Abstract
Background
Little is known about variation in individual cytokines/cytokine profiles for a large healthy, pediatric population. When cytokines in a healthy group are not abnormally high as in a disease state, it is challenging to determine appropriate statistical strategies. Aims were to: 1) describe variation among cytokine concentrations and profiles in healthy adolescent girls, 2) illustrate utility of data reduction approaches novel to cytokine research, [variable-centered (Principal Factor Analysis; PFA), person-centered (Latent Profile Analysis; LPA)], and 3) demonstrate utility of such methods in linking cytokine profiles to health outcomes (e.g., depressive, anxiety symptoms).
Method
Serum was analyzed for 13 cytokines representing adaptive and innate immune responses in 262 girls (11, 13, 15, and 17y).
Results
There was great variation in cytokine concentrations. PFA revealed a 4-factor solution explaining 73.13% of the shared variance among 13 cytokines (e.g., Factor 1 included IL-4, IL-13, IL-5, IFN-γ; 26.65% of the shared variance). The LPA supported classifying girls into subgroups characterized by “High Overall” (7.3% of sample), “High Adaptive” (26.7%), “High Innate (21%) or “Low Overall (45%) cytokine levels. Factors and profiles were useful in describing individual differences in depressive/anxiety symptoms (e.g., Factor 1 positively associated with depressive symptoms but negatively with trait anxiety; increased depressive symptoms or trait anxiety was associated with greater likelihood of being in the “High Adaptive” group).
Conclusions
Healthy girls showed differences in cytokine levels and patterns of variation and important associations with psychological variables. PFA and LPA offer novel approaches useful for examining cytokine panels in healthy populations.
Keywords: cytokine, adolescence, latent profile analysis, principal factor analysis, immune system, inflammation
INTRODUCTION
Cytokines are glycoprotein messengers that have a broad array of functions. Cytokines are characterized by pleiotropic and redundant actions with different cytokines binding to the same receptors and performing similar immune functions. They are important mediators between the immune system and their target organs and are active in various tissues and biological systems representing normal physiology [1]. (Table 1) In clinical research, cytokines have primarily been examined in diseased rather than healthy populations; particularly in pediatrics. For example, individual cytokines or cytokine pathways have described inflammatory bowel disease (IBD) [2], rheumatologic disorders [1, 3], sepsis [4], allergic and respiratory disorders [5]. In disease-focused studies, a healthy comparison group may be included to illustrate when relevant cytokines are abnormally high in the diseased group. Earlier observational studies of healthy participants have used small numbers (e.g., n ~ 36) of children and adolescents [6, 7]. Yet little is known about the variation in cytokine concentrations or profiles in a large, healthy community based pediatric population.
Table 1.
Descriptive Table of Cytokine Features and Functioning
| Cytokine | Source | Target | Function | Pathway/Response |
|---|---|---|---|---|
| IL-1 (α & β) | Monocytes Macrophages Fibroblasts Epithelial cells Endothelial cells |
T cells; B cells Endothelial cells Hypothalamus Liver |
Costimulatory molecule Activation (inflammation) Fever Acute phase reactants |
Innate Pro-inflammatory |
| IL-2 | T cells; NK cells | T cells B cells Monocytes |
Growth Activation |
Cell-mediated Adaptive Th1 |
| IL-4 | T cells Mast Cells Macrophages |
Naive T cells T cells B cells |
Differentiation into a TH2 cell Growth Activation; Isotype switching to IgE |
Humoral-mediated Adaptive Th2 Anti-inflammatory |
| IL-5 | T cells | B cells Eosinophils |
Growth Activation |
Humoral-mediated Adaptive Th2 Anti-inflammatory |
| IL-6 | T cells; Macrophages; Fibroblasts |
T cells; B cells Mature B cells Liver |
Costimulatory molecule Growth (in humans) Acute phase reactants |
Humoral-mediated Adaptive Th2 Pro-inflammatory |
| IL-7 | Bone marrow; Thymic stroma |
T cells; B cells | Growth and differentiation Proliferation Upregulation |
Innate |
| IL-8 | Macrophages; Epithelial cells; Platelets; Leucocytes |
Neutrophils Chemokine | Activation and chemotaxis | Innate Pro-inflammatory |
| IL-10 | T cells (TH2) Macrophages |
Macrophages T cells |
Inhibits APC activity Inhibits cytokine production |
Humoral-mediated Adaptive Th2 Anti-inflammatory |
| IL-12P70 | Macrophages; NK cells Dendritic cells; B cells |
Naive T cells | Differentiation into a TH1 cell Production Potentiates downregulation |
Cell-mediated Adaptive Th1 |
| IL-13 | T cells (TH2) | T cells | Modulation Downregulation |
Humoral-mediated Adaptive Th2 Anti-inflammatory |
| IFN-gamma | T cells (TH1); NK cells | Monocytes Endothelial cells Many tissue cells - especially macrophages Macrophages |
Activation Increased class I and II MHC Inhibits activation |
Cell-mediated Adaptive Th1 Pro-inflammatory |
| GM-CSF | T cells; Macrophages; Endothelial cells, Fibroblasts |
Bone marrow progenitors | Growth Differentiation | Innate |
| TNF-alpha | Macrophages; T cells | Similar to IL-1 | Similar to IL-1 Inflammation Cytotoxic |
Cell-mediated Adaptive Th1 Pro-inflammatory |
Note. The table represents the evolving discovery of how cytokines are listed by adaptive (Th1 and Th2) and innate immune pathways, and inflammatory immune responses as they often occur from various sources in the literature e.g., Mayer, G. (2010) Cytokines And Immunoregulation, Immunology - Chapter 12: 7th edition, The Board of Trustees of the University of South Carolina. Online book http://pathmicro.med.sc.edu/bowers/imm-reg-ver2.htm. and Kindt, T. J., Goldsby, R. A., Osborne, B. A., & Kuby, J. (2007). Kuby immunology. New York, NY: Macmillan.
It is important when examining cytokine profiles in disorders, to fully define what “normal” is. Circulating cytokines in healthy populations are not abnormally high, posing methodological challenges to determine appropriate analytic strategies for using such data. For example, some cytokines may be undetectable or have little variability across healthy individuals. Alternatively, some may represent outliers in these values. Hence we need a better understanding of cytokines in a healthy pediatric sample.
In the current study, two methodological approaches were applied to extend knowledge on variability among 13 individual cytokines and cytokine profiles among a healthy adolescents and to illustrate utility of multivariate techniques in cytokine analysis. Multivariate data reduction methods can be useful when multiple variables (i.e., cytokines) are interrelated and thought to represent some underlying dimension(s) (i.e., immune pathway) within individuals.[8, 9] We incorporated two approaches, variable-centered (principal factor analysis; PFA[10]) and person-centered (latent profile analysis; LPA[11]) to examine individual differences in covariation among 13 cytokines associated with various health and disease outcomes. The contribution of these methods to understanding immune function has benefits for deriving measurements of underlying physiological functioning in healthy and diseased populations and for reducing a large number of physiologic variables into more parsimonious and meaningful constructs. [12, 13]
The purpose of this study was to 1) examine circulating levels and patterns in covariation among cytokine concentrations in a large sample of healthy adolescent girls, 2) illustrate two methodological approaches to meaningfully reduce multivariate cytokine data, and 3) illustrate the utility of these approaches by linking the empirically derived factor and profiles to prototypical health-related variables (e.g., depressive/anxiety symptoms). These two variables seemed appropriate as examples, and are only discussed briefly since the literature contained numerous citations describing associations of several cytokines with these outcomes and the specific health outcomes were not the focus of this report.
Cytokines representing three branches of the immune system were determined: 1) innate immunity (interleukin [IL]-1β, IL-7, IL-8, granulocyte macrophage colony-stimulating factor [GM-CSF]); 2) cell-mediated adaptive immunity: Th1 (IL-2, IL-12P70, interferon [IFN]-γ, tumor necrosis factor [TNF]-α); and 3) humoral-mediated adaptive immunity: Th2 (IL-4, IL-5, IL-6, IL-10, IL-13). Importantly, many of these cytokines serve multiple functions. For instance, although TNF-α is considered a Th1 cytokine due to its important roles in inhibiting tumor growth and viral replication, it is also a principle inducer of inflammation in innate immunity. Similarly, IL-7 stimulates the differentiation and proliferation of natural killer (NK) cells, T- and B-cells which gives it a meaningful function under each of the three above categories, respectively. Due to their multiple important immune functions, an overlap of categories for many of these analytes is unavoidable. For this reason, grouping them based on how they statistically pattern together or covary within a population has potential high merit.
MATERIALS AND METHODS
Participants
This is a secondary analysis from a longitudinal study designed to examine the impact of depressive symptoms and smoking on reproductive and bone health across puberty in healthy girls [14]. Secondary funding allowed for analysis of cytokine levels from existing blood samples. Girls (n = 262) were enrolled by age cohort [11, (n = 52); 13, (n = 52); 15, (n = 87); and 17, (n = 71) years] into a cross sequential design [15]. Data from the first visit are described here. Enrollment took place between December 2004 and October 2007. Girls were recruited through flyers or by care providers from a teen health clinic in Cincinnati Children’s Hospital Medical Center (CCHMC) and its surrounding community. The clinic served as the primary care home for the majority of teens attending the clinic. Those with chronic disorders were not eligible for the study. The initial screen focused on age cohort eligibility and some health variables. Parent study exclusion criteria for a second level of screen was conducted using: 1) pregnancy/breast feeding within 6 months, 2) primary amenorrhea (>16 years), 3) secondary amenorrhea (<6 cycles/year), 4) body mass index (BMI) <1st percentile, weight >300 pounds [DXA cutoff (dual energy x-ray absorptiometry) machine], 5) medication/medical disorder influencing bone health and 6) psychological disabilities impairing comprehension/compliance.
Procedures and Measures
The study was approved by the Institutional Review Board of the CCHMC. Written parental consent was obtained and adolescents provided assent. Visits were conducted in a Clinical Translational Research Center (CTRC) and included a physical examination, anthropometry, phlebotomy, psychosocial questionnaires/interviews, and bone densitometry. Girls came to the CTRC at ~11:30; if menarcheal, they were scheduled during day 5–9 of their menstrual cycle. Visits were rescheduled if girls reported an infection/acute illness. Participants fasted for two hours prior to the IV catheter insertion. Blood was obtained in heparinized tubes. Samples were centrifuged and frozen at −80°C until assayed.
Descriptive variables included height and weight (light clothing, no shoes) using a wall-mounted stadiometer (Holtain Ltd., Crosswell, UK) and a digital scale (Scaletronix, Carol Stream, IL). The mean of 3 measures was used for analyses. Pubertal stage was determined by examination using criteria for breast, via palpation and visualization, and pubic hair stage based on Tanner [16]. Gynecological age was determined via clinical interview [17] asking age at menarche which was then subtracted from chronological age. Race was assessed by self-report and socioeconomic status (SES) from parent report [18].
Cytokines were measured using the Lincoplex™ methodology. The Luminex platform was used with a high sensitivity 13-plex antibody bead array (Millipore, Billerica, MA) permitting simultaneous measurement of multiple cytokines in a single, small volume sample. Level of fluorescence bound to the beads is quantified using the Luminex platform. Quality of the assays is excellent with accuracy of 93–112%, with inter-assay precision of 2.16–14.27% and intra-assay precision of 3.11–5.86%. Sensitivities were: IL-1β (0.06 pg/mL); IL-2 (0.16 pg/mL); IL-4 (0.13 pg/mL); IL-5 (0.01 pg/mL); IL-6 (0.10 pg/mL); IL-7 (0.16 pg/mL); IL-8 (0.11 pg/mL); IL-10 (0.15 pg/mL); IL-12P70 (0.11 pg/mL); IL-13 (0.48 pg/mL); IFN-γ (0.29 pg/mL); TNF-α (0.05 pg/mL); and GM-CSF (0.46 pg/mL). Cytokine values below the level of detection and with no sampling errors had values set to zero for the specific case.
Health Variables
To illustrate use of the statistical techniques, two measures of psychological health were selected that have been reported in the literature to be associated with cytokines [19, 20]. Depressive symptoms were measured via adolescent report using the Children’s Depression Inventory (CDI) [21], a 27-item measure reporting on a 0 to 2 scale in the last two weeks (Cronbach’s α = .89 for this sample). The instrument is designed for ages 7–17 years. T-scores were used in the analyses (M = 50; SD = 10). Anxiety was measured by the State Trait Anxiety Inventory for Children (STAIC) [22] ages <12 and the STAI [23] for ages ≥12 (Cronbach’s α = .85–.89). Only the 20 trait anxiety items were used. Ratings used a 3-point (STAIC) and a 4-point scale (STAI). T-scores were used in the analyses.
Statistical Analysis Plan
Standard descriptive statistics were computed (e.g., mean, standard deviation, skewness) and observed for all variables. Distributions were examined for normality and transformations employed when normality assumptions were violated (e.g., cytokine concentrations were highly skewed; thus both z-score and log-transformations were performed). To examine cytokine patterns and profiles, the goal was to reduce the number of cytokines examined in a manner that made sense physiologically and empirically. Novel to the cytokine field, two statistical approaches were selected to accomplish this task: PFA [10] and LPA [24].
We incorporated variable-centered (PFA) and person-centered (LPA) approaches to examine how the array of 13 cytokines could be used to describe underlying constructs representing immune functioning. Both approaches have utility for data reduction when data are multivariate and multidimensional. Variable-centered approaches (i.e., regression, factor analysis) seek to explain the shared variance among a set of variables [8], and are useful for investigating individual differences in dimensionality, levels, and amount of change in an unmeasured construct(s); the dimensional latent construct is considered to be represented in the shared variance of a given a set of variables of interest. An underlying assumption is that the population from which the data are pooled is homogeneous. Thus, in applying such techniques one can describe how covariation among a set of measureable cytokine concentrations are explained by an underlying unmeasurable latent construct (e.g., Th1 pathway) [9]. PFA estimates continuous latent factors; here, the aim is to empirically derive a factor solution (set of latent constructs) that meaningfully and parsimoniously explains overlap among the cytokines.
Person-centered approaches (i.e., cluster analysis, LPA) describe similarities and differences among individuals in how variables are patterned together [25]. A key tenet of person-centered approaches is that interrelations among variables are, in part, specific to individuals [26]. These methods identify homogeneous sub-groups, within a heterogeneous population, grouping together those who share similar patterns of associations among cytokines. LPA is a mixture model which classifies individuals into distinct profiles (categories of a discrete latent construct), where the covariation among cytokines is conditional on the profile membership and each profile describes the pattern for a homogeneous sub-population within a heterogeneous sample; [27] thus, individuals in the same subgroup are more similar to one another and dissimilar to individuals in other groups. Here the aim was to empirically derive a categorical latent structure that identifies sub-groups of individuals based on levels across the 13 cytokines.
PFAs use 13 cytokines to examine underlying dimensionality. To arrive at a meaningful and interpretable factor solution several steps were carried out:[9] 1) first a principle components analyses was executed and the scree plot, the eigenvector, magnitudes of the eigenvalues and amount of shared variance among the cytokines was examined; 2) Next exploratory PFAs were executed using principle axis factoring and unweighted least squares estimations[28] specifying an increasing number of factors (2, 3,…n factors); extracted communalities, factor loadings, correlations, residual variance of the cytokines, and variance explained were examined for interpretability and parsimony and compared across different factor solutions; 3) The PFA solution was rotated both orthogonally (Varimax) and obliquely (Direct Oblimin and Promax), given the high overlap of the cytokines both conceptually and statistically. The factor solutions was then examined via factor patterns (loading values > .55) as “good”[9], distinguishability (no significant loadings (> .35) on more than one factor, given a sample size of N = 262),[8] and amount of shared variance explained by each latent factor [9]. Factor regression scores were saved from the final solution chosen. Analyses for exploratory PFA were carried out using SPSS Version 22.
LPA identified sub-group differences in patterning of the 13 cytokines. The steps carried out to arrive at a meaningful profile solution included:[27, 29] 1) initial examination of the distribution and pattern of covariation among the 13 continuous cytokines; 2) estimation of LPAs with an increasing number of profiles (1, 2, 3….K); 3) inspection and comparison of model fit indices [log-likelihood ratio, Aikake Information Criteria (AIC), Bayesian Information Criteria (BIC), Sample Size adjusted BIC (SS-BIC) and entropy]; LPA was estimated with different seed values to replicate the local solution;[30] and, 4) profile means and mean cytokine concentrations within each profile were inspected to interpret and label the profiles. Latent class probabilities and profile membership were saved from the best fitting solution. LPA analyses were carried out using Mplus version 7.
Following PFA and LPA model estimations, descriptive information for factor composites was preformed along with latent profiles, their associations among demographic characteristics and mean differences in cytokine levels across the latent profiles. To illustrate the utility of these methods we examined how cytokine factors were linked to depressive symptoms and anxiety using hierarchical regression with theenter (Block) method. In the first block, chronological age, race, body mass index (BMI), gynecological age and smoking were entered; all chosen as conceptually relevant covariates. The second block included the cytokine factor composite scales. The association to cytokine profiles was examined via multinomial logistic regression; in these models profile membership was treated as a categorical dependent variable. Paralleling the regression models with the continuous factor composites, the same covariates were included in the first block of the logistic regression model; the second block included depression and anxiety; predicting the likelihood of being in a certain category of the latent profile.
RESULTS
Descriptive statistics
For the 262 girls, the average age was 14.0 years (± 2.17 SD). Racial/ethnic breakdown shows the majority were Caucasian (62%) or African American (32%) with 6% biracial or other. Most girls were in late puberty (Tanner breast I = 1.5%, II = 1.9%, III = 10.3%, IV = 14.9% and V = 71.4%). Mean BMI was 24.0 (± 6.2) and BMI-z was 0.73 (± 1.0). The cytokines were not normally distributed and both z-score and log-transformation were performed. (Table 2). Analyses were repeated for each variable transformation. Although results were primarily comparable across the raw, z-score, and log-transformed cytokines; analyses with the log-transformed variables yielded a cleaner solution and less violation of model assumptions. Thus, results below are reported for analyses performed on the log-transformed cytokines.
Table 2.
Descriptive Statistics of 13 Cytokines in 262 Healthy Adolescent Girls.
| Cytokine (pg/mL) | Mean | SD | Minimum | Maximum | N(%) ≤ Detection |
|---|---|---|---|---|---|
| Adaptive Th1 | |||||
| IL-12p70 | 51.36 | 318.46 | 0 | 4237.52 | 61(23.3) |
| IFN-γ | 19.94 | 44.14 | 0 | 411.78 | 98(37.4) |
| TNF-α | 10.14 | 21.03 | 0.70 | 336.18 | 0 |
| Adaptive Th2 | |||||
| IL-4 | 203.18 | 2542.37 | 0 | 41098.73 | 137(52.3) |
| IL-5 | 3.25 | 20.49 | 0 | 325.41 | 37(14.1) |
| IL-6 | 16.58 | 40.82 | 0 | 498.82 | 18(6.9) |
| IL-10 | 72.77 | 473.77 | 0 | 6475.82 | 17(6.5) |
| IL-13 | 44.98 | 214.93 | 0 | 3365.27 | 70(26.7) |
| Innate | |||||
| IL-1β | 10.17 | 38.71 | 0 | 417.37 | 47(17.9) |
| IL-2 | 25.93 | 135.00 | 0 | 2087.60 | 28(10.7) |
| IL-8 | 10.34 | 19.74 | 0 | 267.02 | 2(0.8) |
| IL-7 | 27.30 | 77.09 | 0 | 980.40 | 3(1.1) |
| GM-CSF | 95.46 | 427.89 | 0 | 6078.23 | 62(23.7) |
Note. Cytokines are listed by adaptive (Th1 and Th2) and innate immune pathways as they often occur in the literature. A value of 0 was indicated when concentrations were below the level of detection.
Examining Cytokine Profiles Using PFA
The final PFA yielded a 4-factor solution explaining 73.13% of the shared variance among the cytokines (Table 3). The 4-factor model was preferred to the 3- and 5-factor solutions as it described a more interpretable and meaningful solution (i.e., significant factor loadings, no cross-factor loadings). Factor correlations ranged from .482 to .710 and communalities for the 13 cytokines ranges from .370 to .882. Due to the state of the science on immune function, less is known about how cytokines pattern together; thus naming the factors seemed premature. All factors note what part of immune function they represent. Factor 1 (representing Th1/Th2 adaptive immunity) accounted for 26.65% (eigenvalue/sum of eigenvalues reduced correlation matrix = 3.465/13.001) of the explained shared variance and contained four cytokines (IL-4, IL-5, IL-13, IFN-γ) with loadings ranging from 0.744 to 1.015; Factor 2 (representing both innate immunity and the lymphocyte proliferation cytokine IL-2) accounted for 17.81% (= 2.315/13.001) of the explained shared variance and contained three cytokines (IL-1β, IL-2, GM-CSF) with loadings ranging from 0.697 to 0.934; Factor 3 (representing both innate immunity and the lymphocyte development cytokine IL-7) accounted for 14.38% (= 1.870/13.001) of the explained shared variance and contained three cytokines (IL-7, IL-8, TNF-α) with loadings ranging from 0.606 and 0.772. Factor 4 (representing both pro-and anti-inflammatory responses and Th1 polarization) accounted for an additional 14.28% of the explained shared variance (= 1.857/13.001) and contained IL-6, IL-10, and IL-12P70 with loadings ranging from 0.688 and 0.852. Overall the factors closely parallel the adaptive vs. innate pathways, with the strongest statistical loadings (55%) being on Factor 1 (Th1/Th2 adaptive pathway), as would be expected in healthy individuals lacking an acute infectious process. Descriptive analyses showed that all factors were positively correlated. In Factor 1 (representing Th1/Th2 adaptive pathway), mean levels were higher among non-white girls (M = 1.51, SD = 1.77) when compared to white girls (M = 1.04, SD = 1.52), F(1, 261) = 4.81, p = .021. In addition, Factor 3 was negatively correlated with chronological age (r = −.232, p < .001), and gynecological age (r = −.211, p = .002) and positively correlated with family SES (r = .168, p = .007). Factor 4 also showed a negative association with gynecological age (r = −.181, p = .009). No other significant associations were found among the factor composites and demographic characteristics.
Table 3.
Principle Factor Analysis Solution and Cytokine Composite Scales and Descriptive Information for 262 Healthy Adolescent Girls.
| Factors | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| % Variance a | 26.65 | 17.81 | 14.38 | 14.28 | |
| Cytokines | Factor Loadingsb | ||||
| IL-4 | 1.015 | −0.079 | 0.083 | −0.148 | |
| IL-13 | 0.783 | −0.05 | −0.082 | 0.279 | |
| IL-5 | 0.744 | 0.185 | 0.002 | −0.077 | |
| IFN-γ | 0.743 | −0.052 | 0.014 | 0.136 | |
| IL-1β | −0.003 | 0.934 | 0.043 | −0.079 | |
| GM-CSF | −0.127 | 0.788 | 0.068 | 0.014 | |
| IL-2 | 0.236 | 0.697 | −0.083 | 0.130 | |
| IL-8 | 0.263 | −0.068 | 0.772 | −0.071 | |
| IL-7 | −0.046 | 0.156 | 0.664 | 0.126 | |
| TNF-α | −0.117 | 0.037 | 0.606 | 0.074 | |
| IL-10 | −0.055 | −0.066 | 0.216 | 0.852 | |
| IL-12p70 | 0.076 | 0.097 | −0.033 | 0.834 | |
| IL-6 | 0.324 | 0 | −0.087 | 0.688 | |
| Descriptive Statisticsc | |||||
| M | 1.21 | 1.85 | 2.22 | 1.85 | |
| SD | 1.64 | 1.43 | 0.66 | 1.55 | |
| Range | −1.00–6.13 | −1.20–6.75 | −0.27–5.68 | −0.84–7.61 | |
| α | .852 | .720 | .825 | .941 | |
| # of cytokines | 4 | 3 | 3 | 3 | |
| Raw Scale Composites |
M | 82.06 | 43.85 | 15.93 | 46.90 |
| SD | 922.73 | 165.11 | 35.53 | 268.20 | |
| Range | 0–14929.80 | 0–2066.76 | 1.49–420.08 | 0–3137.97 | |
| Skew | 16.09 | 8.93 | 9.11 | 10.61 | |
| Kurtosis | 259.78 | 95.25 | 90.31 | 116.13 | |
| Correlationscd | |||||
| Factor 1 | -- | ||||
| Factor 2 | .495 | -- | |||
| Factor 3 | .518 | .482 | -- | ||
| Factor 4 | .710 | .535 | .599 | -- | |
Note. Cytokines are sorted by loading value size. Shaded values corresponding to each factor are cytokines that were used to compute factor composites. Factor loading values are from the PFA executed on the log-transformed cytokine values.
Reflects the percentage of explained shared variance for each extracted factor.
Factor loadings of the PFA using principle axis factoring extraction method and PROMAX oblique rotation.
Descriptive statistics and correlations reflect log-transformed computed factor composite scores.
All correlations were significant at p < .001.
Examining Cytokine Profiles Using LPA
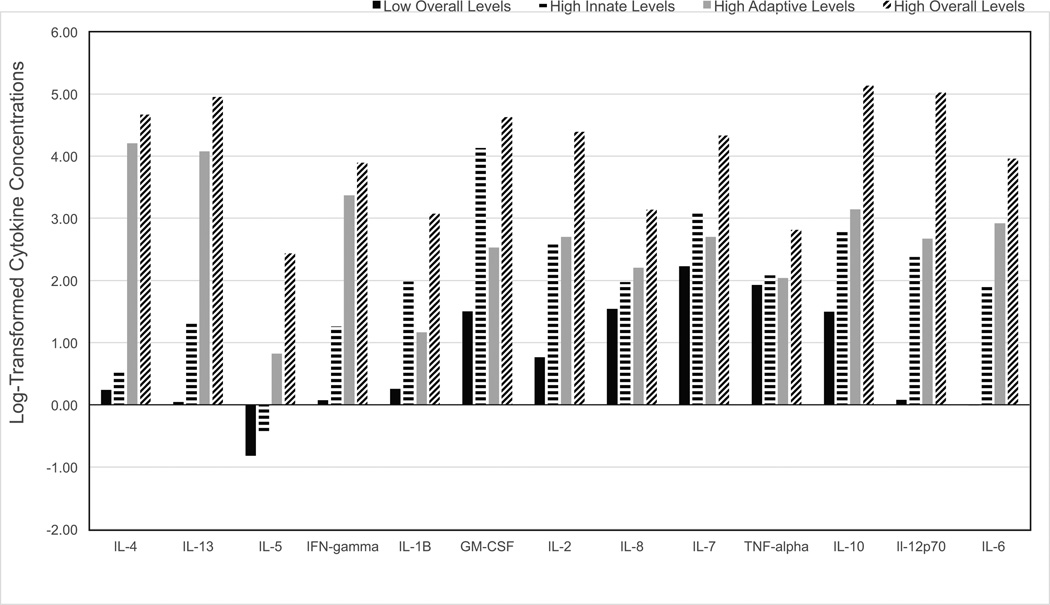
Comparison of the fit indices across LPA solutions resulted in the selection of a four-profile solution with each of the subgroups exhibiting patterns representing different variations in cytokine levels (Table S1, Supplemental Digital Content 1). The first profile was labeled “Low Overall” and comprised 45% (n = 118) of the sample; the average posterior probability for profile membership was .977. Girls classified to this profile had lower than average concentrations for all 13 cytokines. The second profile was labeled “High Innate” and comprised 21% (n = 55) of the sample; average posterior probability for profile membership was .958. Girls were characterized by high concentrations in GM-CSF, IL-2, IL-7, and IL-10. The third profile was labeled “High Adaptive” and comprised 26.7% (n = 70) of the sample; average posterior probability was .995. Girls classified to this profile were characterized by high concentrations of IL-4, IL-6, IL-13, IL-10, IL-12p270, and IFN-γ. The fourth profile was labeled “High Overall” and comprised 7.3% (n = 19) of the sample; average posterior probability was .944. This group was characterized by moderate to high concentrations for all 13 cytokines. Figure 1; Table S1, Supplemental Digital Content 1.
Figure 1.
Profile Solution, Average (log-transformed) Cytokine Concentrations by Profile Membership
The profiles showed significant differences in chronological age, F(3, 258) = 2.97, p = .032; pairwise comparison tests indicated that girls in the “High Overall” (Mage = 14.24, SDage = 2.18 yrs) and “Higher Innate” (Mage = 14.80, SDage = 2.30 yrs) subgroups were younger than girls in the “High Adaptive” (M = 15.33, SDage = 2.10 yrs) and “Low Overall” (Mage = 15.24, SDage = 2.03 yrs) groups. Girls in the “High Overall” and “High Innate” groups were also less physically developed [Tanner Breast Stage, F(3, 258) = 2.73, p = .045; gynecological age, F(3, 258) = 2.95, p = .034] than girls in the “Low Overall” group. Girls classified as “High Overall” were less likely to be white (47.1%) compared to girls classified as “High Innate” (72.6%), “High Adaptive” (55.6%) and “Low Overall” (63.7%), χ2 (3, N = 262) = 9.06, p = .028. There were no group differences on SES [F(3, 258) = 2.15, p = .094] or BMI-z scores [F(3, 258) = 0.70, p = .553].
Linking Cytokines to Depression and Anxiety: Variable-Centered Approach
Hierarchical regression analyses indicated that the cytokine factors significantly improved model fit above covariates (significant increase in adjusted r-squared) for both depressive symptoms (18.4% variance explained; ΔR2 = .126, F(12, 207) = 3.672, p < .001) and trait anxiety symptoms (16.6% variance explained; ΔR2 = .107, F(12, 206) = 3.207, p < .001). Results indicated that Factor 1 (representing Th1/Th2 adaptive immunity) was significantly associated with higher depressive symptoms (B = 3.39, p < .001, 95% CI = 2.00, 4.78). Using clinical terms, a meaningful change in depressive symptoms with treatment may be .5 to 1 SD (5–10 points). This can be shown where a 50% increase in the average value on Factor 1 is associated with an average increase of 1.78 [= 3.39*log(1.50)] points in depressive symptoms. In contrast, Factor 3 (representing innate immunity and the lymphocyte development cytokine IL-7) was negatively associated with depressive symptoms (B = −2.94, p =.030, 95% CI = −5.68, −0.21); where a 50% increase in average value on Factor 3 is associated with an average decrease of 1.55 [= 2.94*log(1.69)] points in depressive symptoms. Factors 2 and 4 were not significantly associated with depressive symptoms (Table S2, Supplemental Digital Content 1).
Additionally, only Factor 1 was negatively associated with trait anxiety (B = 2.84, p < .001, 1.57, 4.11); where a 50% increase in the average value on Factor 1 (representing Th2 adaptive immunity) is associated with an average increase of 1.49 [= 2.84 *log(1.69)] points in trait anxiety symptoms. Factor 3 showed a modest association, but did not reach conventional levels of statistical significance (B = −2.39, p =.067, 95% CI = 1.57, 4.11).
Linking Cytokines to Depression and Anxiety: Person-Centered Approach
In examining the association between depression or anxiety and the cytokine groupings, the “Low Overall” pattern was chosen as the reference group because these individuals had very low activation of the innate and adaptive branches of immunity. Analysis showed that race, depressive symptoms, and trait anxiety were significantly associated with cytokine latent profiles. A unit increase in depressive symptoms increased the likelihood of being in the “High Adaptive” group by a value of 1.10 (95% CI = 1.06, 1.15) when compared to those in the “Low Overall” group. This translates to a 1 SD (10 points) increase in depressive symptoms being associated with a 2.98 (95% CI = 1.90, 4.68) increased likelihood of being classified into the “High Adaptive” group. Similarly, a unit increase in trait anxiety increased the likelihood of being in the “High Adaptive” group by a value of 1.11 (95% CI = 1.06, 1.16), compared to the reference group. This translates to a 1 SD increase in trait anxiety being associated with a 2.91 (95% CI = 1.77, 4.79) increased likelihood of being in the “High Adaptive” group. (Table S3, Supplemental Digital Content 1).
DISCUSSION
Descriptions of cytokines in healthy girls
This study is one of the first to examine the range of multiple cytokine concentrations in a large group of healthy adolescent girls. The few studies conducted in the past on this topic have typically reported on infants or young children [31], while those that have examined healthy children and adolescents have included only small samples, sometimes as few as 10 [5] [6, 7]. Earlier Sack and colleagues [32] reported a wide variability in cytokines across ages, 3–17 years, and in some cases differences compared to adult levels. As echoed by Duramad and colleagues [5], more studies are needed to describe healthy children across age and ethnic background. This will provide information for elucidating mechanisms underlying varied health states.
Girls in the present study were likely typical of individuals that would comprise a healthy comparison group in a clinical study of a specific disorder (e.g., IBD). Our descriptive findings highlight a unique challenge in using cytokine biomarker data in clinical research in that the concentrations have wide ranges, similar to that described in a smaller sample by Chavez-Valdez and colleagues [33]. Being attuned to cytokine distributions in healthy comparison groups, may greatly enhance subject selection and data interpretation in other studies. In the current study, baseline cytokine patterns were statistically dissimilar between individuals and fell within four distinct profiles. This suggests that adolescent comparison groups need be large enough to overcome this heterogeneity in immune profiles.
This study quantified a wide array of cytokines that relate to innate immunity and adaptive Th1 and Th2 immune functions. Since the immune system acts in concert, with some cytokines suppressing and other cytokines promoting inflammation, it is important to understand how to interpret multiple biomarkers simultaneously. Hence, there is a need for methodology that will better analyze and interpret cytokine data as discussed below.
Analytic strategies for examining cytokines
The analytic strategies utilized here offer an alternative approach for examining cytokines and determining cytokine profiles beyond the usual method of examining the association of each individual cytokine on a specific outcome variable or using a standard cut-point to group cytokine families [34]. The 4-factors, representing both innate and adaptive immunity, explained 73.13% of the total variance in the data; hence, these factors provide a strong measure of what is “real” in the 13 individual cytokines. Additionally, variable-centered data reduction techniques can be useful when one has multiple cytokines and is testing hypotheses about how certain cytokines should factor together based on a physiological construct. Such models would be informative to the conceptual understanding of immune function via identifying underlying latent factors that explain joint functioning among groups of cytokines. Once identified, such components can be used in future analyses either as predictor or outcome variables, depending on a study’s hypothesis.
In looking at the individual factors, the PFA reflects what is known about the physiology of cytokines. First, it is encouraging that these different factors incorporated cytokines known to represent different immune pathways (adaptive, innate) supporting the methodology. Second, since these are healthy adolescents one would expect the “weighting” of the various pathways to favor adaptive rather than innate immunity. This is because past illness (bacterial, viral) and vaccinations are events that elicit higher activation of adaptive immune components, whereas higher activation of the innate immune system is suggestive of a current infectious process. Indeed, PFA revealed that the Factor 1 representing higher Th1/Th2 adaptive immunity, accounted for the majority of the explained shared variance (~27%); conversely, Factors 2 to 4, which seemingly represented aspects of innate immunity, lymphocyte development (IL-7) and proliferation (IL-2), accounted for much less variance (~14 to 18%).
Alternatively, LPA provides a person-centered approach by grouping individuals rather than variables (i.e., cytokines) into classes; an approach found infrequently in medical literature. This may be unfortunate as it is a meaningful way of grouping participants based on cytokine profiles; specifically noting that all individuals with X disorder may not be the same but rather they may have distinct cytokine patterns. Importantly LPA appears to be congruent with what is known about the physiology of cytokines. For example, using LPA to group individuals who have similar cytokine profiles (e.g., “High Overall” group pertained to 7.3% of the girls, who were likely to exhibit relatively high levels of 12 of the 13 cytokines) can be useful for further examination. Specifically, the classes could be used as an outcome or as a predictor variable to describe which group may be more likely to exhibit a certain disease or behavior. This LPA method revealed, not surprisingly, that the largest single proportion (45%) of healthy adolescents in this study were found to have low overall cytokine levels. Thus, the two strategies offer unique analytical methods for utilizing multiple cytokines simultaneously. Each technique offers a valuable approach depending upon the hypothesis being tested.
Next the components of the PFA and the classes of the LPA were used to illustrate the utility in examining associations between psychological variables and cytokines based on previously reported associations. Using PFA we found that higher scores on Factor 1 (i.e., reflecting Th1/Th2 adaptive immunity) were associated with higher depressive symptoms and higher state and trait anxiety, while higher scores on Factor 3 (i.e., reflecting both innate immunity and lymphocyte development) were associated with lower depressive symptoms. There is a time-lag for adaptive immune responses to initially develop (5–7 days) and they generally involve a more prolonged activation of the immune system than do innate immune responses (first line of defense against a pathogen).
For LPA, the person-centered approach, the likelihood of being in a certain latent profile group was related to psychological health measures. For example, compared to individuals in the “Low Overall” group, a 1 SD increase in depressive symptoms or trait anxiety increased the odds by nearly three times of being in the “High Adaptive” group. Similar to the PFA analysis, these results suggest that individuals that exhibit higher levels of adaptive immune components in their blood, or higher overall cytokine levels, may be more predisposed to exhibit higher depressive and anxiety symptom levels. Higher levels of systemic inflammation are similar to what is observed in other chronic health problems such as cardiovascular disorders [35, 36]. Conversely, individuals with lower overall cytokine levels, or with higher activation of innate immune components in blood, did not exhibit this relationship. Importantly, associations do not imply causation since data are cross-sectional.
It is critical to emphasize that statistical methods (rather than knowledge of biology) drove the identification of the factors from the PFA and the profiles from the LPA. Such statistical methods are perhaps more stringent and represent a more objective and unbiased approach, since the biology of cytokines is somewhat open to interpretation and often ambiguous due to the overlap and redundancy of functions that occur between analytes (Table 1). Hence, the use of data driven statistical approaches may have stronger merit for the study of healthy populations, and for healthy subgroups that serve as controls in the study of a particular disease. For instance, in the study at hand, a significant number of individuals were classified as having high cytokine levels despite their reporting good health at the time of testing.
In spite of the strengths of this study, limitations should be considered. First, cytokine determinations were made on a single sample taken at approximately 1:30 p.m.; therefore we could not examine individual circadian differences in cytokines. Second, the possibility could not be eliminated that high levels of cytokines in some individuals were related to an emerging (sub-symptomatic) or recent acute infection, or another unidentified physical/psychological stressor. However, participants reported no infections and few girls met diagnostic criteria for major depressive disorder which, in adults, has been associated with altered cytokine levels [37]. It was beyond the scope of the paper to examine anti-cytokine auto antibodies which can regulate cytokine function. Such autoantibodies are present in many healthy and diseased individuals. Importantly, this study calls into question, what is “normal”. Determining “normal” is difficult without knowing what environmental triggers may drive or influence these cytokine profiles. Possibly individuals with low cytokine levels in the LPA analysis had the same exposures but were relatively anergic or had a blunted immune response, or they were not being exposed to the same factors that drove systemic inflammation in the high cytokine group. Finally, longitudinal analyses are needed to determine the stability of these cytokine profiles over time, whether there is a change in factor structure using PFA or in groupings using LPA, as well as if these profiles may be predictive of physical and mental health outcomes. Finally, new developments in statistical techniques allow for the integration of both variable-and person-centered methods for studying development.[12, 38] Although beyond the scope of this paper, future research on cytokines could benefit from the utility of such an approach. With an integrated analysis, researchers can begin to truly delve into the “black box” of physiological functioning and better elucidate how patterns and levels of latent indices of function are associated with health-related outcomes.
In conclusion, healthy adolescent girls show a wider range of variation in levels of cytokines than have been previously reported. Such findings have implications for future studies that include a comparison group of healthy adolescent girls. The study also showed distinct groupings based on cytokine profiles that can be generally classified into innate and adaptive immune responses. PFA and LPA offer novel statistical approaches to the field of cytokine research by examining multivariate cytokine panels. These strategies represent a parsimonious way of using empirically-derived data reduction methods to explain how cytokines covary (PFA) and examine subgroup differences in how cytokines pattern together (LPA). Such approaches would be useful for future studies aimed at objectively examining groups of cytokines, as well as biomarker studies interested in determining how different patterns of immune activity relate to various health outcomes. For example, using PFA and LPA to examine associations with depressive and anxiety symptoms indicated that it may be important consider longitudinally, if some girls with a certain cytokine profile are more likely to become depressed or anxious, if depression or anxiety changes the cytokine profile, or if there is an evolutionary advantage to having a certain cytokine pattern. These analytic strategies also would be useful for classifying healthy individuals into more exact sub-categories when creating a comparison group in a clinical study; by better characterizing what “normal” is, more informative analyses could be performed.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING: This work was supported in part by funding from the National Institutes of Health (R01 DA 16402, R21 DA025312; PI: Dorn) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through Grant 8 UL1 TR000077-04 given to the Institution. We thank Ms. Stephanie Pabst and Dr. Jennifer Hillman for assistance in an earlier draft of the manuscript.
Abbreviations
- DXA
Dual energy x-ray absorptiometry
- GM-CSF
granulocyte macrophage colony-stimulating factor
- IFN-γ
interferon gamma
- IL
interleukin
- IBD
irritable bowel disease
- LPA
latent profile analysis
- NK
natural killer cell
- PFA
Principal Factor Analysis
- SES
socioeconomic status
- Th1
T helper 1
- Th2
T helper 2
- TNF-α
tumor necrosis factor alpha
Footnotes
COI: No competing conflict of interest exists.
References
- 1.McInnes IB. Cytokines, in Kelley's textbook of rheumatology. 8th. Philadelphia, PA: Saunders Elsevier; 2008. pp. 367–377. [Google Scholar]
- 2.Fantini MC, Monteleone G, MacDonald TT. New players in the cytokine orchestra of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(11):1419–1423. doi: 10.1002/ibd.20212. [DOI] [PubMed] [Google Scholar]
- 3.De Jager W, Hoppenreijs EPAH, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66(5):589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Critical Care. 2007;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duramad P, Tager IB, Holland NT. Cytokines and other immunological biomarkers in children's environmental health studies. Toxicol Lett. 2007;172(1–2):48–59. doi: 10.1016/j.toxlet.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. doi: 10.1155/2013/434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pranzatelli MR, Tate ED, McGee NR, Colliver JA. Pediatric reference ranges for proinflammatory and anti-inflammatory cytokines in cerebrospinal fluid and serum by multiplexed immunoassay. J Interferon Cytokine Res. 2013;33(9):523–528. doi: 10.1089/jir.2012.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hair JF, Black WC, Babin BJ, Anderson RE, Tatham RL. Multivariate data analysis. 7th. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. [Google Scholar]
- 9.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th. Boston, MA: Allyn & Bacon; 2007. [Google Scholar]
- 10.Gorsuch RL. Factor Analysis. 2nd. Hillsdale, NJ: Psychology Press; 1983. [Google Scholar]
- 11.Lazarsfeld PF, Henry NW, Anderson TW. Latent Structure Analysis. New York, NY: Houghton Mifflin Boston; 1968. [Google Scholar]
- 12.Laursen BP, Hoff E. Person-centered and variable-centered approaches to longitudinal data. Merrill-Palmer Quarterly. 2006;52(3):377–389. [Google Scholar]
- 13.Ruscio J, Ruscio AM. Categories and dimensions: Advancing psychological science through the study of latent structure. Curr Dir Psychol Sci. 2008;17(3):203–207. doi: 10.1111/j.1467-8721.2008.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorn LD, Pabst S, Sontag LM, Kalkwarf HJ, Hillman JB, Susman EJ. Bone mass, depressive, and anxiety symptoms in adolescent girls: Variation by smoking and alcohol use. J Adolesc Health. 2011;49(5):498–504. doi: 10.1016/j.jadohealth.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson G, Horn JL. Age, cohort, and time developmental muddles: Easy in practice, hard in theory. Exp Aging Res. 1992;18(4):213–222. doi: 10.1080/03610739208260360. [DOI] [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorn LD, Negriff S, Huang B, Pabst S, Hillman J, Braverman P, Susman EJ. Menstrual symptoms in adolescent girls: Association with smoking, depressive symptoms, and anxiety. J Adolesc Health. 2009;44(3):237–243. doi: 10.1016/j.jadohealth.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished working paper. [Google Scholar]
- 19.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol Clinical and Experimental. 2012;27(1):6–14. doi: 10.1002/hup.1259. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs M. Children's Depression Inventory: Manual. Multi-Health Systems; 1992. [Google Scholar]
- 22.Spielberger CD, Edwards CD, Lushene RE, Montuori J, Platzek D. STAIC Preliminary Manual. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- 23.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 24.Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences, in Probability and statistics. Hoboken, NJ: John Wiley & Sons, Inc; 2010. p. 330. [Google Scholar]
- 25.Bergman LR, Magnusson D, El Khouri BM. Studying Individual Development in an Interindividual Context: A Person-Oriented Approach. Psychology Press; 2003. [Google Scholar]
- 26.Bergman LR. A person approach in research on adolescence: Some methodological challenges. J Adolesc Res. 2001;16(1):28–53. [Google Scholar]
- 27.McLachlan GJ, Peel D. Finite Mixture Models. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 28.De Winter JC, Dodou D. Factor recovery by principal axis factoring and maximum likelihood factor analysis as a function of factor pattern and sample size. Journal of Applied Statistics. 2012;39(4):695–710. [Google Scholar]
- 29.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14(4):535–569. [Google Scholar]
- 30.Collins LM, Lanza ST. The Social, Behavioral, And Health Sciences. Vol. 718. John Wiley & Sons; 2010. Latent Class And Latent Transition Analysis: With Applications. [Google Scholar]
- 31.Härtel C, Adam N, Strunk T, Temming P, Müller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142(3):446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sack U, Burkhardt U, Borte M, Schädlich H, Berg K, Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998;5(1):28–32. doi: 10.1128/cdli.5.1.28-32.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavez Valdez R, Ahlawat R, Wills-Karp M, Nathan A, Ezell T, Gauda EB. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. The Journal of Pediatrics. 2011;158(1):57–64.e1. doi: 10.1016/j.jpeds.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simhan HN, Krohn MA. First-trimester cervical inflammatory milieu and subsequent early preterm birth. Am J Obstet Gynecol. 2009;200(4):377.e1–377.e4. doi: 10.1016/j.ajog.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 35.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 36.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 37.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubke GH, Muthén B. Investigating population heterogeneity with factor mixture models. Psychol Methods. 2005;10(1):21. doi: 10.1037/1082-989X.10.1.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.