Abstract
Anthracyclines are the mainstay of treatment of a variety of haematological malignancies and solid tumours. Unfortunately, the clinical use of these drugs is limited by cumulative, dose-related cardiotoxicity which may ultimately lead to a severe and irreversible form of cardiomyopathy. Thus, there is an increasing need for close cooperation among cardiologists, oncologists and haemato-oncologists. As anthracyclines save lives, the logical goal of this cooperation, besides preventing or mitigating cardiotoxicity, is to promote an acceptable balance between the potential cardiac side effects and the vital benefit of anticancer treatment. This manuscript, which is specifically addressed to the cardiologist who has not accumulated much experience in the field of cancer therapy, focuses on several topics, that is old and new mechanisms of cardiac toxicity, late cardiac toxicity, the importance of overall risk assessment, the key role of a cardiology consult before starting cancer therapy, and the pros and cons of primary and secondary prevention programmes.
Keywords: anthracyclines, cardiology consult, cardio-oncology, cardiotoxicity, heart failure
Introduction
Life expectancy after the diagnosis and treatment of cancer has increased significantly in the past two decades, and therefore more patients survive either cancer-free or with cancer as a chronic, manageable disease.1,2
Unfortunately, many anticancer drugs have been associated with the development of cardiovascular complications such as left ventricular dysfunction and heart failure, myocardial, cerebral and peripheral ischaemia, pericarditis and myocarditis, hypertension, thromboembolism, QTc prolongation and arrhythmias.3,4 Each of these is likely to have significant effects on patient outcomes. Therefore, a new discipline, that is ‘cardio-oncology’, was born in an effort to study, prevent, recognize and treat the cardiovascular sequelae of antitumour drugs.5 As anticancer drugs save lives, the logical goal of cardio-oncology, besides preventing or mitigating cardiotoxicity (CTX), is to promote an acceptable balance between the potential cardiovascular side effects and the vital benefit of anticancer treatment.6
This document has been prepared with the main objective of promoting cooperation between the oncologist and the cardiologist and to support the growth of cardio-oncology among cardiologists. It is specifically addressed to the cardiologist who is asked to make strategic decisions in the management of cancer patients, but has not accumulated enough experience in the field of cardio-oncology.
This opinion paper and the others in this issue do not address the wide spectrum of cardiovascular complications of cancer therapy, but rather, they discuss left ventricular dysfunction, focusing on possible strategies to prevent or manage the CTX of the three major classes of drugs: anthracyclines (ANTs), anti-Her-2 and tyrosine kinase inhibitor. Not all treatments affect the heart the same way. In fact, there are important differences regarding the mechanisms, severity, reversibility and time of onset of CTX.7 Furthermore, CTX may occur in many clinical settings which differ in type, stage, clinical presentation and prognosis of cancer and with regard to the presence of other concomitant medication-related types of cardiac and noncardiac toxicity. It is therefore impossible to provide general recommendations on how to manage patients being treated with these drugs: each group would require specific measures and a separate discussion.
Anthracycline cardiotoxicity: mechanisms and pathophysiology
We have known about the cardiotoxic effects of ANT, since they started being used. Depending on when cardiac abnormalities appear, ANT-induced CTX (A-CTX) was initially classified as acute, subacute or chronic.8 It was soon understood that both acute and subacute toxicity are of limited clinical relevance, whereas chronic CTX, which may arise several months after completion of treatment in the form of congestive heart failure, was identified as the most common form of damage caused by ANT and the most important in clinical practice.9 It was then acknowledged that the incidence of chronic A-CTX strongly depends on the cumulative dose of the drug and increases with older age, systemic hypertension or preexisting cardiovascular disease (CVD) and mediastinal irradiation.9,10
Further studies found that both covert left ventricular dysfunction and heart failure may occur in patients treated with ANT after an asymptomatic period lasting longer than 1 year. This event was defined as late A-CTX.11,12
The most accredited interpretation of A-CTX implies the increase, through the formation of iron-complexes, of reactive oxygen species, which results in mitochondrial dysfunction, changes in calcium homeostasis and contractile function, and loss of cardiomyocytes by apoptosis.13–16
Recently, it was suggested that topoisomerase 2β is the key mediator of A-CTX, whose inhibition causes double-strand breaks in DNA, defective mitochondrial biogenesis and increased reactive oxygen species, resulting in cardiomyocyte death.17
A unifying hypothesis that could explain the adverse cardiovascular events in chronic and late forms is that A-CTX is both dose and time dependent. At high doses, ANT induces cardiomyocyte death and dysfunction, which both lead to hypokinetic cardiomyopathy within months. At low doses, they seem to inhibit the progenitor cell-mediated self-healing potential of the heart.18,19 The consequences may become clinically relevant many years later, when the effects of ageing and many other types of stress, including hypertension, diabetes and cardiac ischaemia are not counterbalanced by the renewal of cardiomyocytes by the paracrine repairing mechanisms of progenitor cells. This hypothesis fits well, and offers a mechanistic explanation to the ‘multiple-stress’ hypothesis that was proposed a few years ago, which states that patients treated with ANT have increased susceptibility to cardiac stress which would otherwise be harmless for untreated peers.20,21
As of their CTX, ANTs are currently used much less frequently. Nevertheless, they are still the backbone of the treatment of many solid and haematological tumours, including breast and gastric cancer, sarcoma, leukaemia and lymphoma.
The need for, and purpose of, a cardiology consultation
A number of excellent reviews, editorials and practical recommendations on how to manage patients treated with potentially cardiotoxic drugs emphasize the importance of the cardiological evaluation of patients before and during cancer therapy. Moreover, several practical algorithms, including ECG, dosage of biomarkers and echocardiography have been proposed.22–24 Although they do not specify whether all patients receiving anticancer therapy should be referred for a cardiology consultation, and in fact, the tendency is to request a consult only for patients with known CVD, high-risk profile, or abnormalities of laboratory parameters, including biomarkers, left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) analysis.24
On the other hand, authoritative experts believe that it is up to the cardiologist to perform the baseline cardiology evaluation in all patients, and have a dialogue with the oncologist practicing treatment in patients who are considered at intermediate or high risk for CTX, in an attempt to balance oncologic benefit with the cardiovascular risk prevention.23
It is important to bear in mind that in the past few years it has become very clear that
there are no low-risk patients. The AHA/ACC Guidelines for the Diagnosis and Management of heart failure in Adults state that all patients treated with potentially cardiotoxic drugs are Class A heart failure patients25;
unexpected cardiac complications, be they documented or even suspected, that occur during cancer treatment can have a major impact on the viability of therapies26;
late CTX is a matter of growing concern for patients treated with potentially cardiotoxic drugs11,12,27;
the decision to adopt prophylactic treatment with cardioprotective agents can be substantially effective in several patients28–30; and
appropriate modification of cardiovascular risk factors can provide significant benefits towards limiting the unwanted cardiovascular effects of cancer and its treatment.31
Therefore, even if we do not have guidelines, we suggest carrying out a cardiology consultation for all candidates to ANT treatment.
We see an analogy between the cardiology evaluation before anticancer therapy and the evaluation which precedes noncardiac surgery in moderate-to-high cardiovascular risk patients. The ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery state that ‘the purpose of preoperative evaluation is not simply to give medical clearance but rather to perform an evaluation of the patient's current medical status; make recommendations concerning the evaluation, management, and risk of cardiac problems over the entire perioperative period; and provide a clinical risk profile that the patient, primary physician, anaesthesiologist, and surgeon can use in making treatment decisions that may influence short- and long-term cardiac outcomes’.32 This statement is very well suited for patients who are candidates for anticancer therapy. Mutatis mutandis, the oncologist should inform the cardiologist about the malignancy and the proposed therapy. The cardiologist should not simply approve or deny the proposed treatment, but he/she should consider the overall cardiac risk, suggest how to prevent CTX and then inform the patients, their relatives and the primary physicians about the possible long-term cardiac outcomes.
Baseline cardiology consultation: general approach
The baseline cardiology consultation should be modulated according to the nature of the oncological illness. It is the duty of the oncologist to ensure that the salient information about the overall plan of patient care and prognosis, as well as the clinical circumstances and comorbidities are incorporated into the cardiology assessment. The oncologist should also inform the cardiologist about the risk of noncardiac toxicity, including anaemia, neutropenia or renal toxicity, that could have a strong impact on cardiac patients. All the patient's data, including any previous cardiology records, should be available for the cardiologist to review.
The cardiologist has to obtain the patient's medical history, and should perform a physical examination including a detailed cardiovascular study, supplemented by an ECG and an echocardiogram. Measurement of troponin and BNP should also be carried out.22,24,33,34
Echocardiography is the method of choice for evaluating cardiac risk in patients receiving anticancer therapy as it allows a comprehensive evaluation of the cardiac structure and function.24 It includes, but is not limited to, the measurement of LVEF. It is important to emphasize that LVEF is not the only parameter that must be taken into consideration. Indeed, the oncologist should be informed that this isolated evaluation may be misleading if other echocardiographic parameters, such as myocardial hypertrophy, diastolic function and valvular functions are not critically taken into due consideration. Evaluation of the GLS may contribute to risk stratification, as patients with GLS below the lower limit of normal are at intermediate or high risk, even if LVEF is normal.24
Evidence-based scoring systems to calculate cardiac risk in the context of anticancer therapy are not yet available. Therefore, the cardiologist must refer to the traditional cardiovascular risk factors while keeping in mind that people with hypertension, advanced age or any documented CVD are at increased risk for CTX. If the baseline findings are indicative of severe impairment or active CVD, patients are to be considered at high CTX risk.23
It is the responsibility of the cardiologist to ensure clear communication with patients, who must be as well informed of their cardiac conditions as possible. An intervention aimed at behavioural changes to prevent or correct all cardiovascular risk factors has to be initiated. Hypertension, diabetes and dyslipidaemia have to be aggressively treated. When necessary, beta-blockers and renin-angiotensin-aldosterone system (RAAS) antagonists should be part of treatment.35 Furthermore, the threshold for deciding whether to add statins should be low.35 Lastly, a frank conversation should be reserved to patients at high or intermediate oncological risk so that they may accept the optimal anticancer treatment without excessive concern for CTX. Strategies for switching to less cardiotoxic regimes in the presence of unacceptable cardiac risk should be discussed with the patient's oncologist.
Cardiology management, before and during anthracycline therapy
The algorithm for the management of CTX in patients receiving ANTs is shown in Fig. 1. Before starting treatment with ANTs, the cardiologist should stratify the risk, taking into account the four main factors that influence CTX onset: cumulative doses of ANT9; old age or preexisting heart diseases9,23; favourable cancer prognosis with expected long survival (e.g. breast cancer at an early stage and lymphomas) which increases the risk of late CTX1,27; exposure to further treatment after the end of ANT therapy, as in the case of HER-2+ breast cancer patients designated to receive trastuzumab or similar target therapies. ANT activates the stress pathways of cardiomyocytes, as well as the survival pathways, the most important of which is the neuregulin/HER-2 system. By inhibiting the HER-2 receptor, Trastuzumab impairs this survival pathway and creates an imbalance in favour of the toxic effects of ANTs.36,37 Accordingly, clinical studies showed that the incidence and severity of anti-HER-2-induced myocardial dysfunction significantly increases in women pretreated with ANT.38
Fig. 1.
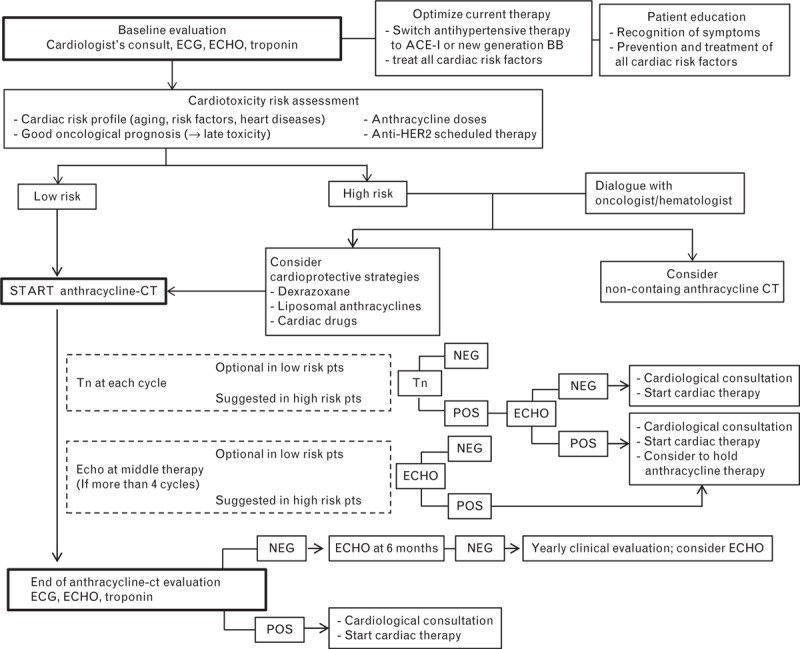
Algorithm for the management of cardiotoxicity in patients receiving anthracyclines. BB, beta-blockers; CT, chemotherapy; ECHO, echocardiograms; Tn, Troponin.
In addition, patients should be informed about the risk of cardiac events, particularly with regard to the late form of CTX. On the other hand, they must fully understand the role of the cardiologist, who is responsible for protecting cardiac function, but whose aim is also to prevent premature discontinuation of therapy. Patients should be aware of the need for repeated medical testing, as the effects of ANTs may appear many years later when the cancer follow-up could be tapered or suspended altogether.
Although the optimal surveillance for patients treated with ANTs is not standardized, it is advisable to repeat echocardiography at the end of ANT therapy in all patients. For patients who are going to receive more than four cycles of chemotherapy—especially those deemed to be at high risk at baseline—an interim analysis must be foreseen after the third cycle. A drop in LVEF of more than 10% compared with baseline, to a value less than 53% during ANT-based chemotherapy, or immediately after its completion, is a rare, but potentially serious event. In these cases, it is essential to assess troponin and BNP, to reassess LVEF after 2 weeks and to request a cardiology consultation in view of starting cardiac therapy. If the decline in LVEF occurs before the end of chemotherapy, the alternative options of discontinuing chemotherapy, switching to a less cardiotoxic regimen, or adapting the ANT therapy, should be discussed with the oncologist as soon as possible.
Cardiac MRI, a noninvasive technique that does not involve exposure to ionizing radiation, has emerged as a sensitive and reproducible alternative to echocardiography for the evaluation of the cardiac structure and function during cancer therapy.39,40 As cardiac MRI is not easily accessible and is costly, the current suggestion is to consider cardiac MRI for patients for whom echocardiography is not technically feasible or optimal, or when highly accurate assessment of LVEF is crucial for assessing possible chemotherapy discontinuation.
Primary prevention
Primary prevention is aimed at avoiding CTX from the very beginning of ANT administration. Although a number of strategies have been proposed, coadministration of dexrazoxane or the use of liposomal preparations has proven to be the most feasible approaches. A third possibility, which is pretreatment with beta-blockers and RAAS antagonists, albeit promising, has limited favourable evidence.
Dexrazoxane
Dexrazoxane is a neutral prodrug that is infused within 30 min prior to ANT administration. It spreads easily in the cardiomyocytes where, upon hydrolytic metabolism, it exerts its two most important cardioprotective activities which are preventing iron-based oxidative stress and inhibiting topoisomerase 2β.41,42
Dexrazoxane is the only U.S. Food and Drug Administration approved cardioprotective agent. Cardioprotection by dexrazoxane has been consistently documented in many clinical studies that investigated its efficacy, in both children and adults.28,43–45 Dexrazoxane is therefore a reliable and effective means for preventing A-CTX.46 Nonetheless, dexrazoxane is only prescribed in a small percentage of patients treated with ANT, and there is considerable discrepancy among oncologists regarding its use.47,48 This is because of the unjustified concern that dexrazoxane may interfere with the antitumour activity of ANTs and that it might increase the risk of secondary tumours.49,50 A number of studies have conclusively refuted this unfounded belief,51,52 which, however, had a negative impact on the decisions of regulatory agencies and some restrictions on its use.
The opinion of the working group of the International Colloquium on Cardio-oncology, a forum that brings together many of the leading experts in cardioncology, must be cited, and in our opinion shared. They recently expressed the view that dexrazoxane should be reassessed for broader clinical use.6
Liposomal anthracyclines
Liposomal ANTs are bound within artificial phospholipid membrane vesicles that are used as drug carriers.53 Large, randomized controlled trials and meta-analyses on metastatic breast cancer patients showed that liposomal formulations significantly reduce the CTX risk that is observed with conventional ANTs, without affecting antitumour activity.29,54–56 The low propensity of liposomal ANTs to cause CTX is well documented by studies evaluating the efficacy and safety of these drugs when administered in selected patients at very high risk of CTX because of advanced age, frailty, ANT pretreatment, preexisting CVD or concomitant trastuzumab treatment.57–60
Reduced CTX of liposomal ANTs should be ascribed to their different biodistribution and pharmacokinetics. Liposomes accumulate in the tumour tissue because of increased intratumour capillary permeability and decreased lymphatic clearance from perivascular space.53 On the contrary, uptake of the drug by the myocardium is diminished because the heart is supplied by vessels with tight junctions and the interstitial spaces are well drained by lymphatic vessels. Pegylated liposomal doxorubicin is a class of liposomal drug systems referred to as Stealth liposomes. It is known that the rate at which the liposome-encapsulated drug can be cleared from the systemic circulation is influenced by the uptake and destruction of circulating liposomes by the reticuloendothelial system. Because of the polyethylene-glycol grafting on the liposome bilayer, pegylated liposomal doxorubicin has lower uptake by the reticuloendothelial system, resulting in a unique pharmacokinetic model characterized by extremely long half-life, slow clearance and small volume of distribution.53 Thus, it is believed that in adult patients at high risk of CTX, the liposomal formulation is a feasible option and, in many cases, the only possibility for performing the ANT-based therapy.
Dexrazoxane and liposomal ANT reduce, but do not eliminate CTX risk. The decision on whether or not to monitor patients during chemotherapy depends greatly on the clinical circumstances. Low-risk patients undergoing primary prevention mainly to limit late CTX do not require monitoring during therapy. Vice versa, high-risk patients, and particularly those in whom the use of primary prevention is deemed to be the only way to receive ANT must be monitored during treatment.
Beta-blockers, RAAS antagonists, statins
This paragraph weighs the pros and cons of using these drugs in cancer patients who are ‘healthy’ from a cardiovascular point of view. At the moment, there are no large randomized, prospective trials that clearly show the benefits of this form of primary prevention.
On the other hand, recent studies on small groups of patients report promising results with the use of third generation beta-blockers (carvedilol or nebivolol) or RAAS antagonists.30,61–66 Cancer and anticancer therapy, however, is associated with fatigue and a variety of haemodynamic modifications such as hyper- or hypotension, hyper- or hypovolemia, and changes in sympathetic or parasympathetic tone, which can fluctuate greatly during treatment. Therefore, the use of vasoactive drugs may cause or exacerbate the distressing and debilitating symptoms, including dizziness, hypotension, and fatigue. Thus, caution is recommended before starting therapy for primary prevention with beta-blockers or RAAS antagonists.67 Although if the cardiologist recommends cotreatment with these drugs, it becomes mandatory to start with very low doses, to closely monitor heart rate, blood pressure and kidney function, and should symptoms appear, to reevaluate patients as quickly as possible.
Retrospective studies document that among patients treated with ANT-based chemotherapy, those who had already received statins to prevent CVD experienced less deterioration in LVEF.68 This finding, together with the lack of adverse haemodynamic effects and the powerful antioxidant and anti-inflammatory ability of statins, suggest a potentially protective effect of these compounds against A-CTX.69 There is currently not enough evidence to warrant recommending statin administration to the general population of patients scheduled for ANT therapy.
Secondary prevention
Secondary prevention aims to prevent left ventricular dysfunction in patients with very early signs of A-CTX. This strategy is based on the following elements: the relatively short duration of therapy—4–6 cycles of drugs administered over 4–6 months—suggests that the optimal strategy for monitoring patients must be planned; a decrease in LVEF is an event that occurs rather late during ANT therapy and therefore, repeated measurement is not a highly sensitive tool for detecting early CTX; an early increase in plasma troponin or decrease in left ventricular systolic deformation indexes, in particular GLS, precedes changes in LVEF and are more sensitive and specific for detecting early CTX70–73; identification of these ‘primordial’ CTX signs suggests an increased cardiac risk, which, however, does not exceed the advantage of maintaining ANT therapy; and in selected patients, treatment with drugs for heart failure may be cardioprotective if administered under the cardiologist's supervision.74,75
Limited scientific data prevent solid recommendations on secondary prevention from being made, although the following information may be helpful to cardiologists in order to determine how to implement the standard management of patients by using secondary prevention measures.
Longitudinal strain
GLS is the most accurate echocardiographic index for detecting subtle changes in myocardial function and it is able to predict the development of ANT-induced cardiomyopathy.72,73 As it is unthinkable to use this technique before each chemotherapy cycle, GLS should be assessed at every scheduled echocardiographic examination (beginning and end of therapy and, where appropriate, at mid-term). Evaluating changes in GLS is particularly useful when LVEF decreases by less than 10% and drops to a value less than 53%. In these cases, a relative decrease in GLS more than 15% from baseline should be considered a sign of CTX, thus requiring a cardiology consultation in order to start cardiac therapy and to determine whether or not to maintain or postpone chemotherapy.24 A relative drop in GLS of 8–15% should be evaluated case by case.
Troponin
A large body of clinical evidence proves that an increase in troponin levels identifies ANT-induced cardiac injury and allows early identification of patients at risk of left ventricular dysfunction or heart failure.22,70,71 Moreover, because of its high negative predictive value, troponin identifies patients at low risk of subsequently developing ANT cardiomyopathy. There are also data from a single centre, which show that patients with troponin elevation greatly benefit from the use of angiotensin-converting enzyme inhibitors.74,75 Despite these promising results, many experts believe that the practical usefulness of serial troponin assessment needs to be definitively established. Many centres are reluctant to rely on troponin in their environment of clinical trials. Although timing of sample collection, cut-off values and comparability between various troponin assays have yet to be resolved, troponin has many of the prerequisites of a good biomarker: tests can be carried out in series with ease, are easily available and inexpensive when compared with imaging, and are already largely employed in many pathological conditions.76 This latter point is important because the more a test is used in current cardiology evaluation, the better it can be used in the specific context of ANT CTX. We suggest that troponin should be measured at each cycle and even one month after the end of CT. It was in fact shown that troponin positivity at the end of chemotherapy is a rare event that however indicates that the patient is at high risk of left ventricular dysfunction or heart failure.71
If troponin is found to be above the cut-off point, it would be appropriate to perform an echocardiogram, dose the BNP, and start cardiac therapy, whereas the ANT therapy should not be discontinued unless a significant decrease in LVEF is observed.
Pharmacological treatment in secondary prevention
The effort to identify patients at risk of CTX would be in vain, if there were no treatment options. Herein, doubts have been expressed concerning the administration of beta-blockers or RAAS antagonists in primary prevention; on the contrary, the use of these drugs should be encouraged in secondary prevention, once patients have been identified by troponin and/or strain evaluation as being at higher risk of left ventricular dysfunction.22
We suggest that therapy with angiotensin-converting enzyme inhibitors should be started first, quickly followed by the addition of beta-blockers. With regard to patient monitoring and dose titration, we refer our readers to current treatment guidelines for heart failure. Although the undesirable effects of these drugs still remain, therapeutic management should preferably be supervised by the cardiologist who, moreover, should pay close attention to ensure that cardiovascular treatments do not endanger the chances of completing ANT therapy.
Cardiomyopathy surveillance for anthracycline-exposed survivors
All patients treated with ANT and their healthcare providers should be aware of the risk of developing ANT-related cardiomyopathy. This event may occur after a long latency period and asymptomatic left ventricular dysfunction, with progressive signs and symptoms of heart failure. Screening for modifiable cardiovascular risk factors, lifestyle modification and, where appropriate, pharmacological treatment to correct the risk factors, are highly recommended. Regular exercise is advisable for all patients, whereas vigorous physical activity that includes intense isometric exercises should not be encouraged in patients at high risk of CTX (high doses, concomitant radiotherapy).
Because of the scarcity of evidence, suggestions for medical surveillance are largely based on consensus. The observation programme that includes two-dimensional echocardiography, the most appropriate instrument for the follow-up of patients, should start 6 months after completion of therapy. For low-risk patients without preexisting CVD who do not undergo treatment with high ANT doses and who show no changes in LVEF, GLS or troponin during treatment, annual cardiovascular clinical evaluation is recommended, as is echocardiographic assessment every 5 years. More frequent monitoring is reasonable for survivors who are at high and moderate risk of ANT cardiomyopathy. In the presence of signs or symptoms suggestive of CVD, an echocardiographic examination is indicated as soon as possible.
The clinical suspicion of heart failure and/or echocardiographic evidence of changes, such as LVEF drop, chamber dilation and valve abnormalities call for a prompt cardiology consultation. Patients with preexisting CVD, as well as those who showed signs of early CTX during chemotherapy and started cardiovascular therapy should periodically be evaluated by a cardiologist.
Periodic assessment of BNP and troponin could play a complementary role in particular circumstances such as in patients with borderline echocardiographic data.
Conclusion
A-CTX has been a well known problem for more than 40 years, but there are still several unresolved issues and unanswered questions. From the patient's point of view, there is an urgent need for specialized care that involves carefully integrating the knowledge of cardio-oncology into daily practice. In order to make this possible, training at least one cardiologist in the management of cardiovascular problems of cancer patients is recommended in all hospitals having an oncological or haemato-oncological Unit. Cardio-oncology is a rapidly growing area. Although keeping up-to-date is certainly necessary, we feel that an even more critical issue is represented by the goal for oncologists and cardiologists to join forces and learn to work side by side.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64:252–271. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010; 46:765–781. [DOI] [PubMed] [Google Scholar]
- 3.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009; 53:2231–2247. [DOI] [PubMed] [Google Scholar]
- 4.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol 2010; 144:3–15. [DOI] [PubMed] [Google Scholar]
- 5.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst 2010; 102:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewer M, Gianni L, Pane F, et al. Report on the international colloquium on cardio-oncology (rome, 12–14 march 2014). Ecancer 2014; 8:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc 2014; 3:e000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med 1996; 125:47–58. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979; 91:710–717. [DOI] [PubMed] [Google Scholar]
- 10.Merrill J, Greco FA, Zimbler H, Brereton HD, Lamberg JD, Pomeroy TC. Adriamycin and radiation: synergistic cardiotoxicity. Ann Intern Med 1975; 82:122–123. [DOI] [PubMed] [Google Scholar]
- 11.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007; 25:3808–3815. [DOI] [PubMed] [Google Scholar]
- 12.Hequet O, Le QH, Moullet I, et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol 2004; 22:1864–1871. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol 2005; 68:261–271. [DOI] [PubMed] [Google Scholar]
- 14.Zuppinger C, Timolati F, Suter TM. Pathophysiology and diagnosis of cancer drug induced cardiomyopathy. Cardiovasc Toxicol 2007; 7:61–66. [DOI] [PubMed] [Google Scholar]
- 15.Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 2010; 53:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisberg C, Pentassuglia L, Sawyer DB. Cardiac side effects of anticancer treatments: new mechanistic insights. Curr Heart Fail Rep 2012; 9:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012; 18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 18.Konorev EA, Vanamala S, Kalyanaraman B. Differences in doxorubicin-induced apoptotic signaling in adult and immature cardiomyocytes. Free Radic Biol Med 2008; 45:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Angelis A, Piegari E, Cappetta D, et al. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 2010; 121:276–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007; 50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 21.Menna P, Salvatorelli E, Minotti G. Cardiotoxicity of antitumor drugs. Chem Res Toxicol 2008; 21:978–989. [DOI] [PubMed] [Google Scholar]
- 22.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2012; 23 (Suppl 7):vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 23.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013; 34:1102–1111. [DOI] [PubMed] [Google Scholar]
- 24.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014; 15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 26.Groarke JD, Cheng S, Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med 2013; 369:1779–1781. [DOI] [PubMed] [Google Scholar]
- 27.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 2015; 12:547–558. [DOI] [PubMed] [Google Scholar]
- 28.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2008; 2:CD003917. [DOI] [PubMed] [Google Scholar]
- 29.van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev 2010; 5:CD005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013; 61:2355–2362. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong GT, Oeffinger KC, Chen Y, et al. Modificable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013; 31:3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol 2007; 50:e159–e241. [DOI] [PubMed] [Google Scholar]
- 33.Colombo A, Sandri MT, Salvatici M, Cipolla CM, Cardinale D. Cardiac complications of chemotherapy: role of biomarkers. Curr Treat Options Cardiovasc 2014; 16:313. [DOI] [PubMed] [Google Scholar]
- 34.Christenson ES, James T, Agrawal V, Park BH. Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin Biochem 2015; 48:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carver JR, Schuster SJ, Glick JH. Doxorubicin cardiotoxicity in the elderly: old drugs and new opportunities. J Clin Oncol 2008; 26:3122–3124. [DOI] [PubMed] [Google Scholar]
- 36.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 2007; 116:954–960. [DOI] [PubMed] [Google Scholar]
- 37.Spallarossa P, Altieri P, Pronzato P, et al. Sublethal doses of an antierbB2 antibody leads to death by apoptosis in cardiomyocytes sensitized by low prosenescent doses of epirubicin: the protective role of dexrazoxane. J Pharmacol Exp Ther 2010; 332:87–96. [DOI] [PubMed] [Google Scholar]
- 38.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 2005; 23:7811–7819. [DOI] [PubMed] [Google Scholar]
- 39.Wassmuth R, Lentzsch S, Erdbruegger U, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J 2001; 141:1007–1013. [DOI] [PubMed] [Google Scholar]
- 40.Jordan JH, D’Agostino RB, Jr, Hamilton CA, et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2014; 7:872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seifert CF, Nesser ME, Thompson DF. Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann Pharmacother 1994; 28:1063–1072. [DOI] [PubMed] [Google Scholar]
- 42.Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 2007; 67:8839–8846. [DOI] [PubMed] [Google Scholar]
- 43.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 2004; 351:145–153. [DOI] [PubMed] [Google Scholar]
- 44.Sparano JA, Speyer J, Gradishar WJ, et al. Phase I trial of escalating doses of paclitaxel plus doxorubicin and dexrazoxane in patients with advanced breast cancer. J Clin Oncol 1999; 17:880–886. [DOI] [PubMed] [Google Scholar]
- 45.Marty M, Espié M, Llombart A, et al. Dexrazoxane Study Group. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol 2006; 17:614–622. [DOI] [PubMed] [Google Scholar]
- 46.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014; 64:938–945. [DOI] [PubMed] [Google Scholar]
- 47.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009; 27:127–145. [DOI] [PubMed] [Google Scholar]
- 48.Walker DM, Fisher BT, Seif AE, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: Analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer 2013; 60:616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol 1997; 15:1333–1340. [DOI] [PubMed] [Google Scholar]
- 50.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007; 25:493–500. [DOI] [PubMed] [Google Scholar]
- 51.Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol 2004; 130:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow EJ, Asselin BL, Schwartz CL, et al. Late mortality after dexrazoxane treatment: a report from the Children's Oncology Group. J Clin Oncol 2015; 33:2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamot C, Drummond DC, Hong K, Kirpotin DB, Park JW. Liposome-based approaches to overcome anticancer drug resistance. Drug Resist Updat 2003; 6:271–279. [DOI] [PubMed] [Google Scholar]
- 54.Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol 2001; 19:1444–1454. [DOI] [PubMed] [Google Scholar]
- 55.Harris L, Batist G, Belt R, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002; 94:25–36. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004; 15:440–449. [DOI] [PubMed] [Google Scholar]
- 57.Rigacci L, Mappa S, Nassi L, et al. Liposome-encapsulated doxorubicin in combination with cyclophosphamide, vincristine, prednisone and rituximab in patients with lymphoma and concurrent cardiac diseases or pretreated with anthracyclines. Hematol Oncol 2007; 25:198–203. [DOI] [PubMed] [Google Scholar]
- 58.Venturini M, Bighin C, Puglisi F, et al. A multicentre Phase II study of nonpegylated liposomal doxorubicin in combination with trastuzumab and docetaxel as first-line therapy in metastatic breast cancer. Breast 2010; 19:333–338. [DOI] [PubMed] [Google Scholar]
- 59.Brain EG, Mertens C, Girre V, et al. Impact of liposomal doxorubicin-based adjuvant chemotherapy on autonomy in women over 70 with hormone-receptor-negative breast carcinoma: A French Geriatric Oncology Group (GERICO) phase II multicentre trial. Crit Rev Oncol Hematol 2011; 80:160–170. [DOI] [PubMed] [Google Scholar]
- 60.Baselga J, Manikhas A, Cortés J, et al. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Ann Oncol 2014; 25:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spallarossa P, Garibaldi S, Altieri P, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol 2004; 37:837–846. [DOI] [PubMed] [Google Scholar]
- 62.Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006; 48:2258–2262. [DOI] [PubMed] [Google Scholar]
- 63.de Nigris F, Rienzo M, Schiano C, et al. Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer 2008; 44:334–340. [DOI] [PubMed] [Google Scholar]
- 64.Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol 2013; 167:2306–2310. [DOI] [PubMed] [Google Scholar]
- 65.Cadeddu C, Piras A, Mantovani G, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J 2010; 160:487.e1–487.e7.doi: 10.1016/j.ahj.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 66.Dessì M, Madeddu C, Piras A, et al. Long-term, up to 18 months, protective effects of the angiotensin II receptor blocker telmisartan on Epirubin-induced inflammation and oxidative stress assessed by serial strain rate. Springerplus 2013; 2:198.doi: 10.1186/2193-1801-2-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spallarossa P, Guerrini M, Arboscello E, Sicbaldi V. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction. J Am Coll Cardiol 2013; 62:2451–2452. [DOI] [PubMed] [Google Scholar]
- 68.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol 2012; 60:2384–2390. [DOI] [PubMed] [Google Scholar]
- 69.Riad A, Bien S, Westermann D, et al. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res 2009; 69:695–699. [DOI] [PubMed] [Google Scholar]
- 70.Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000; 36:517–522. [DOI] [PubMed] [Google Scholar]
- 71.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004; 109:2749–2754. [DOI] [PubMed] [Google Scholar]
- 72.Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail 2014; 16:300–308. [DOI] [PubMed] [Google Scholar]
- 73.Mousavi N, Tan TC, Ali M, Halpern EF, Wang L, Scherrer-Crosbie M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50-59% treated with anthracyclines. Eur Heart J Cardiovasc Imaging 2015; 16:977–984. [DOI] [PubMed] [Google Scholar]
- 74.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006; 114:2474–2481. [DOI] [PubMed] [Google Scholar]
- 75.Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015; 131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 76.Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. noncoronary disease. Eur Heart J 2011; 32:404–411. [DOI] [PubMed] [Google Scholar]


