Abstract
Methamphetamine (MAP) addiction is substantially prevalent in today's society, resulting in thousands of deaths and costing billions of dollars annually. Despite the potential deleterious consequences, few studies have examined the long-term effects of embryonic MAP exposure. Using the invertebrate nematode Caenorhabditis elegans (C. elegans) allows for a controlled analysis of behavioral and neurochemical changes due to early developmental drug exposure. The objective of the current studies was to determine the long-term behavioral and neurochemical effects of embryonic exposure to MAP in C. elegans. In addition, we sought to improve our conditioning and testing procedures by utilizing liquid filtration, as opposed to agar, and smaller, 6-well testing plates to increase throughput. Wild-type N2 C. elegans were embryonically exposed to 50 μM MAP. Using classical conditioning, adult-stage C. elegans were conditioned to MAP (17 and 500 μM) in the presence of either sodium ions (Na+) or chloride (Cl-) ions as conditioned stimuli (CS+/CS-). Following conditioning, a preference test was performed by placing worms in 6-well test plates spotted with the CS+ and CS- at opposite ends of each well. A preference index (PI) was determined by counting the number of worms in the CS+ target zone divided by the total number of worms in the CS+ and CS- target zones. A food conditioning experiment was also performed in order to determine if embryonic MAP exposure affected food conditioning behavior. For the neurochemical experiments, adult worms that were embryonically exposed to MAP were analyzed for (dopamine) DA content using high performance liquid chromatography (HPLC). The liquid filtration conditioning procedure employed here in combination with the use 6-well test plates significantly decreased the time required to perform these experiments and ultimately increased throughput. The MAP conditioning data found that pairing an ion with MAP at 17 or 500 μM significantly increased the preference for that ion (CS+) in worms that were not pre-exposed to MAP. However, worms embryonically exposed to MAP did not exhibit significant drug cue conditioning. The inability of MAP exposed worms to condition to MAP was not associated with deficits in food conditioning, as MAP exposed worms exhibited a significant cue preference associated with food. Furthermore, our results found that embryonic MAP exposure reduced DA levels in adult C. elegans, which could be a key mechanism contributing to the long-term effects of embryonic MAP exposure. It is possible that embryonic MAP exposure may be impairing the ability for C. elegans to learn associations between MAP and the CS+ or inhibiting the reinforcing properties of MAP, however, our food conditioning data suggest that MAP exposed animals can form associations between cues and food. The depletion of DA levels during embryonic exposure to MAP could be responsible for driving either of these processes during adulthood.
Keywords: nematode, drug abuse, prenatal, developmental, classical conditioning, monoamine, neurotoxin, reinforcement, behavior, learning, memory
Introduction
Over 12 million people have tried methamphetamine (MAP) at least once, approximately 1.2 million people reported using MAP in the past year, and 440,000 reported using it in the past month [1]. In the United States, MAP accounted for about 103,000 emergency department (ED) visits in 2011 and it was the fourth most mentioned illicit drug during ED visits [2], with an estimated cost of $23.4 billion in 2005 [3].
Studies examining the effects of MAP abuse during pregnancy are limited because they have utilized small samples and have not been able to control for the possibility that mothers used other drugs in addition to MAP [4]. The effects of prenatal MAP exposure on the developing fetus have not been well characterized [5,6]. However, a series of reports followed a group of amphetamine-exposed children from birth to age 14. Children exposed to amphetamine throughout pregnancy displayed emotional problems, lower IQ, higher aggression, problems with peers, poor school performance, and difficulties performing physical activities [7-10]. One study examined the developmental outcomes in children born to mothers who abused MAP [11], which found that MAP exposure in utero was associated with neurobehavioral patterns of increased physiological stress and increased CNS stress. These neurobehavioral findings are consistent with previous findings in cocaine- [12] and nicotine- [13] exposed children and suggest potential MAP-induced neurotoxic effects.
Although the neurotoxic effects of prenatal MAP exposure have not been well characterized, studies in rodents have shown that MAP is toxic to dopaminergic and serotonergic neurons [14,15]. In addition, MAP-exposed children exhibited increased concentrations of creatine in the striatum, suggesting a possible abnormality in energy metabolism in the brains of exposed children [16]. Studies in MAP-exposed children have demonstrated smaller subcortical volumes in the putamen, globus pallidus and hippocampus [17]. Collectively, these data suggest that areas of the frontal-striatal pathway are vulnerable to prenatal MAP exposure.
Although few clinical and basic research studies have been conducted on prenatal amphetamine or methamphetamine exposure, studies have examined the consequences of prenatal cocaine exposure on the reinforcing properties of cocaine as adults using vertebrate animal models. Increased intravenous self-administration of cocaine has been shown in adult rats and mice following early developmental cocaine exposure [18,19]. In contrast, locomotor sensitization with repeated non-contingent cocaine administration [20,21] and conditioned place preference to a cocaine-paired environment [22,23] are attenuated in adult mice and rats prenatally exposed to cocaine. Similarly, in utero cocaine exposure resulted in a blunted behavioral sensitization to amphetamine in adult rabbits [24]. These findings suggest that there is a divergence of the effects of prenatal cocaine on reinforced behavior, since cocaine operant self-administration increased, while Pavlovian or associative learning decreased in adulthood.
It is well described in previous work that C. elegans demonstrate both unconditioned and conditioned learning [25]. After pairing an unconditioned stimulus (UCS), such as food, with a neutral stimulus (conditioned stimulus; CS), animals show a behavioral change upon subsequent presentation of the CS. Such learning (Pavlovian conditioning) is the basis for models of drug reward in vertebrate animals [26]. C. elegans also show responses to various drugs of abuse [27,28], and recently we have found that C. elegans display a conditioned preference for stimuli previously associated with MAP or cocaine [29].
Although there is evidence that MAP exposure in utero may produce significant neurobiological and behavioral consequences in developing humans and animals, systematic studies have not been conducted. Therefore, the purpose of the present study was to determine how embryonic MAP exposure in C. elegans alters the reinforcing properties of MAP as adults, and if these effects are associated with alterations in dopamine (DA) concentrations.
Materials and Methods
Materials
All reagents and assay materials were purchased from Sigma-Aldrich and Fisher Scientific. (+)-Methamphetamine hydrochloride (MAP) was purchased from Sigma Aldrich (Cat. No. M8750; St. Louis, MO) and all concentrations in the present study include the hydrochloride salt.
Culture and Maintenance of Strains
The N2 Bristol wild-type (WT) strain was used in all assays. All animals were maintained at 22°C, and all general culturing techniques have been described previously by Nass and Hamza (2007). Worms were grown with E. coli strain NA22 as a food source on maintenance plates, produced by filling 60-mm petri dishes with 10-ml regular NGM agar (25g bactoagar, 20g bactopeptone, 3g NaCl, 1L H20, 1ml cholesterol (5mg/ml 95% ethanol), 1ml 1M CaCl2, 1ml 1M MgSO4, and 25ml of potassium phosphate buffer). The potassium phosphate buffer contained 5g of K2HPO4 dibasic/anhydrous, 30g of KH2PO4 monobasic, and 500ml of H20, pH adjusted to 6.0 [30].
Adult worms were used for MAP and food conditioning, preference testing, and determination of dopamine concentrations to control for any effects of different sensitivities and responses to drugs at varying developmental stages. Worms were age synchronized by lysing gravid adults with bleach and sodium hydroxide, allowing eggs to be released into solution and hatched in M9 buffer [30]. For control pre-exposure groups, 1 ml water was added to 9 ml M9 buffer. For MAP pre-exposure groups, 1 ml of a 0.5 or 1.7 mM MAP solution was added to 9 ml M9 buffer for a final concentrations of 50 or 177 μM MAP, respectively. After 18 hours, hatched L1 larvae were washed three times with water to remove any drug, plated, and maintained on NGM plates with NA22 E. coli bacterial lawns until reaching adulthood. Conditioning and testing began approximately 72 hr post-plating the L1 larvae, when worms were adults.
6-well Costar™ cell culture plates were used to determine salt preference (Fisher cat. no. 07-200-80). Clear templates were taped to the bottom each 6-well plate to create two 1.2 cm diameter circular target zones within the 3.5 cm diameter of each well. Salt cue test plates were produced by filling each well of the plates with 3.8ml of NaCl free agar (17g bactoagar, 2.5g bactopeptone, 1L H20, 1ml 1M CaCl2, 1ml 1M MgSO4, and 25ml of potassium phosphate buffer). Cholesterol was not included in the salt-free agar in order to obtain clearer images of worms during testing. Salts were applied to each target zone by placing one 2 μl drop of each ion-spotting solution (2.0 M NaCH3COO and 0.5 M NH4Cl) at the center of either of the 1.2 cm diameter target zones. The drops were placed 1 hr before testing began to allow for the ion gradient to diffuse. New spotting solutions were prepared each day to avoid increased variability in the results due to degradation [31,32].
Preliminary testing in 6-well plates determined that the presentation of 2.5 M Cl- and 2.0 M Na+, concentrations typically used in chemotaxis studies using larger test plates [29,32], in opposing target zones produced a greater preference for Cl- than Na+. Subsequently, it was found that reducing the Cl- concentration to 0.5 M, while keeping the Na+ concentration at 2.0 M, produced a balanced preference response for Cl- and Na+, 30 min after placing worms in the center of each well (data not shown). Therefore, 0.5 M Cl- and 2.0 M Na+ were used in the chemotaxic tests performed in the present experiments using 6-well plates.
MAP salt-cue conditioning procedure
Worms were washed off maintenance plates with 15 ml of water and transferred to 15 ml centrifuge tubes. Adults were allowed to settle on the bottom of each tube for 5 min and then the supernatant was removed. This was repeated two more times to remove the majority of bacteria from the worms. Then, 200 μl of worms were added to each 0.45um disc filter (25 mm, mixed cellulose ester membrane; EMD Millipore . no. HAWG02500) placed on top of the bottom portion of a EMD Millipore Swinnex™ filter holder (. no. SX0002500). A silicone O-ring was placed on top of the filter and the top portion of the filter holder was screwed onto the bottom portion to create an enclosed chamber for the worms. Worms were exposed to water or conditioning solutions in filter holders connected to a Gilson HP-16 peristaltic pump using a flow rate of 1.0 ml/min with PVC tubing (Gilson 1.52mm ID; . no. NC9868575). First, worms were washed with water for 5 min followed by a 2 min wash using a conditioning solution (CS+). Then worms were washed with water again for 5 min followed by a 2 min wash using another conditioning solution (CS-). This process was repeated until worms were exposed to a total of 5 water washes and 4 conditioning solution washes (2 CS+ and 2 CS-; see Table 1). Following this conditioning procedure, worms were washed off filters into 15 ml tubes and the supernatant was removed. Worms were diluted to a ratio of approximately 1 part worms to 2 parts water. Then 4ul of worms were pipetted into the center of each well of a 6-well testing plate and pictures of each well were taken 60 min after placing worms on test plates.
Table 1. MAP salt-cue conditioning procedure in N2 adult C. elegans.
Worms were washed off plates with RO water and transferred to 15 ml centrifuge tubes. The supernatant was removed to remove any remaining bacteria. 200 μl of worms were added to each 0.45um filter. Worms were exposed to water or conditioning solutions in a Swinnex® filter holder connected to a peristaltic pump using a flow rate of 1.0 ml/min. First, worms were washed with water for 5 min followed by a 2 min wash using a conditioning solution (CS+). Then worms were washed with water again for 5 min followed by a 2 min wash using another conditioning solution (CS-). This process was repeated until worms were exposed to a total of 5 water washes and 4 conditioning solution washes (2 CS+ and 2 CS-). Following this conditioning procedure, worms were washed off filters into 15 ml tubes and the supernatant was removed. Then 4ul of worms were pipetted into the center of each well of a 6-well testing plate and pictures of each well were taken 60 min after placing worms on test plates.
| Initial Wash | Conditioning | Testing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 min | 2 min | 5 min | 2 min | 5 min | 2 min | 5 min | 2 min | 5 min | 60 min |
| Water Wash | Drug + CS+ | Water Wash | CS- | Water Wash | Drug + CS+ | Water Wash | CS- | Water Wash | Salt Preference Testing |
Chemosensory conditioning was completed with either a “sodium” or “chloride” cue, similar to previous studies [31,32]. Sodium acetate (NaCH3COO) was used to establish the sodium cue and ammonium chloride (NH4Cl) for the chloride cue. These chemosensory cues have been used in previous associative learning protocols as the Na+ and Cl- [31,32]. Furthermore, additional studies have shown their counterparts (acetate and ammonium ions, respectively) are also attractants in their own right [33]. For the current study, sodium acetate will be referred to as the Na+ cue and ammonium chloride as the Cl- cue. Conditioning solutions were prepared by dissolving either 6.15g/L NaCH3COO or 4.01g/L NH4Cl was to produce 75 mM “Na+” or “Cl-” solutions, respectively. MAP was added to conditioning solutions for the drug + CS+ conditions (see Table 1).
Food conditioning procedure
For food conditioning experiments, NGM agar minus NaCl was used as the plain context for salt ion conditioning. Specifically, 6.15g/L NaCH3COO or 4.01g/L NH4Cl was substituted for NaCl to produce 75 mM “Na+” or “Cl-” plates, respectively (Wen et al., 1997). Food conditioning plates were produced by filling 60-mm petri dishes with 10ml NaCl-free agar (17g bactoagar, 2.5g bactopeptone, either 6.15g NaCH3COO or 4.01 g NH4Cl, 1L H20, 1ml cholesterol (5mg/ml 95% ethanol), 1ml 1M CaCl2, 1ml 1M MgSO4, and 25ml of potassium phosphate buffer). The NA22 strain of E. coli was used when the conditioning required exposure to a food source. The NA22 bacterial culture (NA22: 2g tryptone, 1.2g yeast extract, 0.625g NaCl, and 125-ml water) was pipetted onto hardened salt-free agar plates and spread evenly across the plate to form a consistent lawn. The bacterial lawn was allowed to dry overnight. Four different plates were created for the food conditioning experiment: 1) Na+ plus food, 2) Cl- plus food, 3) Na+ no food, and 4) Cl- no food.
Synchronized, adult worms were washed off maintenance plates with 15 ml of water and transferred to 15 ml centrifuge tubes. Adults were allowed to settle on the bottom of each tube for 5 min and then the supernatant was removed. This was repeated two more times to remove the majority of bacteria from the worms. For the first conditioning treatment (CS- only), separate groups of worms were transferred to plates with Na+ no food or Cl- no food for 3 hrs (see Table 2). Any excess water that was transferred with the worms was absorbed with a Kimwipe to spread the worms over the conditioning plate and to prevent large clumps of animals. Subsequently, worms were washed off plates with 15 ml of water, as described above. For the second conditioning treatment (CS+ plus food or CS+ no food), worms were then exposed to plates with the CS+ plus food or CS+ no food for 1hr. Any excess water that was transferred with the worms was absorbed with a Kimwipe to spread the worms over the conditioning plate and to prevent large clumps of animals. Then, worms were washed again with 15 ml of water, as described above. Worms were tested for salt preference 30 min after they were washed off conditioning plates, using the same salt preference testing methods described in the MAP salt cue conditioning procedure.
Table 2. Food conditioning procedure in N2 adult C. elegans.
Worms were washed off plates with water and transferred to 15 ml centrifuge tubes. The supernatant was removed to remove any remaining bacteria. Worms were placed on CS- conditioning plates for 3 hrs and washed off plates with water. Worms were then placed on CS+ plus food or CS+ no food plates for 1hr. Following this conditioning procedure, worms were washed off plates and 4ul of worms were pipetted into the center of each well of a 6-well testing plate and pictures of each well were taken 30 min after placing worms on test plates.
| Wash off Maintenance Plates | Conditioning | Testing | |||
|---|---|---|---|---|---|
| 3 hr | 1 hr | 30 min | |||
| Water Wash | CS- no food | Water Wash | CS+ plus Food or CS+ no food | Water Wash | Salt Preference Testing |
Imaging and Worm Counting
Worms were imaged by taking pictures with an Olympus 770sw digital camera positioned on top of a light box, which emitted light indirectly and underneath each 6-well plate. Images were analyzed using ImageJ software to count the number of worms in the target zones of the test plates. Using ImageJ, the target zone was cropped from each photo and the color threshold of the image was adjusted. Specifically, threshold color was set to red, color space was set to RGB, and color threshold was adjusted so worms were highlighted in red. Particles were analyzed with a pixel size of 80 to infinity. The number of worms counted in each target zone was recorded and analyzed in Microsoft Excel.
A chemotaxic preference index (PI) for the CS+ was then calculated by dividing the number of worms in the target zone containing the CS+ by the total sum of worms counted in both the CS+ and CS- zones converted to a percentage. To determine if there were statistically significant differences in CS+ preference due to drug or food conditioning, or pre-exposure, PIs were compared to those of respective vehicle exposed groups.
Determination of dopamine (DA) concentrations in C. elegans
Worms were washed from their maintenance plates and then washed three times with water to remove any bacteria. Washed worms were transferred to 1.5 ml eppendorf tubes, excess water was removed after centrifugation (3 min; 2100 × g), and the wet weight of the pelleted worms was determined. Samples were kept on ice during all extraction procedures described below to minimize DA degradation. Perchloric acid (0.5M) was added to each tube just before sonicating (1 μl of PCA: 1mg of worm tissue wet weight). Samples were then sonicated for 30 sec while on ice, then vortexed briefly and a 50 μl aliquot was taken from each sample to determine soluble protein concentrations. Protein concentrations were determined using the Coomassie Plus Protein Assay Reagent (Bio-Rad) [34]. No statistical differences were observed for soluble protein concentrations between treatment groups and the mean (± SEM) concentration was 1.27 ± 0.11 across all groups.
The remaining sample was centrifuged for 5 min to pellet proteins and the supernatant was analyzed for DA concentrations by high performance liquid chromatography with electrochemical detection (HPLC-EC; [35]. Separation was performed on an ESA HR-80-3 C18 (3.2 × 80 mm, 3 μm) column. The mobile phase contained 10% (v/v) methanol, 15% (v/v) acetonitrile, 150 mM sodium dihydrogen phosphate (monohydrate), 4.76 mM citric acid, 3 mM sodium dodecyl sulfate, 50 μM EDTA, and was adjusted to a pH of 5.6 with sodium hydroxide. The mobile phase was maintained at a flow rate of 0.2 ml/min and DA was detected by oxidation at +200 mV using an ESA Coulochem II detector. DA concentrations were quantified by relating peak areas to those of calibrating DA standard solutions. Analysis and quantification of chromatograms (peak area) were performed using Chromperfect software (Justice Laboratory Software, Denville, New Jersey).
Statistical analyses
All analyses were between groups and analyzed using one, two, or three-way ANOVAs, followed by decomposition of factors and post-hoc tests as appropriate and previously conducted [29].
Results
Embryonic MAP Exposure Attenuates MAP Cue Conditioning
A three-way ANOVA examining the effects of CS+ (Na or Cl), pre-exposure (vehicle or 50 μM MAP), and MAP conditioning dose (vehicle, 17 and 50 μM) on CS+ preference indices (PI) found significant effects of CS+ [F(1, 185) = 93.4; p < 0.001] and conditioning dose [F(2, 185) = 8.6; p < 0.001]. This analysis also found significant interactions between CS+ and conditioning dose [F(2, 185) = 0.2; p < 0.008] and pre-exposure and conditioning dose [F(2, 185) = 11.7; p < 0.001] on CS+ PI.
Since a main effect of CS+ was observed, separate two-way ANOVAS were performed to examine the effects of each CS+ (Na or Cl) on PI. When the CS+ was Na, a two-way ANOVA found a significant effect of conditioning dose [F(2, 94) = 5.5; p < 0.006] and a significant interaction between pre-exposure and conditioning dose [F(2, 94) = 6.1; p < 004] on PI. For the vehicle pre-exposed groups, a one-way ANOVA revealed significant effect of MAP conditioning dose on PI [F(2, 46) = 8.8; p < 0.002]. LSD post-hoc tests found significant differences between the vehicle conditioned and either the 17 μM (p < 0.007) or 500 μM (p < 0.001) MAP conditioned groups (Fig. 1). Specifically, when Na was the CS+, vehicle exposed and conditioned worms displayed an enhanced PI when conditioned with 17 and 500 μM MAP, compared to vehicle exposed and unconditioned controls. However, for the MAP pre-exposed groups, a one-way ANOVA did not find a significant effect of MAP conditioning dose on PI [F(2, 46) = 2.8; p < 0.07] (Fig. 1). Specifically, in MAP pre-exposed worms, the PI for the CS+ Na was not enhanced above control levels following conditioning with either the 17 or 500 μM MAP doses.
Figure 1. Effect of embryonic methamphetamine (MAP) exposure (50 μM) on MAP cue conditioning in adult N2 C. elegans.
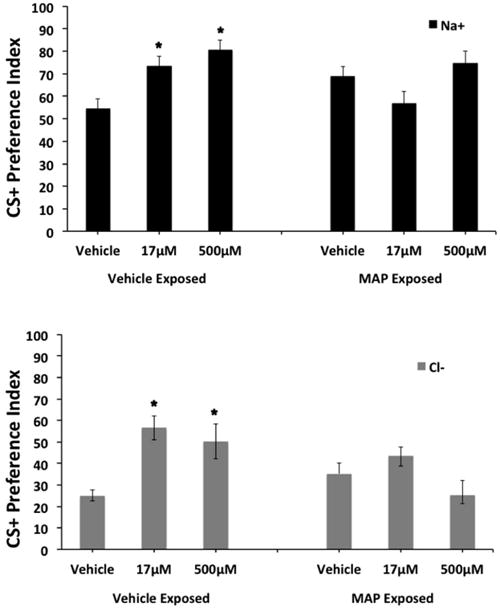
When the CS+ was Na+ (top panel), a two-way ANOVA found a significant effect of conditioning dose [F(2, 94) = 5.5; p < 0.006] and a significant interaction between pre-exposure and conditioning dose [F(2, 94) = 6.1; p < 004] on PI. For the vehicle pre-exposed groups, a one-way ANOVA revealed significant effect of MAP conditioning dose on PI [F(2, 46) = 8.8; p < 0.002]. Post-hoc tests found significant differences between the vehicle conditioned and either the 17 μM (p < 0.007) or 500 μM (p < 0.001) MAP conditioned groups (top panel). However, for the MAP pre-exposed groups, a one-way ANOVA did not find a significant effect of MAP conditioning dose on PI [F(2, 46) = 2.8; p < 0.07] (top panel). When the CS+ was Cl-(bottom panel), a two-way ANOVA found a significant effect of pre-exposure [F(1, 91) = 4.4; p < 0.05], conditioning dose [F(2, 91) = 8.1; p < 0.002] and a significant interaction between pre-exposure and conditioning dose [F(2, 91) = 6.6; p < 0.003] on PI. For the vehicle pre-exposed groups, a one-way ANOVA revealed significant effect of MAP conditioning dose on PI [F(2, 46) = 13.5; p < 0.001]. Post-hoc tests found significant differences between the vehicle conditioned and either the 17 μM (p < 0.001) or 500 μM (p < 0.002) MAP conditioned groups (bottom panel). However, for the MAP pre-exposed groups, a one-way ANOVA did not find a significant effect of MAP conditioning dose on PI for Cl [F(2, 43) = 2.2; p < 0.124] (bottom panel). * indicates significant (p < 0.05) increases in PI compared to respective vehicle conditions.
When the CS+ was Cl, a two-way ANOVA found a significant effect of pre-exposure [F(1, 91) = 4.4; p < 0.05], conditioning dose [F(2, 91) = 8.1; p < 0.002] and a significant interaction between pre-exposure and conditioning dose [F(2, 91) = 6.6; p < 0.003] on PI. For the vehicle pre-exposed groups, a one-way ANOVA revealed significant effect of MAP conditioning dose on PI [F(2, 46) = 13.5; p < 0.001]. LSD post-hoc tests found significant differences between the vehicle conditioned and either the 17 μM (p < 0.001) or 500 μM (p < 0.002) MAP conditioned groups (Fig. 1). However, for the MAP pre-exposed groups, a one-way ANOVA did not find a significant effect of MAP conditioning dose on PI for Cl [F(2, 43) = 2.2; p < 0.124] (Fig. 1).
Embryonic MAP Exposure does not affect Food Conditioning
A three-way ANOVA examining the effects of CS+ (Na or Cl), pre-exposure (vehicle or 50 μM MAP), and conditioning (vehicle versus food) on CS+ preference indices (PI) found a significant effect of CS+ [F(1, 95) = 89.4; p < 0.001]. However, no significant effects of pre-exposure [F(1, 95) = 0.7; ns] or conditioning [F(1, 95) = 2.3; ns] were found. This analysis also found significant interactions between CS+ and pre-exposure [F(1, 95) = 17.8; p < 001], and CS+ and conditioning [F(1, 95) = 6.9; p < 02] on PI.
Since a main effect of CS+ was observed, separate two-way ANOVAS were performed to examine the effects of either the CS+ Na or Cl on PI. When the CS+ was Na, a two-way ANOVA found a significant effect of pre-exposure [F(1, 48) = 13.0; p < 0.002], and conditioning [F(1, 48) = 8.7; p < 006], without a significant interaction between pre-exposure and conditioning [F(1,48) = 0.2; ns] on PI (Fig. 2). These findings indicate that MAP pre-exposure increased Na preference and that both the vehicle and MAP pre-exposed groups conditioned to Na, when previously paired with food. Specifically, MAP pre-exposure enhanced Na PI compared to vehicle pre-exposed controls. However, both the vehicle and MAP pre-exposed groups displayed enhanced PIs for the CS+ Na following food conditioning, compared to their respective controls.
Figure 2. Effect of embryonic MAP exposure (50 μM) on food conditioning in adult N2 C. elegans.
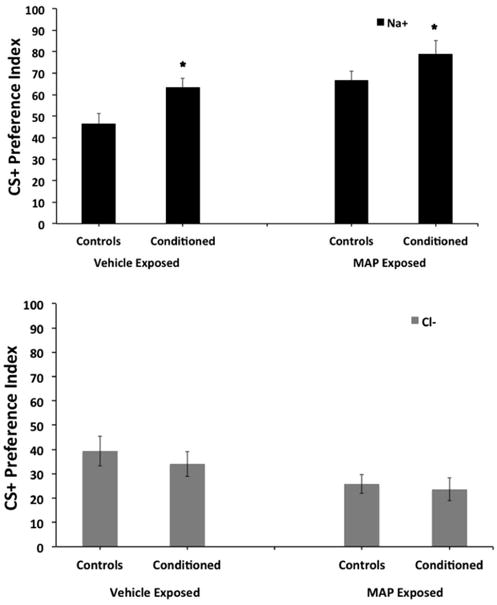
When the CS+ was Na+ (top panel), a two-way ANOVA found a significant effect of pre-exposure [F(1, 48) = 13.0; p < 0.002], and conditioning [F(1, 48) = 8.7; p < 006], without a significant interaction between pre-exposure and conditioning [F(1,48) = 0.2; ns] on PI. These findings indicate that MAP pre-exposure increased Na+ preference and that both the vehicle and MAP pre-exposed groups conditioned to Na+, when previously paired with food. When the CS+ was Cl- (bottom panel), a two-way ANOVA found a significant effect of pre-exposure [F(1, 47) = 5.6; p < 0.05] on PI. However, no significant effect of conditioning [F(1, 47) = 0.6; ns] and no significant interaction between pre-exposure and conditioning [F(1, 47) = 0.1; ns] were found on PI. These findings indicate that MAP pre-exposure decreased Cl- preference, and that both the vehicle and MAP pre-exposed groups did not condition to Cl-, when previously paired with food. * indicates significant (p < 0.05) increase in PI compared to respective control condition.
When the CS+ was Cl, a two-way ANOVA found a significant effect of pre-exposure [F(1, 47) = 5.6; p < 0.05] on PI. However, no significant effect of conditioning [F(1, 47) = 0.6; ns] and no significant interaction between pre-exposure and conditioning [F(1, 47) = 0.1; ns] were found on PI (Fig. 2). These findings indicate that MAP pre-exposure decreased Cl preference, and that both the vehicle and MAP pre-exposed groups did not condition to Cl, when previously paired with food.
Embryonic MAP Exposure reduces DA concentrations in adult N2 C. elegans
DA concentrations (nM) were normalized to % of respective vehicle control condition (50 or 177 μM) as illustrated in Figure 3. A one-way ANOVA examining the effect of MAP dose (0, 50 and 177 μM) found a main effect of MAP dose on % of vehicle treated control DA concentrations [F(2, 48) = 4.6; p < 0.02]. Specifically, embryonic exposure to 50 and 177 μM MAP reduced DA concentrations to 72.0 ± 8.8 and 63.5 ± 10.1 % of vehicle controls, respectively (Fig. 3).
Figure 3. Effect of embryonic MAP exposure (50 and 177 μM) on mean (± SEM) percent (%) of respective vehicle treated control dopamine (DA; nM) concentrations in adult N2 C. elegans.
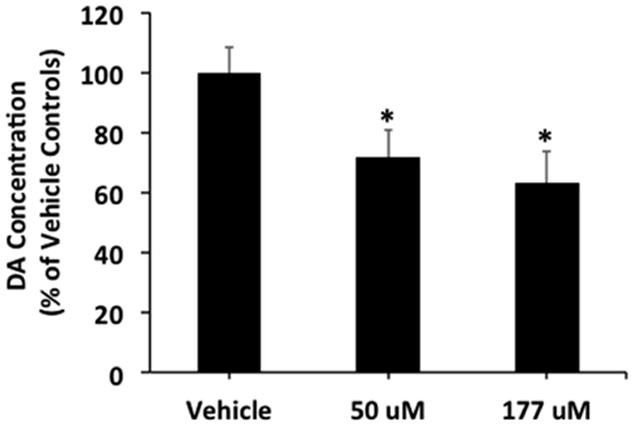
DA concentrations (nM) were normalized to % of respective vehicle control condition (50 or 177 μM). A one-way ANOVA examining the effect of MAP dose (0, 50 and 177 μM) found a main effect of MAP dose on % of vehicle treated control DA concentrations [F(2, 48) = 4.6; p < 0.02]. Specifically, embryonic exposure to 50 and 177 μM MAP reduced DA concentrations compared to vehicle treated controls. * indicates a significant decrease in DA concentrations compared to vehicle controls.
Discussion
As found previously in our lab, adult N2 C. elegans displayed an increased preference for the salt ion that was paired with MAP [29]. However, N2 C. elegans embryonically exposed to MAP failed to display MAP-associated cue conditioning as adults. Furthermore, embryonic MAP exposure did not affect food-associated cue conditioning. Finally, embryonic MAP exposure significantly reduced DA concentrations in adult N2 C. elegans. Overall, these findings suggest that embryonic MAP exposure attenuates the reinforcing properties of MAP, but not food, with a concomitant decrease in DA levels in adult N2 C. elegans. It is possible that the associated depletions in DA after embryonic MAP exposure is a driving mechanism responsible for the attenuation of the reinforcing properties of MAP and that this invertebrate model can be utilized to further investigate the effects of embryonic MAP exposure on behavior and neurochemistry as adults.
Previously, our lab utilized similar conditioning procedures and found that adult N2 C. elegans show enhanced salt cue preference following food or MAP conditioning [29]. This study also found that the cat-1 (CB1) and cat-2 (CB2) mutants, which have reduced levels of DA compared to N2's [36], do not display increases in salt cue preference following food or MAP conditioning. The CB1 mutants are defective in packaging DA for release in vesicular monoamine transporters [37], while the CB2 mutants lack the gene encoding tyrosine hydroxylase, required for DA synthesis [38]. The findings presented here support those by Musselman et al., 2012 [29], as untreated N2 C. elegans demonstrated significant increases in salt cue preference following food or MAP conditioning, and embryonic exposure to MAP, which was associated with decreased DA concentrations as adults (like CB1 and CB2 mutants), prevented their ability to condition to salt cues paired with MAP. Consistent with these findings, exogenous DA exposure rescued the conditioned response to MAP in both CB1 and CB2 mutants [29]. However, in the studies performed here, embryonic MAP exposure, with associated reductions in DA, did not impair the ability of N2 C. elegans to condition to a salt cue paired with food. These divergent findings may be explained by the possibility that CB1 and CB2 mutants have relatively low levels of DA compared to N2's (CB2 60 to 70% reduction in DA vs. wild-type N2 worms [36]; data not shown from our laboratory), while the reductions in DA observed after embryonic exposure are moderate in comparison (28 to 36% decrease in DA vs. untreated controls). Thus, greater reductions in DA neurotransmission observed in the mutants may impair both food and MAP salt cue conditioning, while moderate reductions in DA observed in embryonically exposed N2's may selectively impair MAP salt cue conditioning, without affecting food salt cue conditioning. It is also possible that the DA mutants have more widespread impairments in DA neurotransmission and systems (i.e., more DA neurons are impacted) compared to N2 worms embryonically exposed to MAP, which could explain the differential findings between the present findings and those of Musselman et al., 2012. The present findings are supported in other species, such as drosophila, in which DA neurotransmission was necessary for the development of a conditioned preference response using ethanol as the unconditioned stimulus [39]. Also, monoamine pathway mutants in vertebrates have also been shown to have alterations in stimulant-induced conditioned behavior [40,41].
We have made improvements to our previous methods (as detailed in [29]) for conducting our conditioning and preference testing. We are now able to conduct conditioning trials in liquid, rather than on agar test plates, and have devised a perfusion system to control the drug/cue exposure parings. For the studies presented here, filtration as opposed to centrifugation was used to wash the animals between drug periods cue pairings with drugs and vehicle. In order to establish clear associations between the salt cues and unconditioned stimuli (i.e. drugs), pairings of drugs (or controls) with cues must be done in the complete absence of any previous stimuli (cues or drugs) presented in the previous session. This necessitates multiple washes with sterile water to ensure discrete environments for Pavlovian conditioning to occur. Previously, this required 3 washes between each exposure. Each wash took more than 5 minutes, a significant amount of handling, and resulted in a significant loss of worms. Therefore, we developed a method to use filtration, rather than centrifugation, to wash and expose worms to the requisite stimuli and cues. This significantly reduces the time needed to conduct these experiments, reduces the loss of worms in handling, and increases throughput. In addition, we have downsized our chemotaxic agar test plates by utilizing 6-well plates with 3.5 cm diameter wells with 1.2 cm diameter target zones, instead of 6.0 cm diameter test plates with 1.6 cm diameter target zones used previously [29]. The development of our salt-cue chemotaxis assay in 6-well plates significantly reduces the amount of worms needed for conditioning and testing, reduces the time required for image acquisition, and ultimately increases throughput.
The chemosensory system in C. elegans detects water-soluble (gustatory) cues. Amphid chemosensory organs contain eleven pairs of chemosensory neurons. Each of these sensory neurons express a specific set of candidate receptor genes and detects a characteristic set of attractants, repellants or pheromones. Each chemosensory neuron has a characteristic pattern of expression that is established during embryogenesis. The amphid neurons, single (ASE) neurons are responsible for sensing water-soluble attractants, such as Na+ and Cl- ions [42,43]. It is possible that MAP pre-exposure resulted in sensory damage that prevented the ability of the worms to form an association between salt cues and MAP. However, this is unlikely given that worms embryonically exposed to MAP were able to associate salt cues with food in the present study. In worms that were not conditioned to MAP, embryonic MAP exposure enhanced Na+ and decreased Cl- preference of adult worms compared to those exposed to vehicle/water (Fig. 2). There are several potential reasons for this observed change in salt-ion preference due to embryonic MAP treatment. First, it is possible that embryonic MAP produced alterations in sensory cilium of chemosensory neurons, since behavioral deficits in chemosensation have been observed in cilium structure mutants [44,45]. It is also possible that MAP exposure during embryogenesis produced alterations in ASE neurons. In wild-type worms, ASE neurons possess a rapid form of taste adaptation that can be induced within a few minutes of exposure to salts [46,47]. Na+ or Cl-exposure blocks chemotaxis to Na+ or Cl-, respectively. Mutations in osm-9 and adp-1 blocks ASE adaptation to salts and mutations in G-protein gamma (gpc-1) block salt adaptation [46]. Thus, it is plausible that embryonic exposure to MAP may produce alterations in ASE neurons, which attenuate the ability of the worms to rapidly adapt to salt-ion cues. It is also possible that the enhanced salt-ion preference may be due to HCl salt exposure from the MAP used to treat worms embryonically. However, this seems unlikely, given that the concentration of HCl salt in the dose of MAP used to treat worms was extremely low (10 μM). The present studies were not designed to determine the mechanism by which embryonic MAP exposure enhanced salt-ion preference, therefore, additional studies are required.
In terms of behavioral effects, acute MAP exposure (1hr) in C. elegans decreased egg laying, pharyngeal pumping (feeding behavior) and locomotor activity [48], and this pattern of behavioral effects is very similar to those observed after application of exogenous DA [49-52]. Amphetamine also has similar effects to MAP, which are mediated through DA neurotransmission [53]. McDonald 2007 [51] identified a behavior mediated by DA, swimming-induced paralysis (SWIP), which is observed in nematodes placed in water, and is regulated by the DA transporter DAT-1. Using this assay, Carvelli 2010 [53] found that amphetamine quickly elicits SWIP in wild-type C. elegans, but not DAT-1 knockout worms (dat-1). cat-2 mutants, which are unable to synthesize DA and have reduced DA levels, displayed a significant reduction in amphetamine-induced SWIP compared to wild-type worms. Furthermore, these authors found that amphetamine elicits DA efflux in wild-type C. elegans DA neurons with functional DATs, and could be blocked with DAT-1 inhibitors. In addition, amphetamine was unable to increase DA efflux in DA neurons from dat-1 mutants, suggesting that the DAT plays a key role in the effects of amphetamine on DA efflux in C. elegans.
Protracted MAP exposure (16 hrs) has been shown to result in toxicity and lethality in wild-type C. elegans [48], which has also been observed with prolonged exposure to exogenous DA [50]. MAP exposure can lead to the loss of DA terminals and neurons in the rat brain [14,15], and such effects are also suspected to occur in human MAP abusers [4]. MAP also affects DA neurotransmission in C. elegans [48]. However, the effects of embryonic MAP exposure on the developing DA system are essentially unknown. Thus, we tested the hypothesis that embryonic MAP exposure would reduce tissue DA levels later in development. The present findings, however, indicate that embryonic exposure to MAP reduces tissue levels of DA later in C. elegans development. These data are consistent with our hypotheses and the mechanism by which MAP is known to induce DA neurotoxicity in mammals.
There are two periods of cell production in the developing nervous system of hermaphrodite C. elegans. During the first half of embryogenesis, 222 cells arise and produce a majority of the components of the nervous system [54], and a second period of cell production occurs at the end of the L1 larval stage, as most of the ventral cord motor neurons are generated [55,56]. Since most neurogenesis occurs during embryogenesis in C. elegans, MAP exposure was conducted during this critical period of neurobiological development. Other researchers have utilized similar exposure paradigms to examine the effects of embryonic drug exposure on development in C. elegans [57]. It is well established that MAP is neurotoxic to DA neurons and can lead to neurodegeneration in mammals [15], and the data presented here indicates that similar effects can occur in C. elegans. The reductions in DA levels observed here suggest that MAP exposure during embryogenesis is toxic to DA neurons during this critical period of development.
Future studies should examine the consequences of MAP exposure during embryonic development on drug self-exposure and rewarding behavior throughout the lifespan of C. elegans. MAP exposure is known to have profound behavioral effects in humans and also in animal models. MAP supports self-administration behavior and produces conditioned place preference responses in non-human primates [58] and rodents [59]. However, very little is known about the long-term consequences of MAP exposure during development on subsequent behavior. Consistent with these findings, our published data [29] show that C. elegans also show context conditioning to cues that had been previously associated with MAP or cocaine. These data suggest that MAP exposure during embryonic development may alter the concentration-response curve of drug self-exposure and the rewarding properties of drugs.
Acknowledgments
These studies were supported by the National Institute on Drug Abuse (NIDA); grant DA035468 to Dr. Eric Engleman.
References
- 1.Results from the 2012 national survey on drug use and health: Summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- 2.Drug abuse warning network (dawn) 2011: National estimates of drug-related emergency department visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. pp. 1–93. D-39. [PubMed] [Google Scholar]
- 3.The economic cost of methamphetamine use in the united states. RAND Corporation; 2005. [Google Scholar]
- 4.NIH Publication Number 13-4210. 2013. Methamphetamine National institute of drug abuse Research report 2013. [Google Scholar]
- 5.Plessinger MA. Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstet Gynecol Clin North Am. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson M, Larsson G, Winbladh B, Zetterstrom R. The influence of amphetamine addiction on pregnancy and the newborn infant. Acta Paediatr Scand. 1978;67:95–99. doi: 10.1111/j.1651-2227.1978.tb16283.x. [DOI] [PubMed] [Google Scholar]
- 7.Billing L, Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. Iii. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatr Scand. 1980;69:675–680. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 8.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abuse Negl. 1988;12:503–507. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- 9.Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 10.Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, Fallone M, Liu J, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, Finnegan LP, Maza PL. The maternal lifestyle study: Effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 13.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 14.Fuller RW, Hemrick-Luecke SK. Further studies on the long-term depletion of striatal dopamine in iprindole-treated rats by amphetamine. Neuropharmacology. 1982;21:433–438. doi: 10.1016/0028-3908(82)90027-2. [DOI] [PubMed] [Google Scholar]
- 15.Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 1993;72:325–328. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 16.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Keller RW, Jr, LeFevre R, Raucci J, Carlson JN, Glick SD. Enhanced cocaine self-administration in adult rats prenatally exposed to cocaine. Neurosci Lett. 1996;205:153–156. doi: 10.1016/0304-3940(96)12409-5. [DOI] [PubMed] [Google Scholar]
- 19.Rocha BA, Mead AN, Kosofsky BE. Increased vulnerability to self-administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology (Berl) 2002;163:221–229. doi: 10.1007/s00213-002-1140-0. [DOI] [PubMed] [Google Scholar]
- 20.Byrnes JJ, Pritchard GA, Koff JM, Miller LG. Prenatal cocaine exposure: Decreased sensitization to cocaine and decreased striatal dopamine transporter binding in offspring. Neuropharmacology. 1993;32:721–723. doi: 10.1016/0028-3908(93)90087-j. [DOI] [PubMed] [Google Scholar]
- 21.Crozatier C, Guerriero RM, Mathieu F, Giros B, Nosten-Bertrand M, Kosofsky BE. Altered cocaine-induced behavioral sensitization in adult mice exposed to cocaine in utero. Brain Res Dev Brain Res. 2003;147:97–105. doi: 10.1016/j.devbrainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Heyser CJ, Miller JS, Spear NE, Spear LP. Prenatal exposure to cocaine disrupts cocaine-induced conditioned place preference in rats. Neurotoxicol Teratol. 1992;14:57–64. doi: 10.1016/0892-0362(92)90029-a. [DOI] [PubMed] [Google Scholar]
- 23.Malanga CJ, Pejchal M, Kosofsky BE. Prenatal exposure to cocaine alters the development of conditioned place-preference to cocaine in adult mice. Pharmacol Biochem Behav. 2007;87:462–471. doi: 10.1016/j.pbb.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanwood GD, Levitt P. Repeated i.V. Cocaine exposure produces long-lasting behavioral sensitization in pregnant adults, but behavioral tolerance in their offspring. Neuroscience. 2003;122:579–583. doi: 10.1016/j.neuroscience.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Ardiel EL, Rankin CH. An elegant mind: Learning and memory in caenorhabditis elegans. Learn Mem. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 26.Tzschentke TM. Measuring reward with the conditioned place preference (cpp) paradigm: Update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Jee C, McIntire SL. Ethanol preference in c. Elegans. Genes Brain Behav. 2009;8:578–585. doi: 10.1111/j.1601-183X.2009.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward A, Walker VJ, Feng Z, Xu XZ. Cocaine modulates locomotion behavior in c. Elegans. PLoS One. 2009;4:e5946. doi: 10.1371/journal.pone.0005946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musselman HN, Neal-Beliveau B, Nass R, Engleman EA. Chemosensory cue conditioning with stimulants in a caenorhabditis elegans animal model of addiction. Behav Neurosci. 2012;126:445–456. doi: 10.1037/a0028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi L, Driscoll M. Culture of embryonic c. Elegans cells for electrophysiological and pharmacological analyses. WormBook. 2006:1–15. doi: 10.1895/wormbook.1.122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rankin CH. Context conditioning in habituation in the nematode caenorhabditis elegans. Behav Neurosci. 2000;114:496–505. [PubMed] [Google Scholar]
- 32.Wen JY, Kumar N, Morrison G, Rambaldini G, Runciman S, Rousseau J, van der Kooy D. Mutations that prevent associative learning in c. Elegans. Behav Neurosci. 1997;111:354–368. doi: 10.1037//0735-7044.111.2.354. [DOI] [PubMed] [Google Scholar]
- 33.Frokjaer-Jensen C, Ailion M, Lockery SR. Ammonium-acetate is sensed by gustatory and olfactory neurons in caenorhabditis elegans. PLoS One. 2008;3:e2467. doi: 10.1371/journal.pone.0002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nass R, Hahn MK, Jessen T, McDonald PW, Carvelli L, Blakely RD. A genetic screen in caenorhabditis elegans for dopamine neuron insensitivity to 6-hydroxydopamine identifies dopamine transporter mutants impacting transporter biosynthesis and trafficking. J Neurochem. 2005;94:774–785. doi: 10.1111/j.1471-4159.2005.03205.x. [DOI] [PubMed] [Google Scholar]
- 35.Engleman EA, Keen EJ, Tilford SS, Thielen RJ, Morzorati SL. Ethanol drinking reduces extracellular dopamine levels in the posterior ventral tegmental area of nondependent alcohol-preferring rats. Alcohol. 2011;45:549–557. doi: 10.1016/j.alcohol.2011.02.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH. Dopamine modulates the plasticity of mechanosensory responses in caenorhabditis elegans. EMBO J. 2004;23:473–482. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duerr JS, Gaskin J, Rand JB. Identified neurons in c-elegans coexpress vesicular transporters for acetylcholine and monoamines. Am J Physiol-Cell Ph. 2001;280:C1616–C1622. doi: 10.1152/ajpcell.2001.280.6.C1616. [DOI] [PubMed] [Google Scholar]
- 38.Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among caenorhabditis elegans ray sensory neurons by a tgfbeta family signaling pathway and a hox gene. Development. 1999;126:5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- 39.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc Natl Acad Sci U S A. 2004;101:7781–7786. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: Combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward S. Chemotaxis by the nematode caenorhabditis elegans: Identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci U S A. 1973;70:817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JA, Hodgkin JA. Specific neuroanatomical changes in chemosensory mutants of the nematode caenorhabditis elegans. J Comp Neurol. 1977;172:489–510. doi: 10.1002/cne.901720306. [DOI] [PubMed] [Google Scholar]
- 45.Culotti JG, Russell RL. Osmotic avoidance defective mutants of the nematode caenorhabditis elegans. Genetics. 1978;90:243–256. doi: 10.1093/genetics/90.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen G, Weinkove D, Plasterk RH. The g-protein gamma subunit gpc-1 of the nematode c. Elegans is involved in taste adaptation. EMBO J. 2002;21:986–994. doi: 10.1093/emboj/21.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode caenorhabditis elegans. J Exp Biol. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 48.Schreiber MA, McIntire SL. A caenorhabditis elegans p38 map kinase pathway mutant protects from dopamine, methamphetamine, and mdma toxicity. Neurosci Lett. 2011;498:99–103. doi: 10.1016/j.neulet.2011.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawin ER, Ranganathan R, Horvitz HR. C. Elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 50.Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in caenorhabditis elegans. Nat Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- 51.McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter dat-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 53.Carvelli L, Matthies DS, Galli A. Molecular mechanisms of amphetamine actions in caenorhabditis elegans. Mol Pharmacol. 2010;78:151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 55.Sulston JE. Post-embryonic development in the ventral cord of caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- 56.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 57.Lin CH, Sa S, Chand J, Rankin CH. Dynamic and persistent effects of ethanol exposure on development: An in vivo analysis during and after embryonic ethanol exposure in caenorhabditis elegans. Alcohol Clin Exp Res. 2013;37(Suppl 1):E190–198. doi: 10.1111/j.1530-0277.2012.01856.x. [DOI] [PubMed] [Google Scholar]
- 58.Freeman KB, Wang Z, Woolverton WL. Self-administration of (+)-methamphetamine and (+)-pseudoephedrine, alone and combined, by rhesus monkeys. Pharmacol Biochem Behav. 2010;95:198–202. doi: 10.1016/j.pbb.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizoguchi H, Yamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: Involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:9–14. doi: 10.1254/jphs.fm0070139. [DOI] [PubMed] [Google Scholar]


