Abstract
Objective
This study aimed to compare the clinical response at 36 months and evaluate the adverse events in a cohort of patients with rheumatoid arthritis treated with etanercept, infliximab, or adalimumab.
Methods
An observational retrospective cohort study was performed. Patients older than 18 years with active rheumatoid arthritis, for which the physician had initiated a treatment scheme with etanercept, infliximab, or adalimumab, were included in the study. The follow-up was conducted through at least trimestral evaluations during the course of 36 months. Outcomes evaluated included Disease Activity Score 28, level of disease activity, Health Assessment Questionnaire, and degree of disability.
Results
Three hundred seven subjects were included in the cohort (108 adalimumab, 107 infliximab, and 92 etanercept). The median Disease Activity Score 28 at the onset was 4.1 and 2.39 at month 36. There were no differences among the 3 medications (P = 0.51). The remission rate was of 7.4 per 100 people per month (95% confidence interval [CI], 6.6–8.3) without differences between groups. The initial Health Assessment Questionnaire median was 1.75 and 0.25 at 36 months. No differences per medicine were found (P = 0.54). The most common adverse effect was dermatitis. Eighteen cases of serious adverse effects occurred, including 11 cases of serious infectious events. The adverse events rates were as follows: infliximab, 24 per 100 people per year (95% CI, 19–29); adalimumab, 22 per 100 people per year (95% CI, 18–27); and etanercept, 12 per 100 people per year (95% CI, 8–16).
Conclusions
Etanercept, infliximab, and adalimumab are 3 effective therapeutic anti–tumor necrosis factor alternatives to reduce the level of severity and the degree of disability generated by rheumatoid arthritis. Etanercept presented a rate of adverse events lower than those for infliximab and adalimumab.
Key Words: arthritis, Adalimumab, Etanercept, Infliximab, Tumor Necrosis Factor-alpha and Comparative Effectiveness Research, drug-related side effects and adverse reactions in this cohort
Treatments for rheumatoid arthritis management seek to control the inflammatory process and limit joint damage, maintaining or improving the functional state and the quality of life of those who experience this condition.1,2 Throughout the years, drugs capable of controlling and stopping joint destruction have been developed; these have been described as disease-modifying antirheumatic drugs (DMARDs).3 Despite the effectiveness of DMARDs, some patients do not respond or experience serious adverse events (SAEs) that force them to suspend the therapy.4–6 This situation has given place to the development of a new generation of drugs that inhibit the tumor necrosis factor α (TNF-α) cytokine, which plays an important role in joint inflammation and destruction and that have demonstrated being effective in disease control.6 These drugs are known as biological therapy or biological DMARDs and promise a high effectiveness but without being exempt from presenting adverse events.7,8 This therapeutic class includes etanercept, infliximab, and adalimumab, among others.6 These are molecules capable of linking to the TNF-α receptor, inhibiting its action. These drugs are generally prescribed when DMARDs such as methotrexate alone or in combination have not presented favorable results.7
Most studies that demonstrated the efficacy and safety of biological DMARDs have been controlled clinical trials, which may overestimate their effect, because of their being performed in controlled conditions under which patients adjust to strict administration regimens based on protocols.6,7,9,10 Despite strengths in bias control, it is probable that in real-life settings, these drugs present different results in terms of disease control. The DANBIO study is a clear sample of the impact that systematic registry may have in terms of evidence generated in the context of real application, in which, despite evidence regarding potential selection biases, it is clear that its results reflect with greater certainty the effects of interventions in daily life.11 Thus, a study with conditions similar to those in real life was performed, to assess the clinical response at 36 months and to record the occurrence of adverse events in a cohort of patients with rheumatoid arthritis treated with etanercept, infliximab, or adalimumab.
MATERIALS AND METHODS
Study Design, Patients, and Outcomes
An observational retrospective cohort study was performed in a Colombian reference in rheumatoid arthritis center, including patients older than 18 years with an active rheumatoid arthritis (from moderate-to-high disease activity) despite being under treatment with conventional DMARDs and for whom a treatment scheme was begun with etanercept 25 mg subcutaneous twice per week (Etanar), infliximab 3 mg/kg intravenous at weeks 0, 2, and 6 and then every 8 weeks (Remicade), or adalimumab 40 mg subcutaneous every 2 weeks (Humira).
Treatment selection depended strictly on the treating physician's criteria, who in a real-life setting determined the best option for his or her patient, taking into account, besides the clinical condition, the presence of geographical barriers to transportation, the possibility of greater adherence to less frequent doses, and the learning ability for self-application among others. Follow-up was performed over 36 months, with at least trimestral evaluations. Outcomes evaluated included Disease Activity Score 28 (DAS28) based on erythrocyte sedimentation rate for remission and low disease activity,12,13 Health Assessment Questionnaire (HAQ), level of disability, and adverse events.
The safety evaluation included the search for signs or symptoms suggestive of adverse effects at the physical examination and the performance of biochemical and hematological tests. Adverse events were defined as those injuries that occurred during the study or when the severity or frequency of a preexisting injury increased during the study. An SAE was defined as an effect that caused death, constituted a threat to the patient's life, generated or prolonged hospitalization, was a cause for a surgical intervention, or produced disability, cancer, or an infection associated with death or hospitalization (serious infectious events [SIEs]).
Statistical Analyses
Analyses included the general description of clinical and demographic variables. The distribution of numerical variables was evaluated using the Shapiro-Wilk test. Because of the studied variables not presenting a normal distribution, nonparametric tests were chosen. To observe the behavior of DAS28 and HAQ, the difference between the initial and final evaluations for each subject was calculated, and the difference medians were compared for each drug, using Friedman nonparametric statistic, which is useful for the contrast of 3 or more groups in related samples. In addition, the median of DAS28 and HAQ during the established follow-up times were described. The behavior of the disease's activity level was described during follow-up based on the proposed values for DAS28 (remission, DAS28 < 2.6; low disease activity, DAS28 from 2.6 to 3.19; moderate disease activity, DAS28 from 3.2 to < 5.1; and high disease activity, DAS28 > 5.1). The degree of disability was classified based on HAQ data (<0.5, no disability; 0.5–0.99, mild disability; 1–1.99, moderate disability; ≥2, severe disability). The behavior of DAS28 was evaluated in each follow-up and was considered a remission when a value lower than 2.6 was obtained in a single visit, as proposed by Fransen et al.12
The remission rates were calculated for each group, including in the numerator the number of subjects who reached a DAS28 of less than 2.6 over the sum of persons per follow-up month. In addition, the relapse rate was calculated for each drug, taking into account the sum of subjects for whom after entering remission reactivation of the disease was documented in the follow-up (DAS28 ≥ 3.2).
The adverse event analysis included the description of the cases and the calculus of adverse events rate per 100 people per year of follow-up, the rate of suspension, and the rate of severe adverse events. Confidence intervals (CIs) of 95% were calculated for each rate. A P value of less than 5% was considered for hypothesis contrast.
Ethical Considerations
This study meets the international guidelines related to the recommendations for research with human beings set forth in the Nuremberg Code, the Helsinki Declaration (latest revision Brazil 2013), and the Belmont Report; likewise, it follows the recommendations raised in the Resolution 8430 of 1993 of the Colombian Health Ministry. It was approved by an independent ethics committee, and each patient was granted his or her consent for the use of their information.
RESULTS
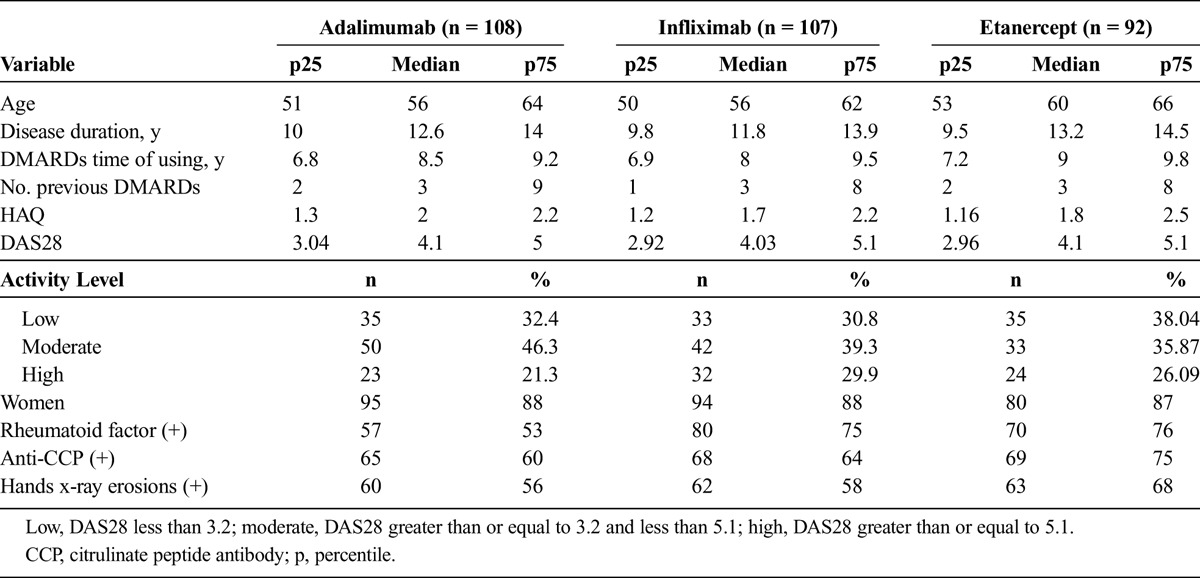
This cohort included 307 subjects, of whom 108 were treated with adalimumab, 107 with infliximab, and 92 with etanercept. One hundred percent of the patients completed the 12-month follow-up, 97% (297/307) of the patients reached 24 months of follow-up, and 95% (292/307) remained at the 36-month follow-up. Table 1 presents the baseline characteristics of patients. In all cases, conventional DMARDs had been used with the usual doses (methotrexate until 20 mg/wk, sulphazalasine until 3 g/d, chloroquine 250 mg/d, and prednisolone until 7.5 mg/d). The median number of conventional DMARDs previously used was of 3 for all 3 groups.
TABLE 1.
General Population Characteristics at the Cohort Onset
Follow-up to Clinical Outcomes
At the beginning of the cohort, the DAS28 median was 4.1, and it reached 2.39 at the end of the follow-up (month 36). The median difference between the baseline DAS28 and the final DAS28 was of 1.16 for infliximab, 1.31 for adalimumab, and of 1.38 for etanercept. No differences were established among the 3 drugs (P = 0.51). Figure 1 shows the comparative curve of the 3 drugs, along with the DAS28 medians behavior along the follow-up.
FIGURE 1.
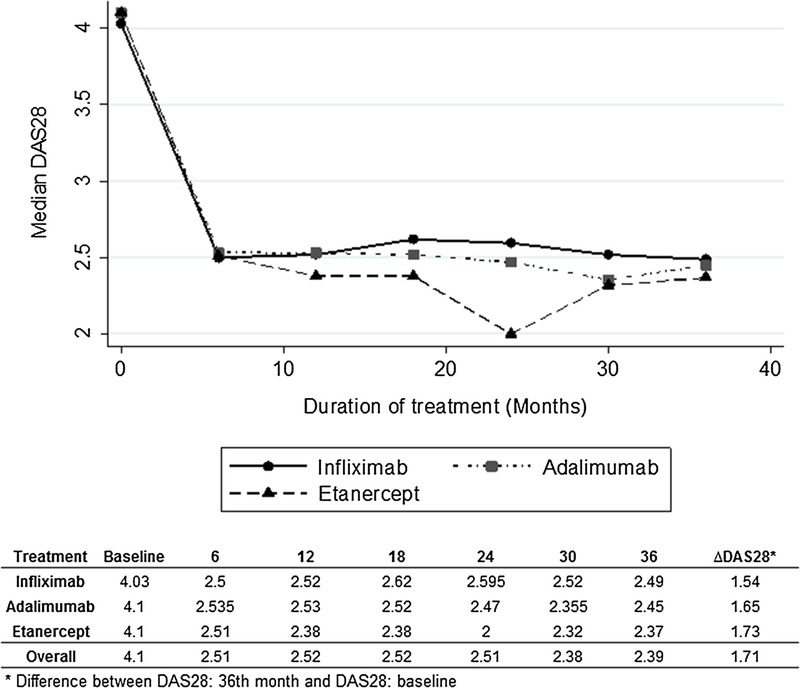
Median DAS28: follow-up during 36 months.
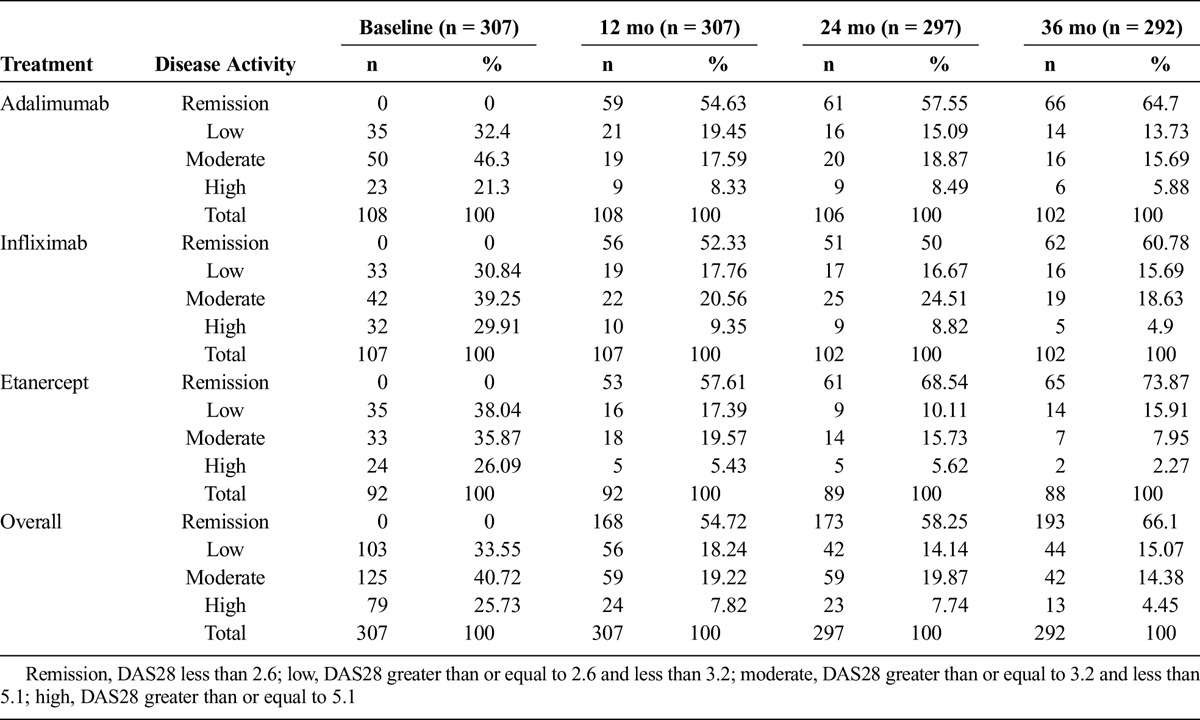
The results of disease activity level were analyzed based on the DAS28 results along the follow-up. A total of 25.7% of the patients entered with a disease level cataloged as high, whereas at month 36 of follow-up, only 4.45% were found in that state. Table 2 presents the behavior of the cohort exposed to each of the drugs, comparing the percentages of subjects in each of the disease activity levels during the 36 months.
TABLE 2.
Disease Activity Level Evolution During 36 Months by DAS28
A remission rate of 7.4 per 100 persons per month (95% CI, 6.6–8.3) was documented, which indicates that 7 new remission cases per month are expected for every 100 patients in treatment. The remission rate was similar for each of the 3 groups (infliximab, 7.3 per 100 persons per month; etanercept, 7.4 per 100 persons per month; adalimumab, 7.4 per 100 persons per month).
The relapse rate of patients who reached the state of remission was of 3.01 per 100 persons per month (95% CI, 2.6–3.6), which indicates that on average 3 new relapse cases are expected for every 100 patients in state of remission. In this case, the relapse rates per drug did not present differences (infliximab, 3.04 per 100 persons per month; etanercept, 3.1 per 100 persons per month; adalimumab, 3.08 per 100 persons per month).
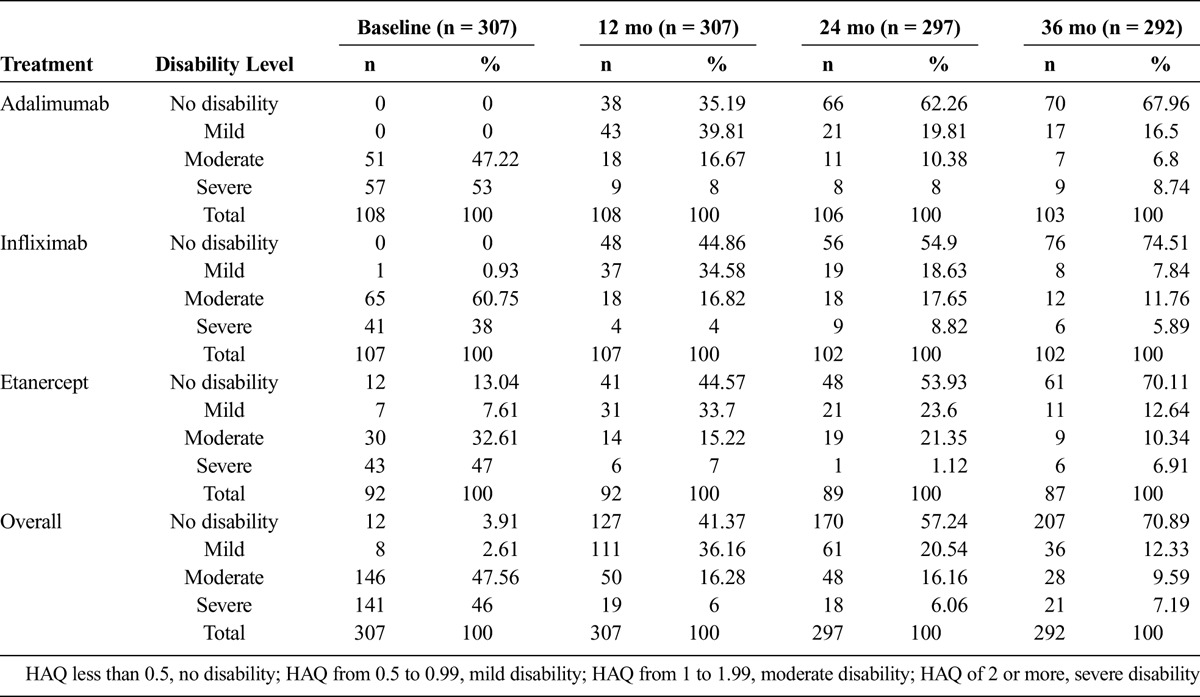
The HAQ median during baseline measuring was 1.75 points. At 36 months of follow-up, the median was 0.25. The median differences between baseline HAQ and final HAQ were 1.45 for infliximab, 1.75 for adalimumab, and 1.52 for etanercept (P = 0.54). Figure 2 presents the behavior of the HAQ median for each drug over follow-up. At the onset, 46% of the patients were in a severe level of disability, whereas at the third year of follow-up, only 7% were found at that level, and 71% reached a state of no disability. Table 3 presents the specific behavior for each drug, detailed by disability level and time of follow-up.
FIGURE 2.
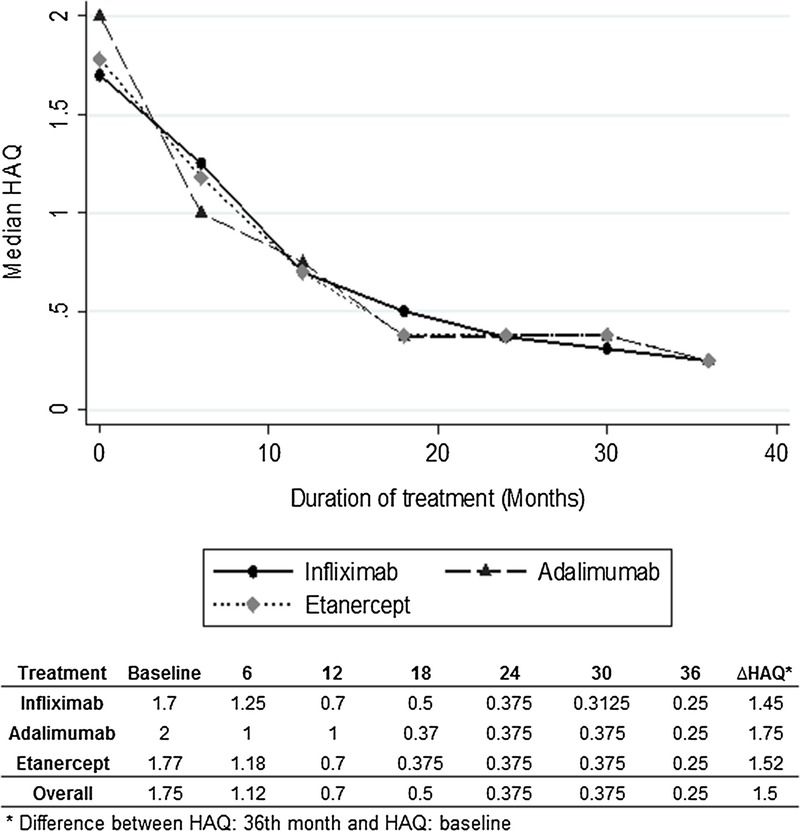
Median HAQ: follow-up during 36 months.
TABLE 3.
Disability Level During 36 Months of Follow-up by HAQ
Adverse Event Report
The most common adverse event was dermatitis, which occurred in 59 cases. The description of adverse events per drug is presented in Table 4. The adverse event rate was 20 per 100 persons per year (95% CI, 17–22). For the infliximab group, it was 24 events per 100 persons per year (95% CI, 19–29); in the adalimumab group, it was 22 per 100 persons per year (95% CI, 18–27); and in the etanercept group, it was 12 events per 100 persons per year (95% CI, 8–16).
TABLE 4.
Adverse Events Description
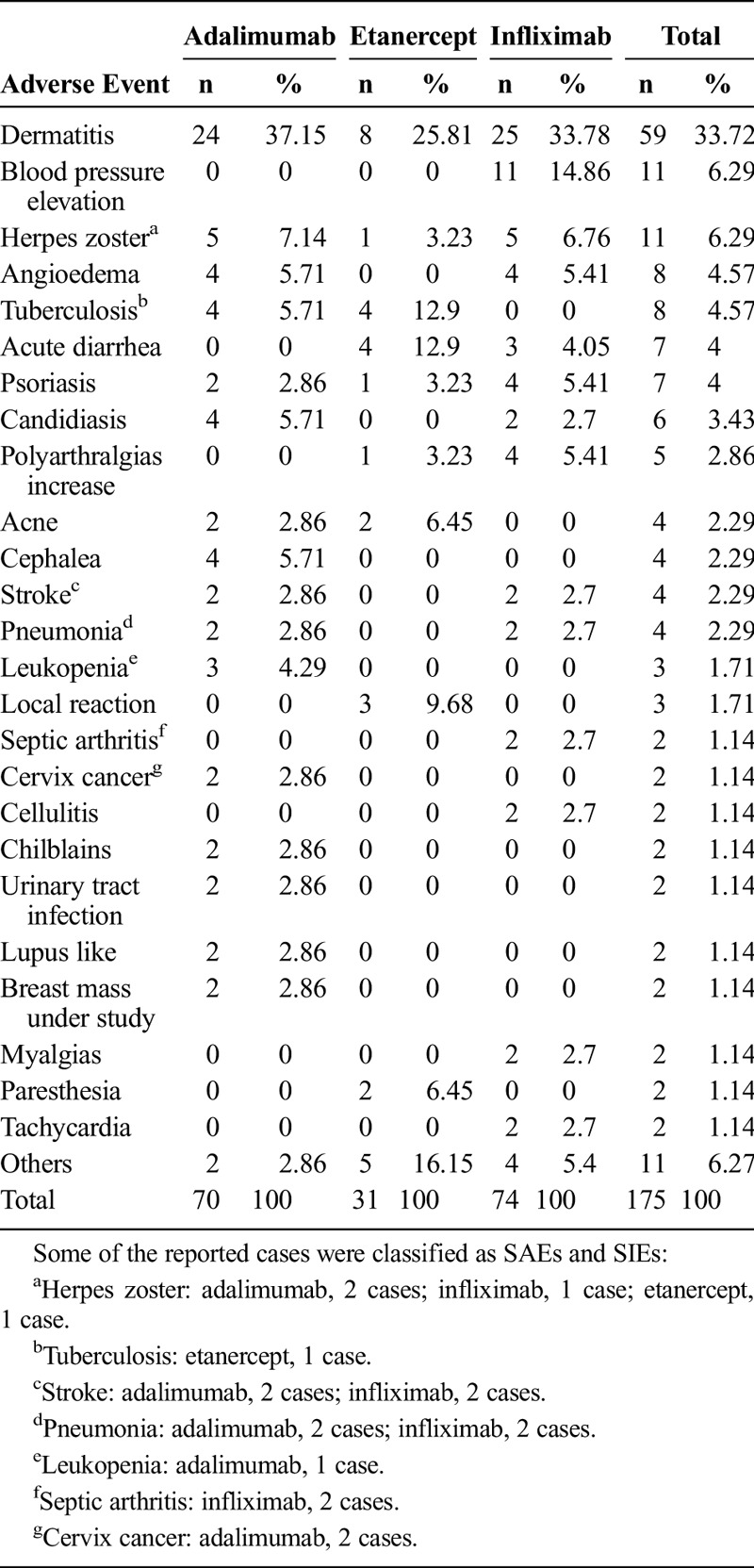
Eighteen SAEs were documented in the cohort, 11 of which were considered SIEs, including 4 cases of pneumonia (2 in the adalimumab group and 2 in the infliximab group), 2 cases of septic arthritis in the infliximab group, a case of tuberculosis in the etanercept group, and 4 cases of herpes zoster (2 in adalimumab, 1 in infliximab. and 1 in etanercept). In the noninfectious SAE, there were 4 cases of cerebrovascular accidents (2 in adalimumab and 2 in infliximab), a case of leukopenia in adalimumab, and 2 cases of cervical cancer in adalimumab (Table 4).
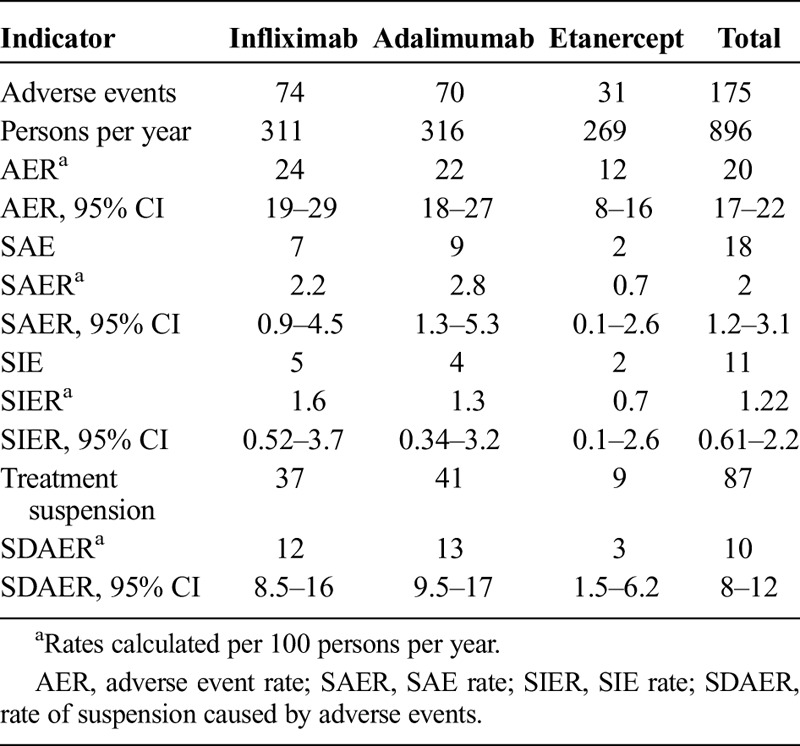
The adverse event per treatment rates, SAEs, and serious infectious events as well as the drug suspension rate due to adverse events are presented in Table 5.
TABLE 5.
Rates of Adverse Events During Follow-up
DISCUSSION
In the context of observational studies, different authors have reported effectiveness findings of biological anti-TNF drugs for the control of rheumatoid arthritis.9,14,15 This study's results, based on the follow-up to a group of patients in a real-life cohort, are consistent and demonstrate that etanercept, infliximab, and adalimumab are drugs capable of reducing the activity and disability level caused by rheumatoid arthritis.
The DAS28 results showed a marked reduction at the sixth month for the 3 drugs, and this evolution remained stable until the third year of follow-up. Despite not finding statistical difference between the DAS28 results of the 3 drugs, etanercept held a slightly higher reduction. The final gradient between the onset and the measuring at 36 months was 1.54 for infliximab, 1.65 for adalimumab, and 1.73 for etanercept. Studies that have compared the effectiveness of the 3 drugs based on real-life follow-up have not been able to establish important clinical among between the 3 drugs.6,16
In terms of remission rates, defined as DAS28 values less than 2.6,12,13 it is important to highlight that the highest percentage of cases that were in remission at month 36 was accomplished with the etanercept group; nevertheless, the results in terms of remission rates were similar in the 3 study groups. These findings are consistent with previous studies.6
The HAQ behavior results presented show how the patients' disability level presents a progressive improvement until month 18, after which it remains stable until month 36. This tendency was similar for the 3 studied molecules. Our findings are consistent with results presented by other authors such as those by Filippini et al,9 who described a marked improvement in the HAQ score during the first year of treatment with anti-TNF, followed by a plateau phase, which was maintained even after 60 months of follow-up.
In accordance with the discussed results, it is possible to propose that infliximab, adalimumab, and etanercept are 3 therapeutic alternatives that demonstrated effectiveness at reducing the level of disease activity and the degree of disability tied to it. These findings coincide with the results described by Chen et al6 in their systematic review, wherein they concluded that the 3 drugs are more effective than the placebo and where a slight superiority effect of etanercept over methotrexate was established.
The literature contains published studies that have evaluated the safety of infliximab, adalimumab, and etanercept as well as the incidence of related adverse events, SAEs, and SIEs. With regard to infliximab, it has been found to have a safety profile similar to that of methotrexate17 and that it can increase the risk of neoplasia and serious infectious diseases, specially pneumonia, cellulitis, sepsis, arthritis,18 and tuberculosis.19 These results coincide with the events reported in this study, where 2 cases of serious infections associated with pneumonia and a case of septic arthritis were reported.
Concerning adalimumab, the literature reports a higher rate of serious infections compared with a placebo, mainly associated with cases of tuberculosis and cellulitis.20,21 Our results reported the highest rate of serious adverse effects for adalimumab, where it is worth highlighting the occurrence of 2 cases of cervical cancer, although the rate of serious infections was less than for infliximab.
Regarding etanercept, reactions at the site of injection and the presence of arterial hypertension have been described as the most frequent adverse events, although they are generally light or moderate. When the incidence of serious or infectious adverse events has been studied, no difference from methotrexate has been determined.22 This research established the lowest rate of SAEs and serious infections events for etanercept.
A systematic review was published in 2014, which included the results of 44 clinical trials, and evaluated the safety of tumoral necrosis factor inhibitors in patients with rheumatoid arthritis, and the authors concluded that the high risk of infection associated with adalimumab and infliximab causes a high rate of treatment desertion, contrary to what occurs with etanercept where lower desertion rates have been documented.23 The highest adverse event rate in our study was reported for infliximab, followed by adalimumab. These rates were almost twice the rate of adverse events presented with etanercept. Regarding SAEs and treatment suspension caused by adverse events, the best safety profile was presented by etanercept, a similar situation to that presented by previous studies, which have demonstrated a superior safety profile for etanercept.8
The limitations in this study are related to the type of observational design in which the effectiveness of the treatment may be influenced by the physicians' decision in the real-life context. In this situation, it is possible that there may be selection biases, brought about by the judgement of the physician involved at the time of prescribing the treatment. It is worthwhile to highlight that the outcomes were evaluated by the same doctors but in the context of their habitual clinical practice. Despite the possible biases inherent to this type of design, it is worth highlighting that the results obtained are consistent with previous studies and have the strength of originating in uncontrolled scenarios, which approximate a tree effectiveness evaluation. The points discussed notwithstanding, the results obtained in similar baseline conditions and in comparable follow-up times demonstrated that all 3 molecules evaluated feature an adequate level of effectiveness in the context of real application, with a safety profile that favors etanercept.
ACKNOWLEDGMENT
The authors thank Adriana Sánchez, Paola Martinez, and Vanessa Giraldo for their contribution in this study.
Footnotes
This study was supported by BIOMAB IPS: Rheumatoid Arthritis Center, Bogotá-Colombia.
The authors declare no conflict of interest.
REFERENCES
- 1. Casado G, Alba P. Treat to target in rheumatoid arthritis [in Spanish]. Rev Fac Cien Med Univ Nac Cordoba. 2010; 67: 133– 134. [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010; 69: 631– 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008; 59: 762– 784. [DOI] [PubMed] [Google Scholar]
- 4. O'Dell JR, Mikuls TR, Taylor TH, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med. 2013; 369: 307– 318. [DOI] [PubMed] [Google Scholar]
- 5. Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007; 370: 1861– 1874. [DOI] [PubMed] [Google Scholar]
- 6. Chen YF, Jobanputra P, Barton P, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;42:iii–iv, xi–xiii, 1– 229. [DOI] [PubMed] [Google Scholar]
- 7. Lethaby A, Lopez-Olivo MA, Maxwell L, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev. 2013; 5: CD004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011: CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filippini M, Bazzani C, Atzeni F, et al. Effects of anti-TNF alpha drugs on disability in patients with rheumatoid arthritis: long-term real-life data from the Lorhen Registry. Biomed Res Int. 2014; 2014: 416892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eng G, Stoltenberg MB, Szkudlarek M, et al. Efficacy of treatment intensification with adalimumab, etanercept and infliximab in rheumatoid arthritis: a systematic review of cohort studies with focus on dose. Semin Arthritis Rheum. 2013; 43: 144– 151. [DOI] [PubMed] [Google Scholar]
- 11. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010; 62: 22– 32. [DOI] [PubMed] [Google Scholar]
- 12. Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. In Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria: Rheumatology (Oxford). 2004; 43: 1252– 1255. [DOI] [PubMed] [Google Scholar]
- 13. Mierau M, Schoels M, Gonda G, et al. Assessing remission in clinical practice. Rheumatology (Oxford). 2007; 46: 975– 979. [DOI] [PubMed] [Google Scholar]
- 14. Bazzani C, Filippini M, Caporali R, et al. Anti-TNFalpha therapy in a cohort of rheumatoid arthritis patients: clinical outcomes. Autoimmun Rev. 2009; 8: 260– 265. [DOI] [PubMed] [Google Scholar]
- 15. Kievit W, Adang EM, Fransen J, et al. The effectiveness and medication costs of three anti-tumour necrosis factor alpha agents in the treatment of rheumatoid arthritis from prospective clinical practice data. Ann Rheum Dis. 2008; 67: 1229– 1234. [DOI] [PubMed] [Google Scholar]
- 16. Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis. 2012; 71: 1134– 1142. [DOI] [PubMed] [Google Scholar]
- 17. Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004; 50: 1051– 1065. [DOI] [PubMed] [Google Scholar]
- 18. St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004; 50: 3432– 3443. [DOI] [PubMed] [Google Scholar]
- 19. Delabaye I, De Keyser F. 74-week follow-up of safety of infliximab in patients with refractory rheumatoid arthritis. Arthritis Res Ther. 2010; 12: R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeuchi T, Tanaka Y, Kaneko Y, et al. Effectiveness and safety of adalimumab in Japanese patients with rheumatoid arthritis: retrospective analyses of data collected during the first year of adalimumab treatment in routine clinical practice (HARMONY study). Mod Rheumatol. 2012; 22: 327– 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti–tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004; 50: 1400– 1411. [DOI] [PubMed] [Google Scholar]
- 22. van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006; 54: 1063– 1074. [DOI] [PubMed] [Google Scholar]
- 23. Michaud TL, Rho YH, Shamliyan T, et al. The comparative safety of TNF inhibitors in rheumatoid arthritis—a meta-analysis update of 44 randomized controlled trials. Am J Med. 2014; 127: 1208– 1232. [DOI] [PubMed] [Google Scholar]


