Abstract
We previously found that oxytocin (OT) receptor (OTR) binding density in the medial amygdala (MeA) correlated positively with social interest (i.e., the motivation to investigate a conspecific) in male rats, while OTR binding density in the central amygdala (CeA) correlated negatively with social interest in female rats. Here, we determined the causal involvement of OTR in the MeA and CeA in the sex-specific regulation of social interest in adult rats by injecting an OTR antagonist (5 ng/0.5 µl/side) or OT (100 pg/0.5 µl/side) before the social interest test (4-min same-sex juvenile exposure). OTR blockade in the CeA decreased social interest in males but not females, while all other treatments had no behavioral effect. To further explore the sex-specific involvement of the OT system in the CeA in social interest, we used in vivo microdialysis to determine possible sex differences in endogenous OT release in the CeA during social interest. Interestingly, males and females showed similar levels of extracellular OT release at baseline and during social interest, suggesting that factors other than local OT release mediate the sex-specific role of CeA-OTR in social interest. Moreover, we found a positive correlation between CeA-OT release and social investigation time in females. This was further reflected by reduced CeA-OT release during social interest in females that expressed low compared to high social interest. We discuss the possibility that this reduction in OT release may be a consequence, rather than a cause, of exposure to a social stimulus. Overall, our findings show for the first time that extracellular OT release in the CeA is similar between males and females and that OTR in the CeA plays a causal role in the regulation of social interest towards juvenile conspecifics in males.
Keywords: oxytocin, oxytocin receptor, central amygdala, medial amygdala, social interest, sex differences
Introduction
Social interest reflects the motivation to investigate a conspecific for the perception and identification of social cues which will, in turn, facilitate context-appropriate social behavior responses. Social interest can therefore be seen as an initial step in mediating the subsequent expression of a wide range of social behaviors, such as aggression, mating, and parental care. Interestingly, there are sex differences in the expression of social interest in both rats and mice. In both species, adult males, compared to adult females, show higher levels of social investigation toward juvenile conspecifics (Thor, 1980; Johnston and File, 1991; Bluthe and Dantzer, 1990; Dumais et al., 2013, 2016). However, the neural mechanisms underlying this sex difference in social interest have not been assessed.
A key candidate for the sex-specific regulation of social interest is the oxytocin (OT) system. OT is primarily synthesized in the paraventricular nucleus and supraoptic nucleus of the hypothalamus. Upon central release, OT modulates the activation of many brain regions via binding to the widely distributed OT receptor (OTR; Gimpl and Fahrenholz, 2001). Importantly, the OT system regulates various social behaviors in humans and rodents (Ross and Young, 2009; Veenema and Neumann, 2008; Guastella and MacLeod, 2012), often in sex-specific ways (for review see Dumais and Veenema, 2015, 2016). The amygdala is of particular interest because it has been shown to be a core region of sex-specific activation by OT. For example, human fMRI studies have shown that exogenous OT modulates amygdala activation in response to social stimuli differently in men compared to women (Domes et al., 2007; Domes et al., 2010; Rilling et al., 2012; Rilling et al., 2014). Furthermore, correlational studies in rodents suggesting that the OT system in subregions of the amygdala, namely the medial amygdala (MeA) and central amygdala (CeA), plays a differential role in mediating male versus female social interest. In detail, male mice showing high levels of social investigation have higher OTR mRNA expression in the MeA compared to males showing low levels of social investigation (Murakami et al., 2011). In rats, males have higher OTR binding density in the MeA compared to females, and OTR binding density in the MeA correlates positively with social investigation time in males, but not females (Dumais et al., 2013). In contrast, OTR binding density in the CeA does not show a sex difference, but correlates negatively with social investigation time in females, but not in males (Dumais et al., 2013). Together, this suggests that OTR activation in the MeA facilitates social investigation in males, while OTR activation in the CeA decreases social investigation in females.
The MeA is part of a network that processes olfactory social cues in rodents (Halpern and Martinez-Marcos, 2003; Guthman and Vera, 2016) and is well-known to regulate various social behaviors (Bergan et al., 2014; Noack et al., 2015). While the CeA is more commonly known for its role in fear and anxiety (Bale et al., 2001; Viviani et al., 2011; Knobloch et al., 2012), it has also been implicated as a region involved in the modulation of social behavior, such that c-Fos expression (an indirect marker for neural activity) in the CeA was higher in adolescent male rats exposed to a social context compared to adolescent rats exposed to a non-social context (Varlinskaya et al., 2013). Importantly, previous studies have implicated the OT system in both the MeA and CeA in mediating social behaviors, further supporting our hypothesis that these amygdala subregions may be involved in modulating social interest. For example, OT in the MeA is known to facilitate social approach and social recognition (Arakawa et al., 2010; Lukas et al., 2013), and OT in the CeA has been implicated in maternal and intermale aggression (Lubin et al., 2003; Bosch et al., 2005; Consiglio et al., 2005; Calcagnoli et al., 2015). However, comparisons between males and females regarding the role of OT in these amygdala subregions in the regulation of social behavior is largely lacking.
In the current study, we aimed to determine the causal role of the OT system in the MeA and CeA in the regulation of social interest in male and female rats. Based on the previously observed correlations of OTR binding and social interest (Dumais et al., 2013), we tested the hypotheses that OT acting on OTR in the MeA facilitates social investigation in males, while OT acting on OTR in the CeA decreases social investigation in females. To this end, we determined the effects of acute pharmacological OTR blockade and OT administration in the MeA and CeA on social investigation time in adult male and female rats. Because these pharmacological manipulations showed a sex-specific effect of OTR blockade in the CeA on social investigation time, we also investigated possible underlying mechanisms for this sex-specific effect by determining potential sex differences in extracellular OT release in the CeA at baseline and during exposure to the social interest test using in vivo microdialysis.
Experimental Procedures
Animals
Male and female Wistar rats were obtained from Charles River Laboratories (Raleigh, NC) at 8–9 weeks of age for experimental rats and at 22 days of age for stimulus rats. Rats were maintained on a 12 h light/dark cycle, lights on at 0700 h, and food and water were available ad libitum. Experimental rats were housed in same-sex pairs in standard rat cages (26.7 × 48.3 × 20.3 cm) unless otherwise mentioned, and were given at least one week to acclimate to our facilities. Stimulus rats were housed in same-sex groups of four per cage, and were used at 25–30 days of age. All experiments were conducted in accordance with the guidelines of the NIH and approved by the Boston College Institutional Animal Care and Use Committee (IACUC).
Stereotaxic surgery
Cannulation
After daily handling for one week to familiarize them with the injection procedure, experimental rats were anesthetized using isoflurane and mounted on a stereotaxic frame. A heating pad was used to regulate body temperature of rats while anesthetized. Guide cannulae (22 gauge; Plastics One, Roanoke, VA) were implanted bilaterally 2 mm dorsal to the MeA (2.8 mm caudal to bregma, 3.3 and −3.3 mm lateral to midline, and 7.3 mm ventral to the skull surface) or CeA (2.5 mm caudal to bregma, 4.2 and −4.2 mm lateral to midline, and 5.9 mm ventral to the skull surface) according to Paxinos and Watson (1998). Guide cannulae were fixed to the skull with four stainless steel screws and acrylic glue and closed with dummy cannulae (26 gauge; Plastics One, Roanoke, VA). After surgery, rats were individually housed in standard rat cages, and behavioral testing was performed 3–4 days after surgery.
Microdialysis probe placement
A separate cohort of rats was used for in vivo measurement of extracellular OT release in the CeA. Handling and surgical procedures were similar to the procedures described above except for the placement of microdialysis probes instead of cannulae. Microdialysis probes (BrainLink, the Netherlands) were implanted unilaterally into the CeA (2.5 mm caudal to bregma, −4.2 mm lateral to midline, and 8.9 mm ventral to the skull surface). Two inch pieces of polyethylene tubing were fixed to the ends of the microdialysis probes to allow for attachment to the microinfusion pumps and eppendorf tubes for sample collection. After surgery, rats were individually housed in standard rat cages. Microdialysis and behavioral testing were performed 2 days after surgery. This short postoperative recovery period is necessary for optimal detection of neuropeptides in microdialysates of chronically implanted probes (Horn and Engelmann, 2001). We have demonstrated that prior surgery and ongoing microdialysis had no effect on social investigation time in rats (Dumais et al., 2016).
Behavioral Testing
Social Interest Test
To test for social interest, the time rats spent investigating an unfamiliar same-sex juvenile rat was measured according to Dumais et al. (2013). A juvenile rat was used in order to assess general social approach of the experimental rat toward a conspecific that does not elicit aggressive or sexual behaviors. A juvenile rat was placed into the experimental rat’s home cage for 4 min, and the time spent investigating the juvenile was measured. Testing was performed during the light phase between 1200 h and 1700 h. Behavior was video recorded and analyzed using JWatcher (http://www.jwatcher.ucla.edu) by an experimenter blind to treatment groups. Behavior was considered social investigation when the experimental rat was actively sniffing the juvenile, including sniffing the anogenital and head/neck regions.
Elevated Plus Maze Test
To determine whether the effects of OTR blockade on social interest were secondary due to generalized effects on anxiety-related behavior, the same rats under the same drug condition were tested for anxiety using the elevated plus maze 2 days after the social interest test. The maze consists of two opposing open (50 × 10 cm) and two opposing closed (50 × 10 cm) plastic arms with a 10 × 10 cm central area. The maze is elevated to 90 cm above the floor. Each experimental rat was placed on the central area of the maze facing a closed arm, and was allowed to explore the maze for 5 min. The maze was washed thoroughly with diluted detergent between each test. Testing was performed during the light phase between 900 h and 1200 h. Behavior was video recorded and time spent on the open arms, closed arms, and central area were measured, along with entries made into the open and closed arms, using JWatcher by an experimenter blind to treatment groups. Rats were considered to be on a particular maze arm when both forepaws and shoulders of the rat were on the respective arm of the elevated plus maze. The percentage of time spent on the open arms ([time on open arms/ (time on open arms + time on closed arms)] × 100) and the percentage of open arm entries ([open arm entries/ (open arm entries + closed arm entries)] × 100] were used as parameters of anxiety-like behavior. The total number of arm entries (open arm entries + closed arm entries) was calculated as a measure of locomotor activity.
Drug Injections and Procedures
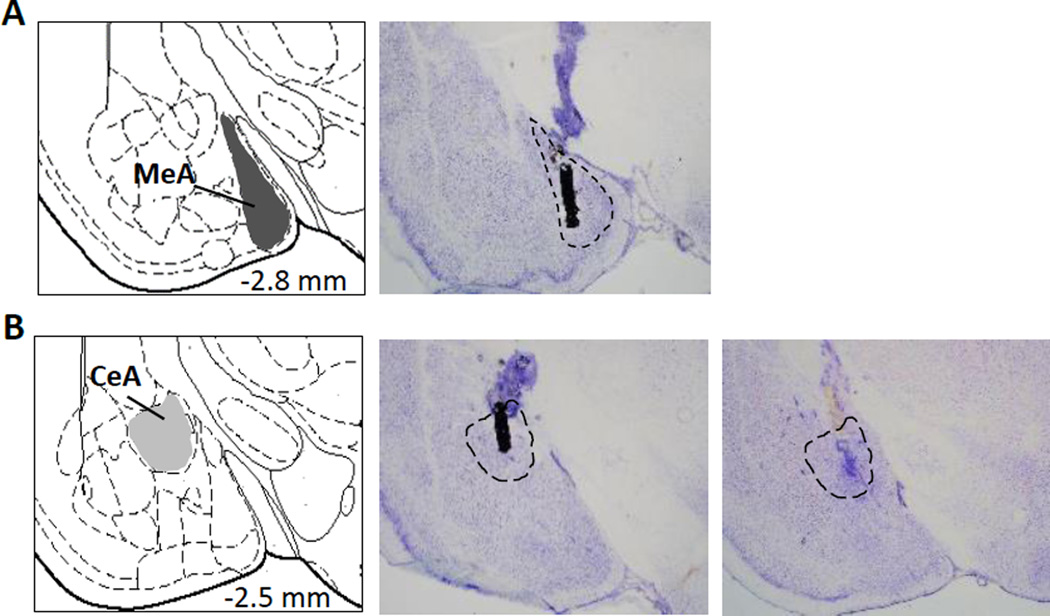
Rats were randomly assigned to receive either the selective OTR antagonist desGly-NH2,d(CH2)5-[Tyr(Me)2,Thr4]OVT (Manning et al., 2008; 5 ng/0.5 µl/side), synthetic OT (Sigma; 100 pg/0.5 µl/side), or vehicle (Ringer’s solution, 0.5 µl/side). Each drug-treated group of rats was tested simultaneously with a group of vehicle-treated rats. Vehicle-treated groups were never paired with drug-treated groups on a post hoc basis nor were they paired with other drug-treated groups. Concentrations for the OTR antagonist and OT were based on prior research in which these doses were effective in altering diverse social behaviors (Guzmán et al., 2013, 2014; Bredewold et al., 2014; Laszlo et al., 2016; Dumais et al.. 2016). Injection systems were composed of polyethylene tubing connected to an injector cannula which extended 2mm beyond the guide cannula. Injections were made using a 10 µl Hamilton syringe (Hamilton, Reno, NV) which was kept in place for 30 sec following injection to allow for tissue uptake. Following the last behavioral test, rats were killed with CO2, a small amount of charcoal was injected into the guide cannulae, and cannulae placement was examined histologically on 30 µm thick Nissl-stained coronal brain sections (see Fig. 1A, B). Cannulae placements were considered correct if they fell within the neuroanatomical borders of the MeA at bregma −2.80 ± 0.2 mm or the CeA at bregma −2.5 ± 0.2 mm. Rats with incorrect placements were removed from analysis.
Fig. 1. Cannula and probe placement in amygdala subregions.
(A) Atlas template indicating the location of the medial amygdala (MeA; dark gray; bregma −2.8 mm; adapted from Paxinos and Watson, 1998; left) and representative photomicrograph of a Nissl-stained coronal section of the rat brain with a representative microinjection location targeted at the MeA shown by charcoal (right). (B) Atlas template indicating the location of the central amygdala (CeA; light gray; bregma −2.5 mm; adapted from Paxinos and Watson, 1998; left), and representative photomicrographs of Nissl-stained coronal sections of the rat brain with a representative microinjection location targeted at the CeA shown by charcoal (middle) and the location of the semipermeable membrane of a microdialysis probe targeted at the CeA (right). Correct placements were considered at bregma −2.80 mm ± 0.2 mm for the MeA and at bregma −2.5 ± 0.2 mm for the CeA according to the brain atlas by Paxinos and Watson (1998).
Microdialysis Procedures
One day after probe implantation, rats were habituated to the sampling procedure for 1 h. The following day, microdialysis probes were connected via polyethylene tubing to 2.5 mm Hamilton syringes mounted on a microinfusion pump. Rats were perfused with Ringer’s solution (with 0.25% BSA; 3µl/min) for 2 h to establish an equilibrium between the inside and outside of the microdialysis membrane. Five consecutive 30-min dialysates were then collected in 0.5 ml Eppendorf tubes containing 10 µl 0.1M HCI to inhibit protein degradation. Dialysates 1 and 2 were taken under baseline conditions, dialysate 3 started concurrently with the 4-min social interest test, and dialysates 4 and 5 were taken thereafter. Dialysates were immediately frozen on dry ice, and stored at −45°C until quantification. OT content was measured using radioimmunoassay, with a detection limit of 0.3 pg OT per dialysate (RIAgnostics, Munich, Germany). Rats were killed with CO2 and proper probe placement was verified histologically on 30 µm thick Nissl-stained coronal brain sections (see Fig. 1B). Probe placements were considered correct if they fell within the neuroanatomical borders of the CeA at bregma −2.5 ± 0.2 mm. Rats with incorrect probe placements were removed from analysis.
Experimental Design
Experiment 1: Effects of pharmacological manipulations of the OT system in the MeA on social interest
To determine the effects of OTR blockade in the MeA on social interest, rats received bilateral injections of either Ringer’s solution (males: n=9, females: n=14) or the OTR antagonist (males: n=5, females: n=9). To determine the effects of OT administration in the MeA on social interest, rats received bilateral injections of either Ringer’s solution (males: n=12, females: n=9) or OT (males: n=6, females: n=7). Rats were injected 20 min before the start of the social interest test.
Experiment 2: Effects of pharmacological manipulations of the OT system in the CeA on social interest and anxiety-related behavior
Experiment 2a: Social interest
To determine the effects of OTR blockade in the CeA on social interest, rats received bilateral injections of either Ringer’s solution (males: n=7, females: n=7) or the OTR antagonist (males: n=6, females: n=7). To determine the effects of OT administration into the CeA on social interest, rats received bilateral injections of either Ringer’s solution (males: n=12, females: n=10) or OT (males: n=9, females: n=6). Rats were injected 20 min before the start of the social interest test.
Experiment 2b: Elevated plus maze
To determine whether effects of OTR blockade in the CeA on social interest are due to effects on general anxiety, the same rats were tested on the elevated plus maze two days after the social interest test. Rats were bilaterally injected with the same drug treatment 20 min before the start of the elevated plus maze test.
Experiment 3: Extracellular OT release in the CeA
Because of the sex-specific effect of OTR blockade in the CeA on social interest, we used in vivo microdialysis to measure extracellular OT release in the CeA of a separate cohort of adult male (n=8) and female (n=8) rats under baseline conditions and during exposure to the social interest test.
Estrus phase measurement
Although previously we found no effect of estrus phase on social interest (Dumais et al., 2013), to control for any effects of estrus cycle in the present study, estrus phase was determined via vaginal smears (according to Dumais et al., 2013) taken from each female immediately following each behavioral test. Estrus cycle phase was determined via cell characteristics based on Goldman et al. (2007). Females were categorized as being in estrus (cells characteristic of proestrus and estrus phases in which females show higher levels of estradiol and progesterone), or non-estrus (cells characteristic of diestrus and metestrus in which females show lower levels of estradiol and progesterone).
Statistical Analysis
For experiments 1 and 2, effect of treatment on social interest (investigation time in seconds), anxiety-related behavior (percent time on open arms, percentage of open arm entries), and locomotor activity (total number of arm entries) were each analyzed using two-way ANOVAs (treatment × sex). When appropriate, Bonferroni post hoc tests were used to examine the nature of interaction effects. For experiment 3, the effects of sex on social investigation time and on baseline OT concentration (pg/dialysate; average of dialysates 1 and 2) were analyzed using one-way ANOVAs. For all 5 dialysate time points, OT release was converted into percent change from the average of the two baseline samples for each rat, and these percentages were analyzed using a two-way ANOVA for repeated measures (sex × dialysate). A bivariate correlation and a curve estimate regression analysis were used to determine correlations of social investigation time with the percentage of OT release during exposure to the social interest test (i.e., dialysate 3). Because of the outcomes of the correlation analysis, we subsequently grouped rats into those that showed low social interest (social investigation time lower than the average; males: < 83 seconds, n=4; females: < 48 seconds, n=4), and those that showed high social interest (social investigation time greater than the average; males: > 83 seconds, n=4; females: > 48 seconds, n=4). Social investigation time was then analyzed using two-way ANOVA (social interest × sex). To confirm a significant difference in social investigation time according to social interest group in each sex, one-way ANOVAs were run separately per sex. Furthermore, the percentage of OT release was analyzed using a three-way ANOVA for repeated measures (social interest × sex × dialysate). When appropriate, LSD post hoc tests were used to examine the nature of interaction effects. Finally, because of low numbers of females in estrus and non-estrus groups, effects of estrus phase on social interest, anxiety-related behavior, and locomotor activity in Experiments 1 and 2 were analyzed separately for each treatment by one-way ANOVAs. Only those treatments with at least an n=3 females in estrus and in non-estrus were included in the statistical analysis. Effect of estrus phase on the percentage of OT release in Experiment 3 was analyzed using a one-way ANOVA for repeated measures with estrus phase as covariate. None of these analyses showed an effect of estrus phase. Data are presented as mean + SEM, and significance was set at p<0.05.
Results
Experiment 1: Effects of pharmacological manipulations of the OT system in the MeA on social interest
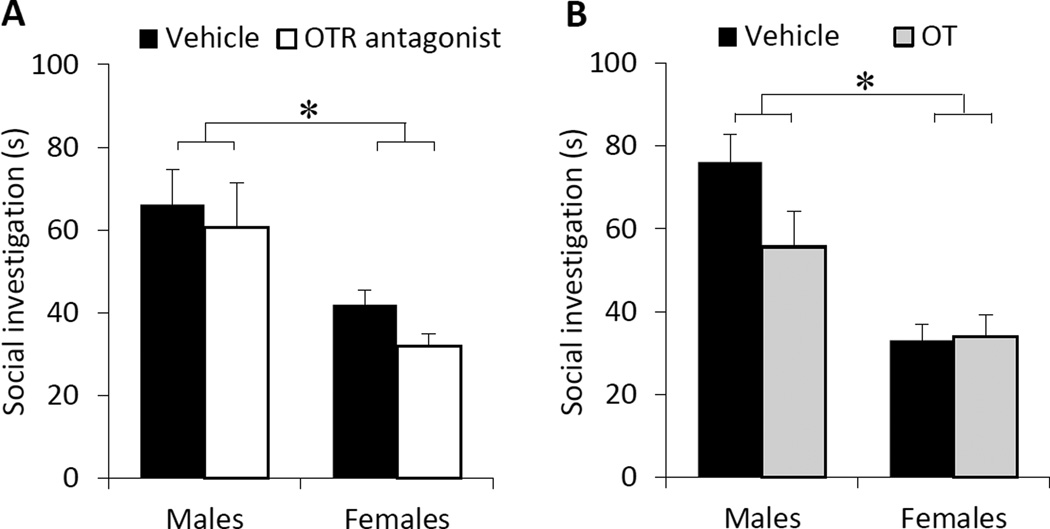
A significant main effect of sex was found for social investigation in both OTR manipulation experiments in the MeA (OTR antagonist: F(1,33)=18.0, p<0.001; OT: F(1,30)=24.8, p<0.0001), in which males showed higher social investigation time compared to females (Fig. 2A, B). However, OTR manipulations in the MeA did not alter social investigation time in either sex (OTR antagonist: treatment, F(1,33)=1.54, p=0.22; sex × treatment, F(1,33)=0.13, p=0.73; OT: treatment, F(1,30)=2.27, p=0.14; sex × treatment, F(1,30)=2.68, p=0.11; Fig. 2A, B).
Fig. 2. OTR manipulations in the medial amygdala do not affect social interest in either sex.
OTR antagonist (A) or OT (B) injections did not alter social investigation time in male and female adult rats. However, males showed higher social investigation time compared to females (A and B). Social investigation time represents the total investigation time in seconds (s) toward a novel, same-sex juvenile rat placed in the home cage of the experimental rat for a 4-min period. Data are presented as mean + SEM; * p<0.01, two-way ANOVA.
Experiment 2: Effects of pharmacological manipulations of the OT system in the CeA on social interest and anxiety-related behavior
Experiment 2a: Social interest
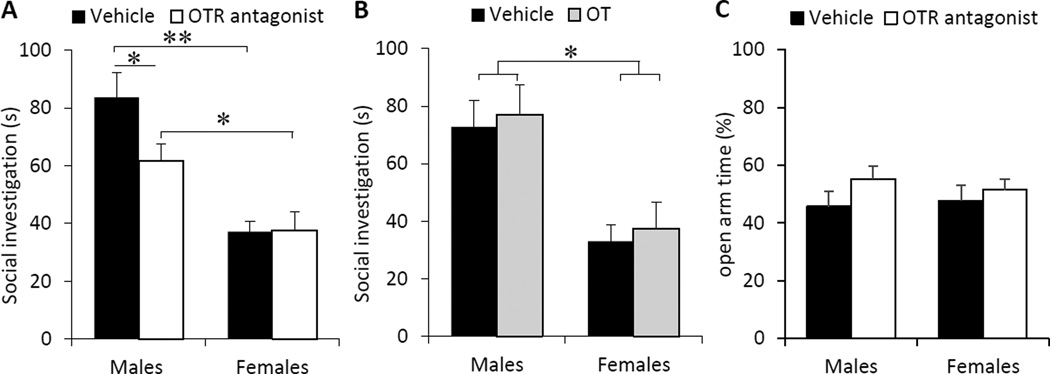
OTR blockade in the CeA decreased social investigation time in males, but not females (Fig. 3A). This is reflected by a significant main effect of treatment (F(1,23)=5.89, p<0.05) and sex × treatment (F(1,23)=6.25, p<0.05), with post hoc tests indicating that OTR antagonist-treated males showed lower social investigation time compared to vehicle-treated males (p<0.01; Bonferroni post hoc test), while the two female groups exhibited similar social investigation times (p=0.96; Bonferroni post hoc test). Furthermore, a main effect of sex was found for social interest, in which males showed higher social investigation time compared to females (F(1,23)=34.8, p<0.00001), and this was true for vehicle-treated rats (p<0.00001, Bonferroni post hoc test) and OTR antagonist-treated rats (p<0.05, Bonferroni post hoc test). OT injections did not alter social investigation time in either sex (treatment: F(1,33)=0.23, p=0.64; sex × treatment: F(1,33)=0.0000002, p=0.99; Fig. 3B), but again, a main effect of sex was found (F(1,33)=19.1, p<0.001) in which males showed higher social investigation time compared to females (Fig. 3B).
Fig. 3. The OTR in the central amygdala (CeA) modulates social interest in sex-specific ways, without an effect on anxiety-related behavior.
(A) OTR antagonist injections reduced social investigation time in male, but not female, adult rats. (B) OT injections had no effect on social investigation time in either sex. Males showed higher social investigation time compared to females (A and B). (C) OTR antagonist injections in the CeA had no effect on the percentage of time spent on the open arms of the elevated plus maze in either males or females. Social investigation time represents the total investigation time in seconds (s) toward a novel, same-sex juvenile rat placed in the home cage of the experimental rat for a 4-min period. Open arm time is the percentage of time spent on the open arms during a 5-min exposure to the elevated plus maze. Data are presented as mean + SEM; * p<0.01, ** p<O.OOOOl, two-way ANOVA followed by Bonferroni post hoc test.
Experiment 2b: Elevated plus maze
Because the OT system in the CeA is known to play a role in anxiety (Bale et al., 2001; László et al., 2016), we determined whether the effects of OTR blockade in the CeA on social investigation time in males were secondary due to effects on anxiety-related behavior. There were no effects of sex or OTR antagonist treatment on the percentage of time spent on the open arms (sex: F(1,23)=0.05, p=0.83, treatment: F(1,23)=1.95, p=0.18, sex × treatment: F(1,23)=0.27, p=0.61; Fig. 3C), or the percentage of open arm entries (vehicle-treated males=45.4 ± 2.8 %, vehicle-treated females= 46.9 ± 3.8 %, OTR antagonist-treated males= 47.1 ± 2.7 %, OTR antagonist- treated females= 46.7 ± 2.5 %; sex: F(1,23)=0.04, p=0.85; treatment: F(1,23)=1.07, p=0.31; sex × treatment: F(1,23)=2.63, p=0.12). Furthermore, there was no effect of sex or OTR blockade on locomotor activity, as reflected by the total number of arm entries (vehicle-treated males=24.7 ± 1.8, vehicle-treated females= 27.1 ± 1.9, OTR antagonist-treated males= 27.7 ± 2.7, OTR antagonist-treated females= 25.4 ± 1.5; sex: F(1,23)=0.002, p=0.96; treatment: F(1,23)=0.10, p=0.76; sex × treatment: F(1,23)=1.41, p=0.25).
Experiment 3: Extracellular OT release in the CeA
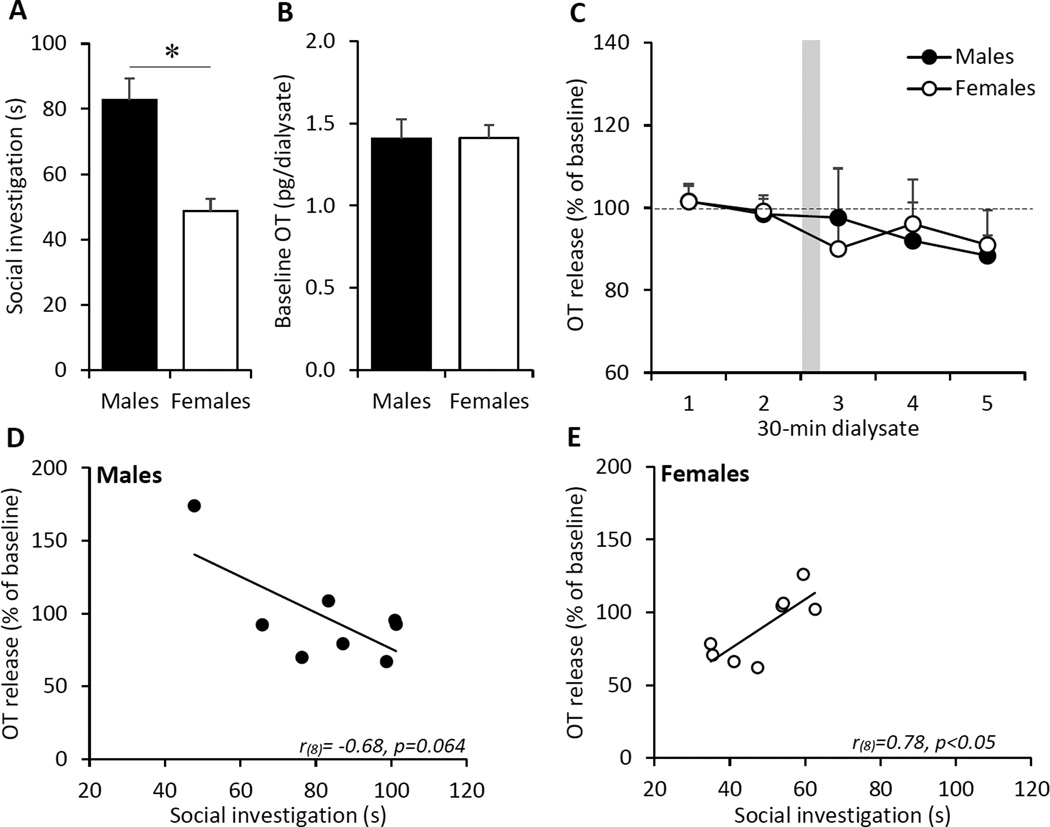
The observed sex-specific effect of OTR blockade in the CeA on social investigation time may suggest sex differences in the endogenous activation of OTR during exposure to the social interest test. Therefore, we determined potential sex differences in endogenous OT release in the CeA under baseline conditions and during exposure to the social interest test. Consistent with the sex difference found for social interest in experiments 1 and 2, males showed higher social investigation time compared to females during ongoing microdialysis (F(1,14)=19.7, p<0.001; Fig. 4A). However, there was no sex difference in baseline extracellular OT concentrations in the CeA (F(1,14)=0.0001, p=0.99; Fig. 4B), nor was there an effect of sex or dialysate on the percent change of OT release from baseline during exposure to the social interest test (sex: F(1,14)=0.15, p=0.71; dialysate: F(4,56)=1.11, p=0.36; sex × dialysate: F(4,56)=0.23, p=0.92; Fig. 4C). Correlation analysis revealed a trend toward a negative correlation between social investigation time and the percentage of OT release during the social interest test in males (r(8)= −0.68, p=0.064; Fig. 4D). However, this trend was mainly carried by one male showing low social investigation and high percentage of OT release. In females, there was a significant positive correlation between social investigation time and the percentage of OT release during the social interest test (r(8)=0.78, p<0.05; Fig. 4E).
Fig. 4. Extracellular OT release in the central amygdala (CeA) of male and female rats exposed to the social interest test.
(A) Male rats showed higher social investigation time compared to female rats in the social interest test during ongoing microdialysis. (B) Baseline extracellular OT concentrations in the CeA is similar between males and females. (C) The percentage of OT release in the CeA did not change in either sex when rats were exposed to the social interest test (dialysate 3). (D) In males, there was a trend toward a negative correlation between social investigation time and the percentage of OT release during the social interest test (p=0.064). (E) In females, social investigation time correlated positively with the percentage of OT release in the CeA during the social interest test (p<0.05). Social investigation time represents the total investigation time in seconds (s) toward a novel, same-sex juvenile rat placed in the home cage of the experimental rat for a 4-min period. The gray bar in (C) indicates the timing of the social interest test during sampling of the third microdialysate. Data are presented as mean + SEM; * p<0.001.
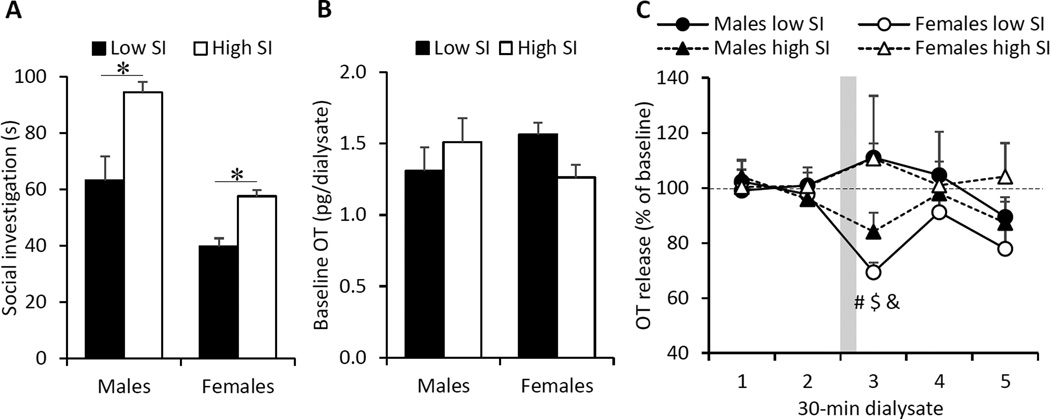
Because of the significant correlation in females, we subsequently divided rats into high and low social interest groups (based on average social investigation times within each sex), and confirmed significant differences between high and low social interest groups (F(1,12)=32.4, p<0.001, two-way ANOVA) within both sexes (males: F(1,6)=15.4, p<0.01; females: F(1,6)=24.6, p<0.01; one-way ANOVAs; Fig 5A). There was no effect of social interest on baseline OT concentrations in the CeA (social interest: F(1,12)=0.05, p=0.83; social interest × sex: F(1,12)=1.10, p=0.32, two-way ANOVA; Fig5B). However, a dialysate × social interest × sex interaction effect was found for the percentage of OT release (F(4,48)=3.30, p<0.05). In detail, the percentage of OT release during the social interest test (dialysate 3) in females with low social interest was lower compared to baseline (dialysate 1, 2, and 4; p<0.05, LSD post hoc) and compared to the percentage of OT release during the social interest test of females with high social interest (p<0.05; LSD post hoc test) and males with low social interest (p<0.05; LSD post hoc test; Fig. 5C).
Fig. 5. Extracellular OT release in the CeA of male and female rats showing high or low social interest (SI).
(A) Male and female rats were divided into high and low SI based on the average social investigation time within each sex. (B) Baseline extracellular OT concentrations were similar between males and females of both low and high SI groups. (C) The percentage of OT release during the social interest test (dialysate 3) in low SI female rats was lower compared to the percentage OT release in dialysate 1, 2, and 4 of low SI female rats, dialysate 3 of high SI female rats, and dialysate 3 of high SI male rats. Social investigation time represents the total investigation time in seconds (s) toward a novel, same-sex juvenile rat placed in the home cage of the experimental rat for a 4-min period. The gray bar indicates the timing of the social interest test during sampling of the third microdialysate. Data are presented as mean + SEM; *p<0.5, two-way ANOVA followed by one-way ANOVA; # p<0.05, versus dialysate 1, 2, and 4; $ p<0.05, versus dialysate 3 of high SI females; & p<0.05, versus dialysate 3 of low SI males; three-way ANOVA for repeated measures followed by LSD post hoc test.
Discussion
We previously observed correlations of OTR binding density in the MeA and CeA with social interest in male and female rats, respectively. This led us to hypothesize that activation of the OTR in the MeA would mediate male social interest while activation of the OTR in the CeA would mediate female social interest. Contrary to our hypotheses, pharmacological manipulations of MeA-OTR or CeA-OTR had no effect on social interest in males or females, respectively. Instead, we found that OTR antagonist (5 ng/0.5 µl/side) injected into the CeA decreased social investigation time in males. We then determined whether this sex-specific effect of CeA-OTR blockade on social interest could be explained by a sex difference in endogenous CeA-OT release. However, no sex differences were found in CeA-OT release at baseline or during exposure to the social interest test. This suggests that factors other than CeA-OT release mediate the sex-specific effect of CeA-OTR blockade on social interest. Yet, females with low social interest showed a decrease in OT release in the CeA during the social interest test. As discussed below, we propose that this change in OT release may be a consequence, rather than a cause, of social stimulus exposure. Together, we show for the first time that the OTR in the CeA plays a causal role in the acute modulation of social interest in male rats.
Causal involvement of OTR activation in the CeA in social interest in males
We previously showed that OTR binding density in the CeA negatively correlates with social investigation time in female, but not male, rats (Dumais et al., 2013). This suggested a role for the OTR in the CeA in regulating social investigation in females. However, in the current study, neither OTR antagonist (5 ng/0.5 µl/side) nor OT (100 pg/0.5 µl/side) administration into the CeA altered social investigation time in females. Although it is unknown whether a higher dose of OTR antagonist or OT would alter social investigation time in females, the same dose of OTR antagonist in the CeA decreased social investigation time in males. This may suggest that, during the social interest test, OTR activation is higher in males than in females to promote social investigation. Therefore, we tested the hypothesis that CeA-OT release is higher in males than in females during social interest. However, males and females showed similar levels of extracellular OT release in the CeA under baseline conditions and during exposure to the social interest test. Moreover, there was no change in extracellular OT release during the social interest test compared to baseline in either sex. It is possible that the relatively short (4-min) duration of the social stimulus exposure compared to the relatively long (30-min) dialysate sampling period (which is required due to detection limits of the radioimmunoassay) could have obscured a possible significant rise in OT release during social investigation. This may also explain why, in two other similarly designed microdialysis studies, OTR blockade in the lateral septum of male rats (Lukas et al., 2013) or OTR blockade in the posterior bed nucleus of the stria terminalis of female rats (Dumais et al., 2016) impaired social recognition without a corresponding change in local extracellular OT release. Alternatively, the absence of a change in OT release may indicate that baseline OT release is sufficient to facilitate social investigation in males. In this scenario, baseline OT release could have a neuromodulatory role in the regulation of social behavior by e.g., altering the actions of fast-acting neurotransmitters (Joëls, 2000). Whether this indeed explains a role for baseline OT release in facilitating social interest in males remains to be tested.
Our finding that OTR blockade in the CeA has a sex-specific effect on social behavior regardless of OTR binding density (Dumais et al., 2013) and OT release (current study) in the CeA being similar between males and females offers a new level of complexity to the mechanisms by which OT may regulate social behavior in sex-specific ways. How, then, is OT acting in sex-specific ways in the CeA? One mechanism may be through a sex difference in OTR-expressing neurons in the CeA. Male rats have fewer GABAergic neurons in the CeA than female rats (Stefanova, 1998) and GABAergic neurons in the CeA express OTR (Huber et al., 2005). This could produce a sex difference in the percentage of OTR-expressing neurons that are GABA-ergic (potentially being lower in males compared to females) which may cause a sex difference in CeA output in response to OTR inhibition, a hypothesis that would require further testing. The sex-specific effect of OTR blockade could also be mediated by sex differences in relative projections from the CeA, such as sex differences in the density of efferents from the CeA, or sex differences in social stimulus-induced activation of specific CeA projections. This would require systematic comparative analysis of CeA efferents in males and females, because current CeA tracing studies have not included a comparison between sexes (Wallace et al., 1992; Petrovich and Swanson, 1997; Zahm et al., 1999; Dong et al., 2001; Petrovich et al., 2001).
Importantly, we showed that OTR blockade in the CeA did not affect anxiety-related behavior in male or female rats as measured on the elevated plus maze. This lack of effect on anxiety is despite the well-known role of the OT system in the CeA in the modulation of both fear and anxiety-related behaviors. For example, an increase in OT activity in the CeA has been found to decrease freezing in fear-conditioned male (Viviani et al., 2011; Lahoud and Maroun, 2013) and female (Knobloch et al., 2012) rats. Furthermore, OT administration in the CeA increased time spent on the open arm on the elevated plus maze in male rats (László et al., 2016) and in the middle quadrant in the open field test in female rats (Bale et al., 2001). However, our finding is consistent with several studies showing that acute OTR antagonist injections in the CeA or lateral ventricle have no effect on anxiety-related behaviors (Neumann et al., 2000; Slattery and Neumann, 2010; László et al., 2016). Therefore, although exogenously administered OT in the CeA may reduce anxiety-related behavior, blockade of endogenous OT release via OTR antagonist has not been found to affect anxiety-related behavior. This suggests that the sex-specific effect of OTR blockade in the CeA on social interest is less likely due to an effect on general anxiety.
The lack of an effect of OT administration in the CeA on social investigation time in either sex could be a dose-dependent effect. Because recent studies have shown that OT can mediate its behavioral effects via the vasopressin Via receptor (Schorscher-Petcu et al. 2010; Sala, et al. 2011; Ramos, et al. 2013; Qiu, et al. 2014; Song et al., 2014) and the CeA expresses OTR and Via receptor (Huber et al., 2005), it is also possible that OT activated both the OTR and the Via receptor in the CeA. Given that OTR and Via receptor are expressed on CeA neurons that mediate opposing behavioral effects (Viviani and Stoop, 2008), this could have resulted in an overall null effect. Such cross-reactivity of the OTR antagonist with the Via receptor is less likely to have occurred in the current study given that the OTR antagonist is about 20 times more potent to bind OTR than the Via receptor (Manning et al., 1989).
Dynamic change in CeA-OT release in females may be a consequence of low social investigation time
We recently reported a negative correlation between OTR binding density in the CeA and social investigation time in female rats (Dumais et al., 2013). We now find a positive correlation between the percentage of OT release in the CeA during the social interest test and social investigation time in female rats. A possible explanation could be that higher OTR binding density compensates for lower OT release. Determining OTR binding density and OT release in the CeA as well as social interest in the same rat may provide an answer to this possibility.
Furthermore, our data suggests that there may not be a causal role of the correlations between OTR binding/OT release and social investigation time in females, as neither OTR antagonist (5 ng/0.5 µl/side) nor OT (100 pg/0.5 µl/side) administration into the CeA altered social investigation time in females. Despite that, low social interest females did show a significant decrease in OT release in the CeA during exposure to the social interest test. We therefore suggest the possibility that the decrease in OT release in the CeA of females with low social interest is a consequence rather than a cause of low social investigation time. Indeed, previous studies provide evidence that exposure to a social stimulus can be associated with a change in OT release that does not seem to have an immediate effect on the behavior tested. For example, OT release in the lateral septum increased during retrieval of social memory in male rats, but blocking the actions of OT had no effect on the retrieval of social memory (Lukas et al., 2013). Likewise, OT release in the bed nucleus of the stria terminalis increased in male rats during exposure to a juvenile, but blocking the actions of OT had no effect on the behavior towards the juvenile (Dumais et al., 2016). In these studies, and in our present study, it could be that the change in OT release brings about changes in neural processes that will serve future behaviors. Support for this comes from studies with prairie voles, in which females given an OTR antagonist immediately prior to exposure to a male showed normal mating behavior, but impaired partner preference formation when tested 14 or 24 hours later (Insel and Hulihan, 1995), suggesting that OT activity during mating is critical for the subsequent formation of later partner preference. It would be of interest to determine whether the decrease in OT release in the CeA of females with low social interest has effects on subsequent behaviors by, e.g., affecting social memory for the exposed juvenile.
OTR in the MeA may not be involved in regulating social interest toward juvenile conspecifics
We recently showed that OTR binding densities in the MeA are higher in male rats compared to female rats, and that OTR binding density in the MeA correlates positively with social investigation time in males (Dumais et al., 2013). In addition, OTR mRNA expression in the MeA correlates positively with social investigation time in male mice (Murakami et al., 2011). These findings suggested a role for the OTR in the MeA in mediating social investigation in males. However, in the current study, neither OTR antagonist (5 ng/0.5 µl/side) nor OT (100 pg/0.5 µl/side) administration into the MeA altered social investigation time in either sex. Although the same dose of OTR antagonist and OT were effective in altering social behavior when administered into the CeA (present study) or the bed nucleus of the stria terminalis (Dumais et al., 2016), respectively, we cannot exclude that higher drug doses could be effective in altering social interest. Future research is therefore required to determine whether different doses of OT or OTR antagonist in the MeA modulates social interest towards juvenile conspecifics. Alternatively, this lack of effect could be due to the low social salience of juvenile conspecifics. In support, OTR antagonist administered into the MeA of adult male rats was found to reduce the investigation of soiled bedding from unfamiliar adult male conspecifics (Arakawa et al., 2010), but did not affect investigation toward juvenile or ovariectomized female rats (Lukas et al., 2013). Furthermore, extracellular recordings revealed that the MeA responds to social stimuli in both male and female mice, but stronger to opposite-sex stimuli compared to same-sex-stimuli (Bergan et al., 2014). Finally, MeA regulation of social recognition also depends on the salience of the social stimulus, as OTR blockade in the MeA impaired recognition of an ovariectomized female in male mice (Ferguson et al., 2001) and rats (Lukas et al., 2013), but not the recognition of a juvenile conspecific (Lukas et al., 2013). Taken into account the difference in social stimuli (i.e., adult male, adult ovariectomized female, and juvenile conspecific) an interesting pattern emerges, such that the OT system in the MeA facilitates social interest and social recognition depending on the salience of the social stimulus. This hypothesis requires further testing.
Relevance of comparing males and females in studying the role of the amygdala-OT system in behavioral regulation
Given the key role of the OT system in the CeA in mediating incentive learning and emotion processing (Balleine and Killcross, 2006; Ledoux, 2000), fear and anxiety responses (Bale et al., 2001; Viviani et al., 2011; Knobloch et al., 2012; Lahoud and Maroun, 2013; László et al., 2016), and aggression in rodents (Lubin et al., 2003; Bosch et al., 2005; Consiglio et al., 2005; Calcagnoli et al., 2015), it is surprising that, to the best of our knowledge, we are the first to compare the role of the OT system in the CeA between males and females. This comparison is important given that human studies demonstrated that OT modulates amygdala function differently in men and women. Specifically, it was found that intranasal OT administration altered amygdala activation in opposite ways in men and women toward socially-relevant stimuli, such as exposure to fearful faces and during cooperative social interactions (Domes et al., 2007, 2010; Rilling et al., 2012, 2014). Importantly, the idea that the amygdala may be a site for sex-specific action of OT could be of relevance for the treatment of sex-biased psychiatric disorders of social dysfunction. Indeed, altered amygdala functioning is a main characteristic of autism spectrum, social anxiety, and borderline personality disorders (Baron-Cohen et al., 2000; Kleinhans et al., 2016; Kim et al., 2015; Evans et al., 2008; Goldin et al., 2009; Bruhl et al., 2014; Donegan et al., 2003; Herpertz et al., 2001) and has been found to be normalized in response to intranasal OT (Domes et al., 2013; Bertsch et al., 2013; Labuschagne et al., 2010). Therefore, it is imperative to further understand the sex-specific role of the OT system in the amygdala and its subregions in the regulation of social behavior in both rodents and humans.
Conclusion
Sex differences in the effects of the OT system on various social behaviors are well known (for review, see Dumais and Veenema, 2015, 2016). Our current finding adds to this growing body of literature by showing a sex-specific role of the CeA-OT system in the regulation of social interest, in which OTR blockade (5 ng/0.5 µl/side) in the CeA of rats alters social interest in males but not females. This sex-specific modulation of social interest by the CeA-OTR is found despite there being no sex differences in OTR binding density or extracellular OT release in the CeA. Further research is required to investigate the underlying mechanisms, as well as whether the sex-specific function of the OT system in the CeA extends to the regulation of other social behaviors. Overall, we show that in rodents, much like humans, the amygdala is a key brain region that is differentially modulated by the OT system in males and females during the processing of social cues.
Highlights.
OT receptor blockade in the CeA decreased social interest in males, but not females
There is no sex difference in CeA-OT release at baseline or during social interest
CeA-OT release correlated positively with social investigation time in females only
CeA-OT release decreased during social investigation in females with low social interest
The OT system in the CeA regulates social interest in sex-specific ways
Acknowledgments
We would like to thank the Veenema Lab for critically reading the manuscript, and Marisa Immormino, Daniel Cho, and Tessa Gillespie for technical assistance. We would also like to thank the animal caretakers at Boston College for excellent animal care, and Dr. Maurice Manning for kindly providing the OTR antagonist. This research is supported by NRSA Predoctoral Fellowship F31MH100891 to KMD and NIMH R15MH102807 to AHV.
Abbreviations
- OT
Oxytocin
- OTR
Oxytocin receptor
- MeA
Medial amygdala
- CeA
Central amygdala
Glossary
- Social interest
social investigation toward a novel conspecific for the assessment of social cues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Authors Kelly M. Dumais and Alexa H. Veenema designed the study and wrote the manuscript. Author Kelly Dumais performed all experiments and analyzed the data. Authors Andrea G. Alonso and Remco Bredewold assisted in the experiments. Author Remco Bredewold edited the manuscript. All authors have approved the final manuscript.
References
- Arakawa H, Arakawa K, Deak T. Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience. 2010;171(4):1141–1151. doi: 10.1016/j.neuroscience.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29(5):272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. Elife. 2014 doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kastel T, Schnell K, Buchel C, Domes G, Herpertz SC. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry. 2013;170(10):1169–1177. doi: 10.1176/appi.ajp.2013.13020263. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535(2):301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in Beh Neurosci. 2014;8(216):1–11. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder – a meta-analytic review resulting in a new neurofunctional model. Neurosci Behav Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology. 2015;90:74–81. doi: 10.1016/j.neuropharm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85(3):354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus D, Herpertz S. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossman A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Filbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54(11):1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nucleus of the stria terminalis. Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2015;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Presence and absence of sex differences in structure and function of the brain oxytocin system: Implications for understanding social behavior. In: Shansky R, Johnson J, editors. Sex Differences in the Central Nervous System. Elsevier; 2016. pp. 247–284. [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25(6):496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):630–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61(3):410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guthman EM, Vera J. A cellular mechanism for main and accessory olfactory integration at the medial amygdala. J Neurosci. 2016;36(7):2083–2085. doi: 10.1523/JNEUROSCI.4304-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16(9):1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, Radulovic J. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology. 2014;231(10):2097–2105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70(3):245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50(4):292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Horn TF, Engelmann M. In vivo microdialysis for nonapeptides in rat brain- a practical guide. Methods. 2001;23(1):41–53. doi: 10.1006/meth.2000.1104. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Joëls M. Modulatory actions of steroid hormones and neuropeptides on electrical activity in brain. Eur J Pharmacol. 2000;405:207–216. doi: 10.1016/s0014-2999(00)00554-9. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Kim SY, Choi US, Park SY, Oh SH, Yoon HW, Koh YJ, Im WY, Park JI, Song DH, Cheon KA, Lee CU. Abnormal activation of the social brain network in children with autism spectrum disorder: an FMRI study. Psychiatry Investig. 2015;12(1):37–45. doi: 10.4306/pi.2015.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Greenson J, Dawson G, Aylward E. Altered dynamics of the fMRI response to faces in individuals with autism. J Autism Dev Disord. 2016;46(1):232–241. doi: 10.1007/s10803-015-2565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch SH, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud N, Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology. 2013;38(10):2184–2195. doi: 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- László K, Kovacs A, Zagoracz O, Ollmann T, Peczely L, Kertes E, Lacy DG, Lenard L. Positive reinforcing effect of oxytocin microinjection in the rat central nucleus of amygdala. Behav Brain Res. 2016;296:279–285. doi: 10.1016/j.bbr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117(2):195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38(6):916–926. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Manning M, Kruszynski M, Bankowski K, Olma A, Lammek B, Cheng LL, Klis WA, Seto J, Haldar J, Sawyer WH. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem. 1989;32(2):382–391. doi: 10.1021/jm00122a016. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A. Segregated pathways to the vomeronasal amygdala: differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci. 2007;25(7):2065–2080. doi: 10.1111/j.1460-9568.2007.05472.x. [DOI] [PubMed] [Google Scholar]
- Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci. 2011;34(3):469–477. doi: 10.1111/j.1460-9568.2011.07761.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95(2):567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Noack J, Murau R, Engelmann M. Consequences of temporary inhibition of the medial amygdala on social recognition memory performance in mice. Front Neurosci. 2015;9(15) doi: 10.3389/fnins.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. San Diego: Academic Press; 1998. [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38(1–2):247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763(2):247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z, Li JD, Zhou QY, Hu WP. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171(12):3065–3076. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38(11):2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young U. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacological rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young U, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30(24):8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58(1):56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JK, 4th, Larkin TE, 2nd, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N. Gamma-aminobutyric acid-immunoreactive neurons in the amygdala of the rat – sex differences and effect of early postnatal castration. Neurosci Lett. 1998;255(3):175–177. doi: 10.1016/s0304-3940(98)00735-6. [DOI] [PubMed] [Google Scholar]
- Thor DH. Testosterone and persistence of social investigation in laboratory rats. J Comp Physiol Psychol. 1980;94(5):970–976. doi: 10.1037/h0077831. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt BA, Spear LP. Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Dev Psychobiol. 2013;55(7):684–697. doi: 10.1002/dev.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:260–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Viviani D, Stoop R. Opposite effects of oxytocin and vasopressin on the emotional expression of the fear response. Prog Brain Res. 2008;170:207–218. doi: 10.1016/S0079-6123(08)00418-4. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopamine, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull. 1992;28(3):447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., 3rd Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci. 1999;11(4):1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]