groenewald CB, Dietz NM, et al. Perioperative opioid requirements are decreased in hypoxic children living at altitude. Paediatr Anaesth 2010; 20:1078–1083. [DOI] [PubMed] [Google Scholar]
- 50.Brown KA, Laferrière A, Moss IR. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology 2004; 100:806–810. [DOI] [PubMed] [Google Scholar]
- 51.Laferrière A, Liu JK, Moss IR. Neurokinin-1 versus mu-opioid receptor binding in rat nucleus tractus solitarius after single and recurrent intermittent hypoxia. Brain Res Bull 2003; 59:307–313. [DOI] [PubMed] [Google Scholar]
- 52.Moss IR, Laferrière A. Central neuropeptide systems and respiratory control during development. Respir Physiol Neurobiol 2002; 131:15–27. [DOI] [PubMed] [Google Scholar]
- 53.Brown KA. Intermittent hypoxia and the practice of anesthesia. Anesthesiology 2009; 110:922–927. [DOI] [PubMed] [Google Scholar]
- 54.Moss IR, Brown KA, Laferrière A. Recurrent hypoxia in rats during development increases subsequent respiratory sensitivity to fentanyl. Anesthesiology 2006; 105:715–718. [DOI] [PubMed] [Google Scholar]
- 55.Sadhasivam S, Chidambaran V, Ngamprasertwong P, et al. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics 2012; 129:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders JC, King MA, Mitchell RB, Kelly JP. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg 2006; 103:1115–1121. [DOI] [PubMed] [Google Scholar]
- 57.Kajimura S, Aida K, Duan C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc Natl Acad Sci U S A 2005; 102:1240–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪.Doufas AG, Tian L, Padrez KA, et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One 2013; 8:e54807. [DOI] [PMC free article] [PubMed] [Google Scholar]; The experimental study provides evidence for increased potency of opioids in OSA patients. There was an association with lower nadir oxygen saturation and higher serum markers of hypoxia with higher analgesic sensitivity to remifentanil infusion.
- 59▪.Turan A, You J, Egan C, et al. Chronic intermittent hypoxia is independently associated with reduced postoperative opioid consumption in bariatric patients suffering from sleep-disordered breathing. PLoS One 2015; 10:e0127809. [DOI] [PMC free article] [PubMed] [Google Scholar]; The retrospective study adds to the growing body of evidence describing increased opioid sensitivity in patients with intermittent hypoxia.
- 60.Guzy RD, Hoyos B, Robin E, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 2005; 1:401–408. [DOI] [PubMed] [Google Scholar]
- 61.Lavie L. Obstructive sleep apnoea syndrome: an oxidative stress disorder. Sleep Med Rev 2003; 7:35–51. [DOI] [PubMed] [Google Scholar]
- 62.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008; 28:5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010; 16:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruehl S, Burns JW, Gupta R, et al. Endogenous opioid function mediates the association between laboratory-evoked pain sensitivity and morphine analgesic responses. Pain 2013; 154:1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gislason T, Almqvist M, Boman G, et al. Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest 1989; 96:250–254. [DOI] [PubMed] [Google Scholar]
- 67.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 2005; 103:1268–1295. [DOI] [PubMed] [Google Scholar]
- 68.Lydic R, Keifer JC, Baghdoyan HA, Becker L. Microdialysis of the pontine reticular formation reveals inhibition of acetylcholine release by morphine. Anesthesiology 1993; 79:1003–1012. [DOI] [PubMed] [Google Scholar]
- 69.Mortazavi S, Thompson J, Baghdoyan HA, Lydic R. Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal. Anesthesiology 1999; 90:1070–1077. [DOI] [PubMed] [Google Scholar]
- 70.Nelson AM, Battersby AS, Baghdoyan HA, Lydic R. Opioid-induced decreases in rat brain adenosine levels are reversed by inhibiting adenosine deaminase. Anesthesiology 2009; 111:1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu M, Sahbaie P, Zheng M, et al. Opiate-induced changes in brain adenosine levels and narcotic drug responses. Neuroscience 2013; 228:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cronin A, Keifer JC, Baghdoyan HA, Lydic R. Opioid inhibition of rapid eye movement sleep by a specific mu receptor agonist. Br J Anaesth 1995; 74:188–192. [DOI] [PubMed] [Google Scholar]
- 73.Cronin AJ, Keifer JC, Davies MF, et al. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep 2001; 24:39–44. [DOI] [PubMed] [Google Scholar]
- 74.Moore JT, Kelz MB. Opiates, sleep, and pain: the adenosinergic link. Anesthesiology 2009; 111:1175–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernards CM, Knowlton SL, Schmidt DF, et al. Respiratory and sleep effects of remifentanil in volunteers with moderate obstructive sleep apnea. Anesthesiology 2009; 110:41–49. [DOI] [PubMed] [Google Scholar]
- 76.Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology 1990; 73:52–61. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg J, Wildschiødtz G, Pedersen MH, et al. Late postoperative nocturnal episodic hypoxaemia and associated sleep pattern. Br J Anaesth 1994; 72:145–150. [DOI] [PubMed] [Google Scholar]
- 78.Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care 2011; 39:545–558. [DOI] [PubMed] [Google Scholar]
- 79▪.Chung F, Liao P, Yegneswaran B, et al. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology 2014; 120:287–298. [DOI] [PubMed] [Google Scholar]; In this prospective cohort study, the authors demonstrate significant sleep disturbances in both OSA and non-OSA patients after surgery. These findings reveal the pattern, timing, and severity of sleep-disordered breathing and hypoxia in patients with OSA.


