Abstract
Background and Purpose
The utility of prophylactic antiepileptic drug (AED) administration following spontaneous subarachnoid hemorrhage (SAH) remains controversial. AEDs have not clearly been associated with a reduction in seizure incidence and have been associated with both neurologic worsening and delayed functional recovery in this setting.
Methods
We retrospectively analyzed a prospectively collected database of SAH patients admitted to our institution between 2005 and 2010. Between 2005 and 2007, all patients received prophylactic AEDs upon admission. After 2007 no patients received prophylactic AEDs or had AEDs immediately discontinued if initiated at an outside hospital. A propensity score-matched analysis was then performed to compare the development of clinical and/or electrographic seizures in these two populations.
Results
353 patients with spontaneous SAH were analyzed, 43% of whom were treated with prophylactic AEDs upon admission. Overall, 10% of patients suffered clinical and/or electrographic seizures, most frequently occurring within 24-hrs of ictus (47%). The incidence of seizures did not vary significantly based on the use of prophylactic AEDs (11 vs. 8%, p=0.33). Propensity score-matched analyses suggest that patients receiving prophylactic AEDs had a similar likelihood of suffering seizures as those who did not (p=0.49).
Conclusions
Propensity score-matched analysis suggests that prophylactic AEDs do not significantly reduce the risk of seizure occurrence in patients with spontaneous SAH.
Keywords: Seizure, SAH, Antiepileptic Drug
Subject Terms: Intracranial Hemorrhage, Cerebral Aneurysm, Pharmacology, Treatment, Quality and Outcomes, Clinical Studies, Complications
INTRODUCTION
The development of seizures following spontaneous subarachnoid hemorrhage (SAH) is a well-documented phenomenon. Pathophysiologic processes involved in the development of post-SAH seizures include both acute biochemical dysfunction and delayed gliotic cellular reorganization. Seizure activity has been associated with secondary neurologic injury including reduced cerebral blood flow and increased intracranial pressure.1 Seizures occurring after SAH have been associated with clinical and radiographic markers of hemorrhage severity (higher SAH grade/extent of SAH blood burden, lower Glasgow Coma Scale score at presentation, etc.), as well as rebleeding and delayed ischemic neurologic deficits (DIND, or cerebral vasospasm).2,3 Literature has reported seizure rates to be as high as 27% in this population.4 These high rates of seizures, as well as concern over the possible consequences of a seizure in the setting of an unsecured aneurysm, has led to routine prophylactic administration of antiepileptic drugs (AEDs) following SAH in many centers.2,3
More recently published studies have found seizure rates to be significantly lower than previously described (1–10%).2,5 Of added importance, newer literature has suggested increased adverse effects associated with post-hemorrhagic AED exposure; including serious drug related complications as well as worse cognitive and functional outcomes.6 Systematic reviews examining AEDs and seizures following SAH found no recent literature supporting the effectiveness of AED prophylaxis.3,7 Furthermore, up to 21% of those receiving AED prophylaxis suffered adverse medication side effects, including impaired liver function, thrombocytopenia, rash, and Stevens-Johnson syndrome. Few studies to date have specifically evaluated prophylactic AED treatment protocols, and only one has detailed the incidence and risk factors of clinical seizures in patients not receiving prophylactic AED medications.4
The purpose of this study was to evaluate whether prophylactic administration of AEDs significantly decreased the incidence of post-SAH seizures. We hypothesized that the prophylactic AEDs would not be associated with a decreased risk of seizure following SAH. To evaluate this, we performed a propensity score-matched analysis of patients with spontaneous SAH treated with or without prophylactic AEDs to assess the incidence of clinical and electrographic seizures during initial hospitalization.
METHODS
Patient Population
We carried out a retrospective review of prospectively collected data on all patients presenting to UPMC Presbyterian Hospital for spontaneous SAH from February-2005 to October-2010. The Institutional Review Board of the University of Pittsburgh approved this study (IRB#021039), all participants (and/or their representative) gave informed consent, and all procedures were in accordance with institutional guidelines. Patients diagnosed with a cerebral aneurysm as well as those without an identifiable etiology for SAH on angiography were included for analysis. Patients were excluded if SAH was secondary to trauma, arteriovenous malformation or fistula, spontaneous intraparenchymal hemorrhage, or inflammatory vasculopathy. Baseline demographic information, clinical characteristics at presentation, aneurysm morphology and treatment modality, AED prescription characteristics (type, timing, and duration of use), and clinical course were all recorded prospectively and analyzed. A universally accepted grading scheme for hemorrhage severity does not exist, as such, cisternal SAH burden was defined as scant (≤1mm of layering in ≤3 basal cisterns or <2 contiguous axial CT slices), diffuse (≤1mm in >3 basal cisterns on multiple slices), or severe (>1mm in all basal cisterns on contiguous slices) as demonstrated on admission head CT (standard axial non-contrast protocol performed at ≤5mm slice thickness).
Antiepileptic Drugs
Antiepileptic drug administration at our hospital or the referring facility was defined as either prophylactic (prior to documented clinical or EEG seizure activity) or therapeutic. Between February-2005 and July-2007, departmental protocol was to administer prophylactic AEDs upon presentation for all patients suffering spontaneous SAH. The dose and duration of treatment were left to the discretion of the attending neurosurgeon. Phenytoin (PHT) was the predominant prophylactic AED administered (81%), and included a loading dose of 1,000mg followed by 100mg every 8-hours. The remaining patients received prophylactic levetiracetam (LEV), given as 1,000mg doses every 12-hours. Prophylactic AEDs were administered for 7 to 30 days (mean 14±8 days) with the duration at the discretion of the treating neurosurgeon. As part of an institutional SAH Quality Improvement initiative and revision of departmental protocol in July-2007, patients presenting with spontaneous SAH no longer received prophylactic AEDs on admission, and AEDs initially administered at the referring hospital were immediately discontinued. Regardless of when or where the patient initially presented, those who received prophylactic AED were analyzed as the treatment cohort (including only a one-time bolus dose). Serum levels of AEDs administered for prophylaxis were not checked. Patients who did not receive AED were analyzed as controls.
Clinical or electrographic seizure activity at UPMC was treated with an AED, with type, dose, and duration dependent upon preferences of the managing physicians and response to medications. Patients undergoing craniotomy for aneurysm clipping and/or clot evacuation that received prophylactic AEDs post-op were assigned to the treatment cohort; duration was at the discretion of the treating neurosurgeon (range 7 to 30 days).
Outcomes
The primary endpoint was seizure occurrence diagnosed clinically and/or through electroencephalography (EEG) during the initial hospitalization period. A neurologist, neurosurgeon, and/or neurointensivist must have witnessed clinical seizure activity in order to be recorded as clinically positive. As such it was impossible for adjudication of clinical seizure to be performed without knowledge of AED treatment status (i.e. masked). Clinical seizure activity included intermittent twitching, episodic unidirectional nystagmus, perioral unilateral twitching, paroxysmal autonomic signs, or motor automatism with suspicion of seizure. Electroencephalographic monitoring was performed in all patients unable to be followed clinically due to depressed mental status or in whom a high clinical suspicion of seizure activity existed. Initial monitoring was performed over a 2-hour period for acute evaluation and repeated 12- to 24-hours later if clinical suspicion of seizure activity remained. Patients suffering electrographically confirmed seizures were continuously monitored until epileptiform activity was controlled. EEG output was recorded digitally using 21 scalp electrodes placed according to the international 10–20 system. Results were interpreted by an attending neurophysiologist/neurologist without knowledge of AED treatment status (i.e. masked). Positive seizure activity was defined as focal or generalized epileptiform discharges according to 2012 American Clinical Neurophysiology Society (ACNS) Standardized Critical Care EEG Terminology.8,9 Consequently, generalized spike and wave patterns slower than 3/s and/or evolving discharges that remained slower than 4/s were not recorded as positive.
Secondary outcomes evaluated included timing (acute, subacute or delayed) and type of seizure activity (clinical vs. electrographic vs. both), incidence of DIND (defined as neurological deterioration due to impaired cerebral blood flow on angiogram and/or elevated transcranial Doppler velocities), and 12-month functional outcome as measured by modified Rankin Score (mRS, assessed by trained research staff in-person during routine 12-month follow-up clinic appointments).
Statistical Analysis
Acceptable Type I error was set a priori at α=0.05 for all statistical tests. Patients with missing and/or incomplete clinical, radiographic, or outcome data were excluded from all analyses. Continuous demographic characteristics were assessed for normality using the Kolmogorov-Smirnov test; normally distributed data were analyzed by t-test while the remainders were compared using the Wilcox Rank-Sum test. Categorical data were analyzed with Pearson’s chi-squared or Fisher’s exact test. Univariable logistic regression analysis was performed to assess for predictors of seizure activity. Multivariable logistic regression used to adjust for variables identified by univariate analysis to be marginally different between the outcomes (the probability value for inclusion was set at p<0.2). Secondary endpoints, including DIND were analyzed using multivariable logistic regression adjusting for known predictors of functional outcome.
Because prophylactic AED treatment was not randomly assigned the decision was made a priori to perform analyses using propensity score matching to account for clinical covariates associated with prophylactic AED administration. The covariates used to generate this propensity score were clinical characteristics (admission Hunt-Hess score, cisternal SAH thickness, intraventricular hemorrhage, and intraparenchymal hemorrhage), procedural characteristics (aneurysm occlusion modality, craniotomy for hemorrhage evacuation), and monitoring characteristics (use of EEG monitoring). After propensity score generation, patients underwent 1:1 nearest neighbor (Greedy-type) matching of the logit of the propensity score with a caliper width of 0.25. Matching between those receiving prophylactic AEDs (treatment) and those who did not (control) was performed without replacement and unpaired treated and control patients not meeting matching criteria were excluded. Each propensity score-derived matched pair was assigned a unique pair ID. Improvement in covariate balance after matching was determined using conditional logistic regression, conditioned on the pair ID. Occurrence of seizure was then compared between treatment and control propensity-matched patients using univariate statistics. Data were analyzed using Stata version 14 (StataCorp, College Station, TX).
RESULTS
Baseline Characteristics
From July-2005 through October-2010 353 patients were admitted with a diagnosis of spontaneous SAH. Baseline clinical characteristics for these patients are displayed in Table 1 by prophylactic AED status. Forty-three percent (n=152/353) of patients received prophylactic AEDs either on admission or following craniotomy (Figure 1). Clinical characteristics that varied significantly between those receiving prophylactic AEDs or not included age, aneurysmal etiology, and presence of acute hydrocephalus (all p<0.01). The remaining baseline demographic characteristics, as well as clinical and radiographic surrogates of SAH severity (Hunt-Hess score, cisternal SAH burden, intraventricular hemorrhage, intraparenchymal hemorrhage, etc) were not significantly different between treatment cohorts.
Table 1.
Demographic and clinical characteristics
| Characteristic | No AEDs %(n=201) |
Prophylactic AEDs %(n=152) |
p value |
|---|---|---|---|
| Baseline | |||
| Age, mean±SD yrs | 56±13 | 52±11 | 0.01 |
| Sex,% female | 61 (122) | 69 (105) | 0.15 |
| Aneurysmal SAH etiology,% | 75 (150) | 99 (150) | 0.01 |
| SAH severity on admission | |||
| Hunt-Hess score, median(IQR) | 3 (1) | 3 (1) | 0.92 |
| Severe SAH,% Hunt-Hess>3 | 22 (45) | 22 (34) | 0.99 |
| Severe cisternal SAH burdena,% | 90 (180) | 67 (102) | 0.01 |
| Intraventricular hemorrhage,% | 9 (18) | 18 (27) | 0.01 |
| Intraparenchymal hemorrhage,% | 22 (44) | 20 (31) | 0.73 |
| Acute hydrocephalus,% | 65 (131) | 38 (58) | 0.01 |
| Treatment | |||
| Craniotomy for any reason,% | 14 (29) | 37 (56) | 0.01 |
| Coil occlusion,% | 65 (130) | 63 (95) | 0.67 |
>1mm hemorrhage in all basal cisterns on contiguous CT slices IQR: interquartile range
Figure 1.
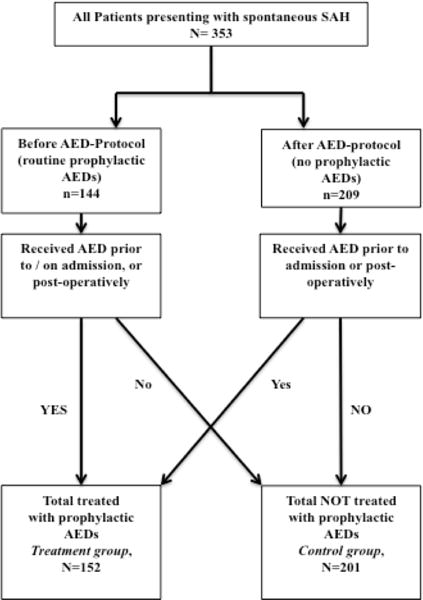
Flux diagram of treatment cohorts.
Seizure Characteristics
Overall, clinical and/or electrographic seizure activity was noted in 10% (n=34/353) of patients (Table 2). Mean seizure onset was 3.6±4.4 days post-ictus, and occurred up to 16 days post-ictus. Seizure onset occurred in 47% (n=16/34) within 24 hours of ictus, 35% (n=12/34) perioperatively (1–7 days), and 17% delayed (>7 days; n=6/34)); >75% (n=27/34) of seizures occurred within 4 days of hemorrhage.
Table 2.
Distribution of seizure characteristics and secondary outcomes
| No AEDs %(n=201) |
AEDs %(n=152) |
p value | |
|---|---|---|---|
| Seizures,% overall | 9 (22) | 11 (12) | 0.59 |
| Clinical | 5 (11) | 3 (4) | |
| Electrographic | 3 (6) | 5 (7) | |
| Both | 2 (5) | 1 (1) | |
| EEG monitoring,% | 39 (78) | 34 (52) | 0.38 |
| Seizure,% of those monitored | 14 (11) | 15 (8) | 0.84 |
| DIND,% | 12 (24) | 36 (51) | 0.01 |
| 12-month mRS, median (IQR) | 2 (5) | 1 (3) | 0.45 |
| Poor outcomea,% | 32 (50) | 25 (33) | 0.20 |
mRS 4–6. mRS: Modified Rankin Scale. DIND: delayed ischemic neurologic deficit. IQR: interquartile range
Of the patients suffering post-ictal seizures, 44% (n=15/34) were diagnosed on clinical exam alone, 38% on EEG alone (n=13/34), and 18% had clinical findings confirmed on EEG (n=6/34). Of the 130 patients who underwent EEG monitoring during the initial post-ictal hospitalization, 15% (n=19/130) displayed epileptiform changes. Patients undergoing EEG monitoring tended to be older (OR1.03, 95%CI 1.01–1.05), of poor Hunt-Hess grade (OR4.84, 95%CI 2.83–8.25), suffer worse SAH burden (OR2.12, 95%CI 1.17–3.85), and have intraparenchymal hemorrhage (OR2.38, 95%CI 1.14–4.01). Notably, diagnosis of electrographic seizure did not vary significantly according to the timing of EEG (i.e. at which point during the hospital patient’s course).
Factors Associated with Risk of Seizure
Univariate analysis of demographic and clinical characteristics revealed that risk of clinical and/or electrographic seizure was significantly associated with poor neurologic grade on admission, cisternal SAH burden, and intraventricular hemorrhage (p<0.05; Table 3). Treatment with prophylactic AED was not significantly associated with risk of seizure. Furthermore, admission epoch (during or after management change), aneurysmal SAH source, and aneurysm treatment modality (craniotomy and clip occlusion vs. coil embolization) were also not significantly associated with risk of seizure.
Table 3.
Overall seizure risk by clinical characteristic
| Univariate | Adjusteda | |||
|---|---|---|---|---|
| Characteristic | OR; 95%CIb | p value | OR; 95%CIb | p value |
| Baseline | ||||
| Agec | 1.02;0.99–1.05 | 0.09 | 1.02;0.98–1.05 | 0.27 |
| Female | 1.29;0.62–2.66 | 0.48 | 1.35;0.63–2.89 | 0.44 |
| Aneurysmal SAH etiology | 1.92;0.57–6.52 | 0.30 | 1.79;0.51–6.04 | 0.36 |
| Aneurysm location, anterior vs. posterior circ. | 1.15;0.93–1.42 | 0.19 | 1.16;0.93–1.44 | 0.18 |
| Severe Hunt-Hess scored | 2.24;1.54–3.24 | 0.01 | 2.78;1.27–6.09 | 0.01 |
| Severe SAH burdene | 4.41;1.03–18.9 | 0.04 | 2.79;0.63–12.4 | 0.18 |
| Intraparenchymal hemorrhage | 1.62;0.74–3.57 | 0.22 | 0.81;0.33–2.01 | 0.64 |
| Intraventricular hemorrhage | 2.40;1.15–5.03 | 0.02 | 3.27;1.40–7.67 | 0.01 |
| Acute Hydrocephalus | 1.67;0.79–3.49 | 0.17 | 1.33;0.61–2.94 | 0.48 |
| Treatment period, no prophylactic AEDsf | 0.86;0.43–1.75 | 0.68 | 0.95; 0.42–2.11 | 0.89 |
| Aneurysm treatment | ||||
| Aneursym clipping | 1.15;0.52–2.57 | 0.73 | 1.20;0.51–2.81 | 0.68 |
| Seizure prophylaxis | ||||
| AED administration | 0.70;0.33–1.46 | 0.34 | 0.58;0.25–1.32 | 0.25 |
| AED duration | 1.01;0.93–1.10 | 0.85 | 1.02;0.93–1.13 | 0.56 |
Adjustment for neurologic grade on admission, cisternal SAH burden, and intraventricular hemorrhage;
Significant predictors (p≤0.05) are reported as odds ratio & 95% confidence intervals;
Wilcoxon-Mann-Whitney test;
Admission Hunt-Hess score >3;
>1mm hemorrhage in all basal cisterns on contiguous CT slices;
Treatment period before versus after protocol change to no prophylactic AEDs.
After adjustment for neurologic grade on admission, cisternal SAH burden, and intraventricular hemorrhage the results of multivariable regression analysis did not reveal prophylactic AED therapy to be a significant predictor seizure risk (Table 3). Of important note, sensitivity analyses also failed to point out significant interactions between timing of prophylactic AED administration (beginning at outside hospital versus at our institution), duration of administration, method of seizure detection, and disease severity covariates.
Risk of Seizure Following Propensity Score Adjustment
The risk for seizure development was analyzed in relation to prophylactic AED treatment using propensity score matching, and calculated for each patient using a logistic regression model with 6 covariates (Hunt-Hess grade, SAH burden, intraventricular hemorrhage, intraparenchymal hemorrhage, hydrocephalus, craniotomy, and aneurysmal etiology). Following 1:1 matching, 152 treated and 201 control patients were matched to at least one patient of the other treatment cohort based on similarities in clinical characteristics previously listed. There were no non-matched cases in either treatment or control groups. Following matching, all covariates were statistically indistinguishable between the prophylactic AED treatment and control cohorts.
After propensity score matching prophylactic AED treatment did not significantly impact the incidence of seizure following SAH (p=0.49). Similarly, the incidence rate of seizure activity by diagnostic modality (clinical or EEG) was also not significantly different in those receiving prophylactic AEDs (p=0.86). Adjusted analyses for secondary outcome measures suggested prophylactic AED treatment was significantly associated with DIND (OR1.21, 95%CI 1.05–1.40); however, not 12-month functional outcome. As well, diagnosis of seizure by any means was also not significantly associated with worse 12-month neurologic outcome after adjustment for known predictors of poor outcome (p=0.15).
Post hoc analysis was performed to explore the significant association observed between prophylactic AED treatment and the development of DIND. Multivariable logistic regression analysis of AED type (PHT vs. LEV) adjusting for known predictors of DIND (age, aneurysmal SAH etiology, and cisternal SAH burden) revealed the risk of DIND was significantly greater among those following prophylactic LEV compared to PHT or no AED treatment whatsoever (LEV 45% vs. PHT 29% vs. 12%; OR2.10, 95%CI 1.02–4.35).
DISCUSSION
This retrospective analysis of prospectively collected data revealed that the administration of prophylactic AEDs after spontaneous SAH was not associated with a significant change in seizure incidence. This finding persisted despite adjustment for clinical characteristics which have been consistently associated with a higher risk of seizure occurrence, including severity of neurologic injury, higher hemorrhage burden, and need for craniotomy. Previous studies have identified several risk factors for the development of seizures during the acute hospitalization period following spontaneous SAH, including aneurysmal etiology, middle cerebral artery aneurysms, thickness of SAH clot, associated intracerebral hematoma, rebleeding, infarction, and poor neurological grade.2,3,10 Univariate and adjusted logistic regression analyses of this sample parallel these prior findings; in our study poor neurologic grade on admission, cisternal SAH burden, and intraventricular hemorrhage were all significant predictors of seizure occurrence.
Prior recommendations for prophylactic AED treatment following spontaneous SAH had been based on the concern for seizure in the setting of an unsecured aneurysm and the possible impact of seizures on long-term functional outcomes. Compounding this, early observational trials also reported seizure rates to be high as 20–30%.5 However, this current study exhibited seizures occurrence in only 10% of our sample population, a finding consistent with many other recent trials.11 One such analysis of the Nationwide Inpatient Sample database identified a 10% to 11% incidence of seizures or epilepsy after clipping or coiling of ruptured intracranial aneurysms, further corroborating the results of our trial.3,7,12 Despite data such as these, surveys suggest up to 84% of practitioners routinely use prophylactic AEDs following aneurysmal SAH, with nearly a quarter of neurosurgeons prescribing AEDs up to 3-months after SAH.13
Continued justification for prophylactic AEDs has focused on the unsubstantiated risk of seizures contributing to aneurysmal re-rupture, a finding that has not been chronologically proven as the cause rather than the product of aneurysmal re-rupture.7 In this current study only 4.5% of patients suffered acute, post-ictal seizures (<24 hours after SAH), and of these patients only 12% (n=2/16) suffered documented re-rupture. Importantly, neither the incidence of acute seizure nor the occurrences of aneurysm re-rupture significantly differed between AED treatment cohorts (p=0.26), at the same time sensitivity analysis did not reveal an underlying interaction. These findings, along with other literature reported seizure rates as low as 3% in the acute post-ictal period do not support routine prophylaxis even for the short-term in the current era of early aneurysm control.3
Moreover, the efficacy of routine prophylactic use of AEDs in patients with SAH has never been proven in a randomized control trial.14 Prior recommendations for routine administration were based on retrospective trials evaluating patients undergoing elective craniotomy for aneurysm clipping and studies on delayed epilepsy.1,7,14 Previous retrospective studies and systematic reviews specifically evaluating prophylactic AED therapy have failed to demonstrate a clear benefit for prophylactic use of AED after aSAH.3,14,15 A Cochrane systematic review found insufficient evidence to support the routine use of prophylactic AEDs; however, the trials reviewed all lacked cohorts not receiving prophylactic AEDs.7 This current study contains the largest reported patient sample not treated with prophylactic AEDs following SAH.
Further confounding the results of prior trials use and duration of AED therapy was associated with severity of SAH, suggesting there may have been bias in who was selected for AED treatment. To solve these previous shortcomings, we implemented propensity-matched analysis to reduce the possibility of selection bias contributing to our findings (e.g. bias from factors that may have made it more likely that a patient would have been administered prophylactic AEDs). Propensity matching mimics randomization by sampling a treatment cohort that is comparable across these factors to a control cohort who did not receive the treatment. In this study 6 covariates found in early trials to be associated with AED administration were utilized; Hunt-Hess grade, SAH burden, intraventricular hemorrhage, intraparenchymal hemorrhage, hydrocephalus, craniotomy, and aneurysmal etiology.2,3,10,15 Propensity score-matched analyses revealed that prophylactic AED treatment did not significantly impact the incidence of seizure following spontaneous SAH providing the most robust support to date for discontinuing the routine, prophylactic administration of AED following spontaneous SAH.
A controversial topic has been the effect of aneurysm treatment on the risk of seizure following SAH.4,12 Craniotomy and clip occlusion for aneurysm treatment has been inconsistently associated with a higher seizure risk in the perioperative period, while those treated solely by coil embolization have been shown to suffer no additional seizures in the periprocedural period (despite an 11% incidence of acute, post-ictal seizures).16 Interestingly, this current study did not corroborate these findings. After controlling for the higher rate of post-operative prophylactic AED treatment following craniotomy through both adjusted logistic regression and propensity-matched analysis, aneurysm treatment modality was not associated with seizure risk.
Recent trials have shown prophylactic AED themselves may contribute to poor outcome.5,6,17–20 Rosengart et al. analyzed 4 independent SAH trials and found patients who had received AEDs were 1.6 times more likely to have a poor outcome at 3 months, as well as being at increased risk for radiographic vasospasm, neurological deterioration, cerebral infarction, and elevated temperature during hospitalization.5 Phenytoin, the first-line prophylactic AED traditionally used in SAH, has been shown to not only have a variety of adverse effects, but has also been correlated with poor neurologic recovery following SAH.19 Our propensity score matched analysis observed a significant association between prophylactic AED treatment and DIND, corroborating many of these prior trials. Importantly however, this is the first time either PHT or LEV has been compared against an AED-naive control group. One mechanism postulated for the association between AEDs and DIND has been PHT’s interference with cerebral vasospasm prophylaxis through induction of the cytochrome P-450 isozyme and subsequently increased first-pass metabolism of nimodipin.18,19 However, Karamchandani et al. found no significant difference in DIND between PHT vs. LEV treatment.17 Surprisingly, adjusted analysis of our sample suggests LEV may actually be associated with the greatest risk of DIND- twice that of not receiving prophylactic AEDs. Although post hoc findings require cautious interpretation, they are nonetheless important given that current AHA/Stroke Guidelines state that the use of anticonvulsants is reasonable, without specifically recommending a particular anticonvulsant.15
Limitations
Although baseline, treatment, and outcome clinical characteristics were similar between groups, the cohorts were sampled from 2 separate epochs possibly introducing sampling bias; however, this was robustly controlled for through propensity score-matched analyses. Residual confounding may still exist as a product of covariates not considered or unable to be assessed (e.g. serum AED levels), immeasurable differences despite propensity matching, and possible classification errors with respect to covariates. This study may have also suffered from detection bias, as not all the patients underwent EEG monitoring. The incidence of non-convulsive seizure activity in conscious SAH patients is unknown and it is highly unlikely that these patients would not display even subtle neurologic findings prompting further work-up; 28% of good-grade patients underwent EEG monitoring, and only 9% of which revealed seizure activity. Of greater concern is the incidence of non-convulsive seizure/epilepsy in obtunded or comatose patients. A meta-analysis evaluating the diagnostic ability of continuous EEG in SAH found that non-convulsive seizure and/or epilepsy was diagnosed in 3 to 18 % of poor-grade SAH patients, most occurring by post-bleed day 9 (IQR 4–17 days).21 In our study, poor neurologic-grade patients (n=79) who did not improve after 24-hours underwent continuous EEG monitoring, 22% of these patients suffered seizures (5 diagnosed clinically, 12 suffered electrographic seizures). Despite this increased seizure incidence, AEDs were not associated with lower risk in this patient subset, a finding corroborated by other reports.21,22 Sensitivity analyses were also performed to evaluate and control for characteristics that may have increased the likelihood of having EEG monitoring (i.e. interaction effects). Detection method was again not associated with treatment, nor did it correlate with rate of seizure detection, suggesting any detection bias may be inconsequential in this situation.
PHT was generally utilized for seizure prophylaxis in our series, an agent only recently recommended against by AHA/Stroke Guidelines.15 Subgroup analysis from this study did not find an association between type of prophylactic AED and seizure risk; however, LEV or other newer agents may yet prove to be more effective without an increase in side effects. The use of PHT as a first-line prophylactic AED is hampered by its unpredictable, nonlinear pharmacokinetics, requiring assessment of serum PHT levels. Unfortunately the general practice during both treatment epochs was not to routinely assess PHT levels during prophylactic AED administration as PHT may take up to 1 week to achieve steady-state serum concentrations, well outside the peak post-ictal seizure period.23 Instead serum levels were drawn only in the setting of suspected or confirmed seizures and analyzing these levels in isolation would introduce significant bias. Antiepileptic burden (dose and number of days administered) is a surrogate covariate for AED exposure; however, sensitivity analyses in this study did not show a significant interaction between duration of AED administration and risk of seizure (all patients administered PHT received the same loading and maintenance dose).
SUMMARY/CONCLUSIONS
In a sample of patients suffering spontaneous SAH, the risk of seizure during the acute hospitalization period was low. The utilization of propensity score-matched analysis suggests prophylactic AED therapy did not significantly reduce the risk of seizure occurrence. After controlling for markers of SAH severity, patients receiving prophylactic AED therapy did display an increased risk of DIND; however, AEDs were not associated with overall neurologic outcome. There is no substantial evidence to support the routine administration of prophylactic AEDs in patients with aneurysmal SAH.
Acknowledgments
Sources of Funding: No support tendered from a granting organization
Disclosures: Dr.Crago receives NIH grants R01NR004339 & R01NR04221
References
- 1.Rhoney DH, Tipps LB, Murry KR, Basham MC, Michael DB, Coplin WM. Anticonvulsant prophylaxis and timing of seizures after aneurysmal SAH. Neurology. 2000;55:258–265. doi: 10.1212/wnl.55.2.258. [DOI] [PubMed] [Google Scholar]
- 2.Chumnanvej S, Dunn IF, Kim DH. Three-day phenytoin prophylaxis is adequate after SAH. Neurosurgery. 2007;60:99–102. doi: 10.1227/01.NEU.0000249207.66225.D9. [DOI] [PubMed] [Google Scholar]
- 3.Raper DM, Starke RM, Komotar RJ, Allan R, Connolly ES., Jr Seizures after aneurysmal SAH: A systematic review of outcomes. World Neurosurg. 2013;79:682–690. doi: 10.1016/j.wneu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Lin YJ, Chang WN, Chang HW, Ho JT, Lee TC, Wang HC, et al. Risk factors and outcome of seizures after spontaneous aneurysmal SAH. Eur J Neurol. 2008;15:451–457. doi: 10.1111/j.1468-1331.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosengart AJ, Huo JD, Tolentino J, Novakovic RL, Frank JI, Goldenberg FD, et al. Outcome in patients with SAH treated with antiepileptic drugs. J Neurosurg. 2007;107:253–260. doi: 10.3171/JNS-07/08/0253. [DOI] [PubMed] [Google Scholar]
- 6.Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, et al. Phenytoin exposure is associated with functional and cognitive disability after SAH. Stroke. 2005;36:583–587. doi: 10.1161/01.STR.0000141936.36596.1e. [DOI] [PubMed] [Google Scholar]
- 7.Marigold R, Gunther A, Tiwari D, Kwan J. Antiepileptic drugs for the primary and secondary prevention of seizures after subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013;(6):CD008710. doi: 10.1002/14651858.CD008710.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furbass F, Hartmann MM, Halford JJ, Koren J, Herta J, Gruber A, et al. Automatic detection of rhythmic and periodic patterns in critical care eeg based on american clinical neurophysiology society (acns) standardized terminology. Neurophysiol Clin. 2015;45:203–213. doi: 10.1016/j.neucli.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:1288–1295. doi: 10.1001/jamaneurol.2013.3475. [DOI] [PubMed] [Google Scholar]
- 10.Hasan D, Schonck RS, Avezaat CJ, Tanghe HL, van Gijn J, van der Lugt PJ. Epileptic seizures after SAH. Ann Neurol. 1993;33:286–291. doi: 10.1002/ana.410330310. [DOI] [PubMed] [Google Scholar]
- 11.Lin CL, Dumont AS, Lieu AS, Yen CP, Hwang SL, Kwan AL, et al. Characterization of perioperative seizures and epilepsy following aneurysmal SAH. J Neurosurg. 2003;99:978–985. doi: 10.3171/jns.2003.99.6.0978. [DOI] [PubMed] [Google Scholar]
- 12.Hoh BL, Nathoo S, Chi YY, Mocco J, Barker FG., 2nd Incidence of seizures or epilepsy after clipping or coiling of ruptured and unruptured cerebral aneurysms in the nationwide inpatient sample database: 2002–2007. Neurosurgery. 2011;69:644–650. doi: 10.1227/NEU.0b013e31821bc46d. [DOI] [PubMed] [Google Scholar]
- 13.Dewan MC, Mocco J. Current practice regarding seizure prophylaxis in aneurysmal SAH across academic centers. J Neurointerv Surg. 2015;7:146–149. doi: 10.1136/neurintsurg-2013-011075. [DOI] [PubMed] [Google Scholar]
- 14.Riordan KC, Wingerchuk DM, Wellik KE, Zimmerman RS, Sirven JI, Noe KH, et al. Anticonvulsant drug therapy after aneurysmal SAH: A critically appraised topic. Neurologist. 2010;16:397–399. doi: 10.1097/NRL.0b013e3181efc92f. [DOI] [PubMed] [Google Scholar]
- 15.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal SAH: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 16.Choudhari KA. Seizures after aneurysmal SAH treated with coil embolization. Neurosurgery. 2004;54:1029–1030. doi: 10.1227/01.neu.0000117122.32806.b0. [DOI] [PubMed] [Google Scholar]
- 17.Karamchandani RR, Fletcher JJ, Pandey AS, Rajajee V. Incidence of delayed seizures, delayed cerebral ischemia and poor outcome with the use of levetiracetam versus phenytoin after aneurysmal SAH. J Clin Neurosci. 2014;21:1507–1513. doi: 10.1016/j.jocn.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Shah D, Husain AM. Utility of levetiracetam in patients with SAH. Seizure. 2009;18:676–679. doi: 10.1016/j.seizure.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Wong GK, Poon WS. Use of phenytoin and other anticonvulsant prophylaxis in patients with aneurysmal SAH. Stroke. 2005;36:2532. doi: 10.1161/01.STR.0000190837.63350.84. author reply 2532. [DOI] [PubMed] [Google Scholar]
- 20.Murphy-Human T, Welch E, Zipfel G, Diringer MN, Dhar R. Comparison of short-duration levetiracetam with extended-course phenytoin for seizure prophylaxis after SAH. World Neurosurg. 2011;75:269–274. doi: 10.1016/j.wneu.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Kondziella D, Friberg CK, Wellwood I, Reiffurth C, Fabricius M, Dreier JP. Continuous eeg monitoring in aneurysmal SAH: A systematic review. Neurocrit Care. 2015;22:450–461. doi: 10.1007/s12028-014-0068-7. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor KL, Westover MB, Phillips MT, Iftimia NA, Buckley DA, Ogilvy CS, et al. High risk for seizures following SAH regardless of referral bias. Neurocrit Care. 2014;21:476–482. doi: 10.1007/s12028-014-9974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayegh ET, Fakurnejad S, Oh T, Bloch O, Parsa AT. Anticonvulsant prophylaxis for brain tumor surgery: Determining the current best available evidence. J Neurosurg. 2014;121:1139–1147. doi: 10.3171/2014.7.JNS132829. [DOI] [PubMed] [Google Scholar]


