Abstract
The advent of fluorescent proteins (FP) for genetic labeling of molecules and cells has revolutionized fluorescence microscopy. Genetic manipulations have created a vast array of bright and stable FPs spanning the blue to red spectral regions. Common to autofluorescent FPs is their tight β-barrel structure, which provides the rigidity and chemical environment needed for effectual fluorescence. Despite the common structure, each FP has its own unique photophysical properties. Thus, there is no single “best” fluorescent protein for every circumstance, and each FP has advantages and disadvantages. To guide decisions about which FP is right for any given application, we have characterized quantitatively over 40 different FPs for their brightness, photostability, pH stability, and monomeric properties, which permits easy apples-to-apples comparisons between these FPs. We report the values for all of the FPs measured, but focus the discussion on the more popular and/or best performing FPs in each spectral region.
Introduction
Live cell fluorescence microscopy offers a powerful way to examine tissues, cells, and subcellular components at functionally important time and length scales1. The cloning of green-fluorescent protein from the jellyfish Aequorea victoria2 and its expression in non-jellyfish systems3 empowered fluorescence microscopy in cell biology and physiology. A major leap in fluorescent protein (FP) technology came with the discovery of homologous fluorescent proteins from corals and other Anthozoans4. Genetic manipulations performed on A. victoria GFP5 as well as non-jellyfish proteins6 have led to FPs covering blue to red spectral regions with improved brightness and better stability5,7,8. Detailed discussions of FP mutations are found elsewhere1,5,8.
Some general properties of FPs are critical for understanding their function and utility. FPs are ~25 kD, which is large compared to organic fluorophores, and the entire protein structure appears to be essential to its fluorescence9. FP structures consist of 11 β-strands surrounding a central α-helix10, where the fluorophore arises from a few amino acids. During protein biogenesis, a reaction occurs among FP residues to form an extended conjugation5, and fluorescence depends on rigidity and chemical environment within the protein structure11. Within the β-barrel fold, mutations can change the local fluorophore environment, and lead to variations in spectral characteristics, photostability, acid resistance, and other physical properties. Mutational work on FPs initially focused on improved maturation of fluorescence at 37°C7,12, enhanced protein maturation and stability13,14, and improved stability in response to chloride, acidity, and photoexcitation15. Beyond simply improving the photophysical properties of FPs, silent mutations have been made to optimize expression in host organisms16. Finally, virtually all FPs are oligomeric (either dimeric or tetrameric) in their natural environment, so mutations have been made towards creating monomeric FPs derived from jellyfish17 and other species6,18. After over 20 years of research, there is still no perfect fluorescent protein. Each FP has advantages and disadvantages, so there is no “best” one for all situations. To provide consistent data for comparisons across different FP performance, we characterized over 40 different FPs for their brightness, photostability, pH stability, and monomeric properties. While we report the values for all the FPs, discussion will be focused on some of the more popular and/or best performing FPs in various spectral regions.
Results
The fluorescent protein color palette
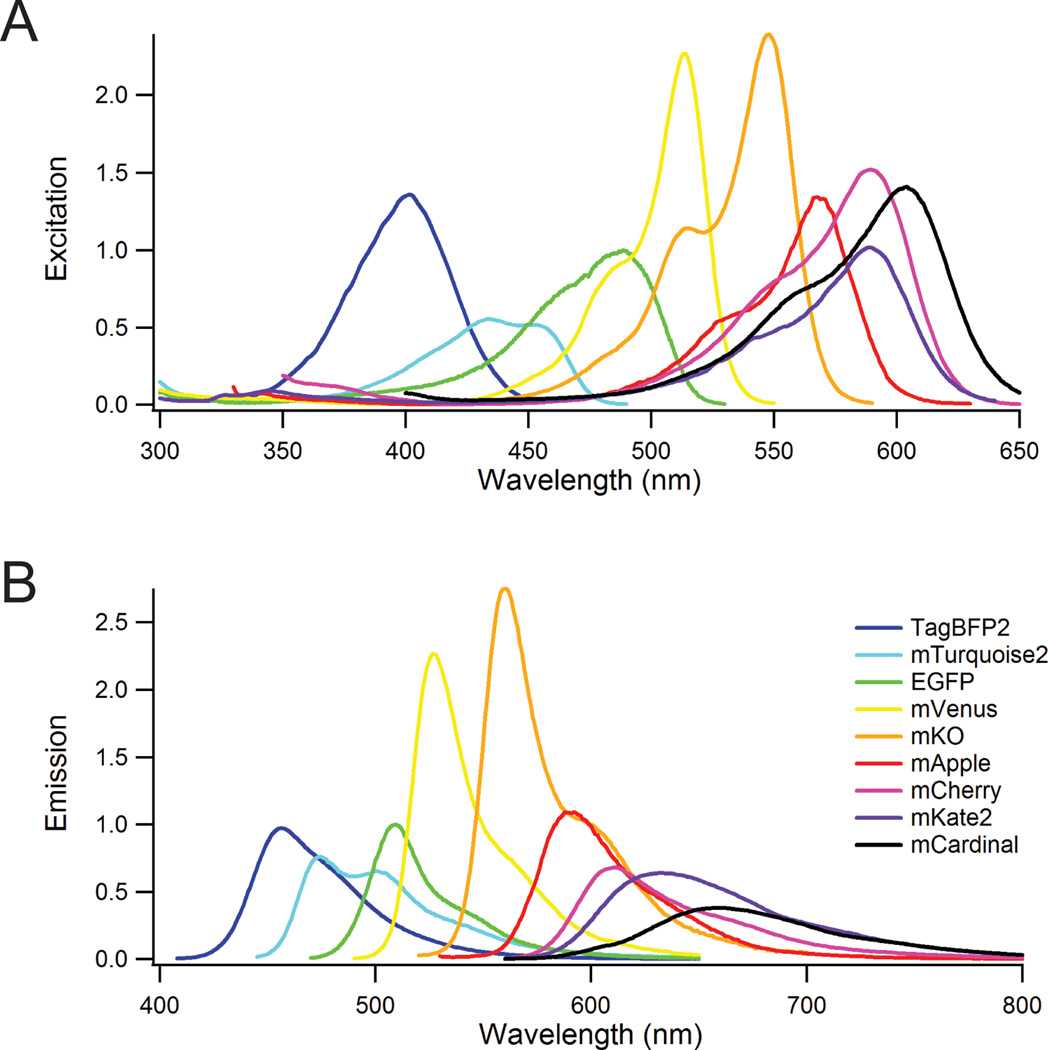
A major advantage of fluorescence is the ability to resolve multiple labels by their fluorescence color. FP variants span the visible spectrum, broadly categorized into blue, cyan, green, yellow, orange, red, and far-red. Within a given color range, the simplest delineation is brightness, defined as the combination of light absorbance (extinction coefficient) and fluorescence quantum yield (ratio of fluorescent photons per absorbed photon). To compare brightness, FPs were purified and their extinction coefficients, quantum yields, and spectral properties measured7,19. The enhanced green-fluorescent protein (EGFP) was the first robust and reliable FP12, and it remains the gold standard for FPs. Excitation (Fig. 1a) and fluorescence (Fig. 1b) spectra of FPs can be normalized to EGFP for relative absorbance and brightness. Beyond the excitation and emission maxima and brightness of the FP, the specific spectral shape is important. A striking example is the excitation spectra for mKate2 and mCardinal (Fig. 1a). The excitation peaks of these two spectra are separated by 15 nm, but the broader spectral width of mCardinal gives significant absorption at ~635 nm (a typical laser line), but the absorption of mKate2 at 635 nm is negligible. Thus, knowledge of spectral shapes is important for choosing between FPs.
Figure 1.
Excitation and emission spectra of several commonly used FPs across the visible region. The peak values of the excitation spectra (A) are normalized to EGFP = 1 by the extinction coefficient, and the emission spectra peaks (B) are normalized to EGFP = 1 by their relative brightness (extinction coefficient × quantum yield).
FP brightness peaks in the middle of the visible spectrum with the yellow and orange FPs (mVenus and mKO FPs in Fig. 1), which follows from general fluorophore properties. Optical transition energy depends on charge separation in the fluorophore with larger the separation leading to lower energy transitions. Blue fluorophores (higher energy) are necessarily smaller than red fluorophores, so blue ones have smaller extinction coefficients. On the other hand, quantum yield is usually inversely correlated with fluorophore size. High quantum yield depends on fluorophore rigidity, and since larger molecules have more degrees of freedom, they are less rigid. Therefore, despite the higher absorption of red FPs, their brightness is reduced by lower quantum yields. The extinction coefficients, quantum yields, relative brightness and pKa values are given in Supplementary Table 1.
Photobleaching
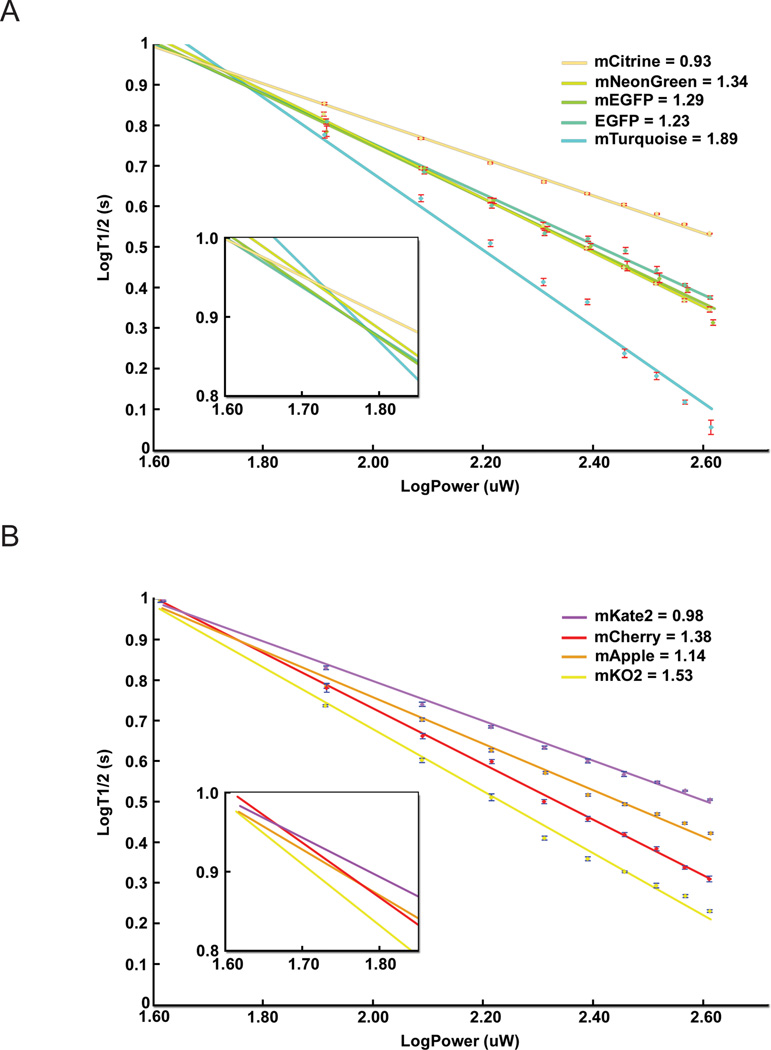
A major consideration in fluorescence microscopy is fluorophore photostability. We measured photobleaching rates of FP variants embedded in poly-acrylamide20 or microdroplets19,21, which allow for comparisons between microscopies (Supplementary Table 2). Under identical conditions (80 µW laser illumination), we measured bleaching half-times from 530 sec for mCardinal range down to 2.7 sec for DsRed2. We also used different illumination intensities and modalities (widefield metal halide lamp or LED, and laser scanning). We verified that fluorescence intensity is a linear function of excitation power, and based on 40 years of literature22,23, we expected photobleaching rates to also be linear (i.e., for twice the intensity, the photobleaching rate doubles). Instead, we found that almost all FPs exhibit supra-linear (“accelerated”) photobleaching, which is shown for selected FPs in Fig. 2. This accelerated photobleaching is similar to what is measured under two-photon excitation21. We can fit these data to log(F) = −α·log(P) + c, which yields a photobleaching rate of kbleach = b·Iα, where F is the fluorescence intensity, P is the illumination power, and b and c are constants. The slope of the log-log plot gives the value of α, and these values are listed in Supplementary Table 2 for each modality.
Figure 2.
Log-Log plot of photobleaching rates vs. illumination power as measured by laser scanning microscopy – similar power dependence is seen with widefield lamp or LED illumination (see Table 2). Data points are plotted as average and standard deviation as described in the methods. Panel (A) shows the excitation intensity dependence for a few common cyan, green, yellow FPs, while (B) shows the behavior for orange and red FPs. For each FP, the fit value of the exponent α is given on the legend.
Accelerated FP photobleaching has significant implications, as even a small α can make a big difference in the total photobleaching depending on the imaging method. Widefield microscopy uses low power spread over every pixel for the full duration of the image, but laser scanning confocal microscopy focuses all of the light on to a single pixel for a short period of time and then raster scans the pixels. Using identical total power for widefield and confocal imaging, the instantaneous power (i.e., intensity) is much higher in laser scanning. For a one second 512×512 image, the laser-scanning intensity is 250,000-fold larger than in widefield illumination. For α = 1.07 (mEGFP), the laser scanning bleaching rate (kbleach) goes as 250,0001.07 ≈ 596,750, but the pixel is only illuminated 1/250,000 of the image time, which yields bleaching (kbleach · time) ~2.4 fold greater in laser-scanning than widefield microscopy. Photobleaching differences between laser scanning and widefield illumination grow rapidly as a function of the exponent, α: α = 1.2 leads to >10 fold faster photobleaching in laser scanning, and α = 1.35 leads to >100 fold faster bleaching. Despite accelerated photobleaching disproportionate effects on laser scanning approaches, confocal imaging is superior for many studies of intact tissue because of its 3D signal discrimination24, and many long-term confocal imaging studies have been successful with FPs. Accelerated photobleaching is not as bad for spinning disk approaches (where multiple spots are simultaneously, thus lowering the intensity at each spot), but photobleaching in these instruments can still be several fold faster than for widefield imaging25. The mechanism underlying accelerated photobleaching remains unknown, but it is hypothesized that additional photons interacting with the excited-state fluorophore increase the photobleaching probability.
Fig. 2 and Supplementary Table 2 data were acquired in vitro, but accelerated photobleaching of FPs is also seen in cells. We measured laser scanning confocal bleaching rates of four widely-used FPs, mCerulean (α = 1.12), mEGFP (α = 1.29), mVenus (α = 1.13), and mCherry (α = 1.38), at 60 µW and 4-fold higher power, 240 µW. Free FPs were expressed, and bleaching was measured over entire cells (>100 cells each condition). Linear photobleaching would yield 4-fold faster bleaching for 240 µW over 60 µW, so deviations from the factor of 4 yield a value for α. For mCerulean, the bleaching half-time was 108 sec with 60 µW and 24 sec with 240 µW, an apparent α = 1.09. For mEGFP, bleaching half-times were 239 sec at 60 µW and 46 sec at 240 µW (α = 1.19); for mVenus, 58 sec at 60 µW and 12 sec at 240 µW (α = 1.14); for mCherry, 348 sec at 60 µW and 49 sec at 240 µW (α = 1.41), all consistent with our in vitro measurements. Absolute photobleaching rates and accelerated photobleaching appear to be independent. In cells, mCherry bleaches ~50% more slowly than mEGFP with 60 µW, but their photobleaching rates are nearly identical at 240 µW.
Photobleaching is a complex phenomenon that depends on many factors, especially oxidizing or reducing environments, but accelerated photobleaching appears independent of the local environment21. However, photobleaching rates in cells can vary as a function of the incubation medium26. Similar differences can be expected for FPs in various intracellular compartments, but slowly photobleaching FPs are generally more slowly photobleached in all compartments. Photobleaching can also be exacerbated durings multicolor imaging, as some FPs photobleach faster in the presence of additional illumination wavelengths, even when those additional wavelengths would not excite the FP on their own27.
Several consequences arise from this accelerated photobleaching phenomenon. First, it is difficult to choose between FPs based on any single photobleaching rate. An FP that bleaches too fast at low excitation intensity may actually be preferable for higher intensity experiments if it demonstrates minimal accelerated photobleaching. Second, the most common approach for time-lapse, three-dimensionally resolved measurements confocal microscopy, but these instruments are susceptible to accelerated photobleaching problems. Several alternatives offer reduced photobleaching susceptibility given supra-linear bleaching. These alternatives do not require scanned illumination or a pinhole for detection, and they include total internal reflection (TIRF), selective plane illumination (SPIM), deconvolution, and structured illumination microscopies. Some of these approaches do not create out-of-focus background in the first place (TIRF and SPIM), and others utilize the out-of-focus light to generate a more complete view of an entire three-dimensional data volume (deconvolution and structured illumination). TIRF is ideal for cell imaging28, especially events involved in apoptosis, but can only measure things within ~100 nm of the surface. Both deconvolution29 and structured illumination30 microscopies can achieve high lateral and axial resolution (100 nm in x&y and <500 nm in z, respectively), but in practice for scattering samples, such as cell clusters, these approaches lose effectiveness at depths <10 µm into the sample31,32. SPIM is a more recent development that permits imaging deep into samples with minimal photobleaching33. These alternatives are widely used in cell biology research, and with the potential severity of accelerated photobleaching, even more researchers should consider using them.
Monomeric quality
Virtually all FPs are oligomeric (either dimeric or tetrameric) in their natural environment. Wild-type A. victoria GFP is part of a heterotetrameric complex with aequorin34, whereas most coral and anemone FPs occur naturally as tetramers6. To prevent oligomerization, mutations have been developed to practically eliminate dimerization of jellyfish derived FPs17, and though eliminating tetramers in coral FPs has proven more difficult, significant progress has been made. For some applications such as cell-type identification, monomeric proteins are not required, so any bright FP of the appropriate color can be used. Monomerized FPs work well in most applications, even though some can form low-affinity oligomers when expressed at high levels or constrained within subcellular compartmens. To evaluate FP oligomeric tendencies, we utilized FPs fused onto an endoplasmic reticulum (ER) membrane protein (CytERM)35. These ER-associated FP-fusion proteins can be expressed at high effective concentrations, where homo-oligomerization can lead to cross-linking between FPs and reconfiguration of ER from a tubular network into an organized smooth ER (OSER) whorl structure. Live cells included in the OSER assay were first evaluated to eliminate gross overexpression artifacts, and representative images of live cells used for the OSER assay of different FPs are shown in Fig. 3. Approximately 10,000 cells were analyzed for each FP (using a minimum of 7,000, but up to 20,000 cells), and the percentage of observed cells exhibiting OSER whorls was determined over at least 15 preparations by three individual scorers.
Figure 3.
Widefield fluorescence images of FP-CytERM fusion proteins expressed in HeLa cells. (A) mEGFP (A206K); (B) mCherry; (C) mCitrine (A206K); (D) mOrange2; (E) mNeonGreen; (F) mKate2; (G) EGFP (A206); (H) mKO2; (I) mTagBFP2; (J) mTagRFP-T; (K) Citrine (A206); (L) DsRed2. Scale bar = 10 µm.
We performed the OSER assay on 63 FPs, including known oligomeric FPs. Many FPs appear strongly monomeric, although others that have been previously reported as monomeric were found to oligomerize (Table 1). The two monomeric forms of EGFP (mEGFP containing either the A206K or the L221K mutation17) are the best performing FPs in the OSER assay. There is a continuum of monomeric quality, but we suggest that FPs scoring ~90% or higher can be reasonably assumed to be monomeric. We expect these FPs to perform well in most fusion constructs, but their behavior should be evaluated in each specific construct and cell type. Many widely used and promising FPs received scores below 90%. In the same way that a high score in the OSER assay does not mean an FP will always perform well, a lower score does not mean that an FP can never be used. We have had success using mKO2 and mCardinal in protein fusions, although each has also failed in some circumstances. Such results would be consistent with their OSER assay scores of 68% and 41%, respectively.
Table 1.
Percentage of cells scored without visible OSER whorls as a function of FP-CytERM fusion protein, ranked from the most monomeric (100%) to the most strongly oligomeric (0%).
| Fluorescent Protein | % Normal Cells | % StDev |
|---|---|---|
| mEGFP (L221K) | 98.8 | 1.2 |
| mEGFP (A206K) | 98.1 | 1.6 |
| mEmerald (A206K) | 96.6 | 1.1 |
| mRFP1 | 95.8 | 1.1 |
| mT-Sapphire (A206K) | 95.5 | 0.6 |
| mApple | 95.3 | 1.7 |
| mPapaya | 95.1 | 1.1 |
| mCherry | 95.0 | 0.8 |
| CyPet | 94.0 | 2.4 |
| mKate2.5 | 93.9 | 1.7 |
| mCitrine (A206K) | 93.8 | 2.6 |
| mTurquoise2 (A206K) | 93.8 | 1.0 |
| mTurquoise (A206K) | 93.3 | 1.2 |
| mRuby | 93.1 | 2.1 |
| mCerulean (A206K) | 92.6 | 2.0 |
| mTFP1 | 92.0 | 1.5 |
| mTopaz (A206K) | 91.9 | 2.0 |
| mOrange2 | 91.8 | 1.4 |
| FusionRed | 91.5 | 3.0 |
| mPlum | 91.5 | 2.4 |
| mOrange | 91.4 | 1.7 |
| mCerulean3 (A206K) | 91.0 | 1.8 |
| mStrawberry | 90.6 | 2.5 |
| mClover (A206K) | 90.5 | 4.5 |
| mNeonGreen | 90.4 | 2.1 |
| mEos3d.2G16 | 90.4 | 2.4 |
| mAzamiGreen | 90.3 | 4.7 |
| mAmetrine | 90.0 | 4.5 |
| mRaspberry | 89.6 | 4.0 |
| mPapaya2 | 87.6 | 3.2 |
| mRuby2 | 87.4 | 5.8 |
| mWasabi | 86.4 | 2.5 |
| mVenus (A206K) | 83.9 | 4.4 |
| mKate2 | 81.1 | 6.1 |
| Cerulean (A206) | 78.3 | 4.8 |
| EGFP (A206) | 76.5 | 6.9 |
| Clover (A206) | 72.9 | 5.5 |
| mKO2 | 68.4 | 4.2 |
| Topaz (A206) | 63.8 | 8.0 |
| tdTurboRFP | 62.5 | 9.4 |
| YPet (A206) | 62.5 | 5.2 |
| Superfolder GFP (A206V) | 62.4 | 6.3 |
| Emerald (A206) | 58.9 | 5.5 |
| mTagRFP | 57.7 | 7.5 |
| tdTomato | 57.6 | 4.9 |
| EBFP2 (A206) | 57.0 | 2.7 |
| mKate | 54.5 | 4.8 |
| mEos3dG16 | 53.3 | 7.8 |
| mTagBFP2 | 49.8 | 1.9 |
| mKO | 46.9 | 6.1 |
| MiCy | 41.7 | 10.7 |
| mCardinal | 41.3 | 3.6 |
| mTagRFP-T | 41.2 | 4.0 |
| mNeptune | 40.5 | 3.8 |
| Venus (A206) | 36.5 | 3.5 |
| Citrine (A206) | 36.2 | 3.9 |
| tdKatushka | 30.6 | 5.9 |
| acGFP | 29.5 | 12.4 |
| TurboRFP | 0.0 | 0.0 |
| dKatushka | 0.0 | 0.0 |
| DsRed2 | 0.0 | 0.0 |
| zsYellow | 0.0 | 0.0 |
| AsRed | 0.0 | 0.0 |
Beyond brightness, pH- and photo-stability, or monomeric quality, other factors can affect FP performance in specific situations, but these are difficult to assess across all FPs. First, some experiments require a fast maturation rate or high maturation efficiency of the FP fluorophore, for which some FPs are optimal (e.g., sfGFP14 and mVenus36). However, this property can be cell-type dependent, and is difficult to recapitulate accurately in vitro. Second, another property that is difficult to assess broadly is protein stability. Some FPs have been designed to degrade37 or change color38, but protein stability and color switching can be confounding factors. While the photoactivation properties intrinsic to the original GFP7 have been leveraged for lineage tracing and super-resolution microscopy, many FPs show photo-switching properties that complicate experimental results39,40. Not every FP exhibits photoswitching, and those that do have properties that can depend on both excitation intensity and pH40. Third, the fidelity of any FP in fusions relies on more than oligomerization, as FPs can interfere with proper trafficking of the target protein. Such interference can often be specific to each FP and cell-type. Finally, FPs exhibit different expression levels under identical conditions41. These differences might reflect mRNA stability, translation efficiency, or FP stability, and can be critical for FP use in transgenic animals.
Discussion
As demonstrated by these data, there is no single “best” FP for every circumstance. One interesting observation is that even though EGFP was the first generally useful FP, mEGFP remains one of the best and most versatile FPs available. Considering how the growth of FP use depended on this early variant, it seems fortuitous that it is one of the best performers. When choosing an FP, it is important to note that every FP measured here (as well as previous variants that were dimmer and less photostable) can be used effectively in many experiments. For this reason, it may not be worth the effort to switch to an improved FP if the one currently in use is yielding reliable data. For new projects, though, our comprehensive data set provides a guide to which FPs are likely to be the best choice. Based on overall performance, mTagBFP2 and mTurquoise2 are considered the best of the blue and cyan FPs, respectively. However, unless these colors are needed for specific reasons, it is usually better to use more red-shifted FPs to avoid the greater autofluorescence in these spectral regions. In particular, blue FPs have proven difficult to use and should be used with caution. In the green, EGFP was the first generally reliable FP, and because of its combination of positive attributes, mEGFP remains the gold standard to which the performance of other FPs is compared. For the yellow spectrum, mVenus and mCitrine have proven reliable, but because mCitrine is more strongly monomeric, it is likely better for the most demanding situations.
The orange FPs are the brightest, due to their combination of large extinction coefficients and relatively high quantum yields, but choosing the “best” orange FP is not straight forward. The brightest orange FPs, mKO and mKO2, show a propensity to oligomerize and are prone to accelerated photobleaching. mOrange2 is not as bright as the other orange FPs, but it shows excellent photostability, is strongly monomeric, and may therefore be the best choice, even though it also exhibits accelerated photobleaching. In the red spectral region, mCherry is widely used for many applications, but has been reported to aggregate when expressed within some fusions42, despite its excellent performance as a monomer in the OSER assay. mApple can be used effectively as a brighter substitute for mCherry in most cases. mApple is strongly monomeric and does not show a lot of accelerated photobleaching. However, the mApple emission is blue-shifted from that of mCherry by approximately 18 nm, which increases spectral overlap with the orange FP variants. In the far-red region, mKate2 is currently the brightest and most monomeric FP. However, mCardinal can be a better choice because its red-shifted excitation profile allows it to be used with 635 nm (or Cy5 cube) excitation, which is unique among current autofluorescent FPs. mCardinal is photostable, but it does show accelerated photobleaching and is not strongly monomeric.
Blue, green, and yellow FPs have reached near-maximal performance, as many have very high quantum yields and are strongly monomeric. Some improvements in photostability may still be forthcoming, although many of these FPs are sufficiently photostable for long-term live cell imaging. Looking forward, significant improvements are likely for orange, red, and especially far-red FPs, which are less bright because of their low quantum yields. Because tissue absorption of light and autofluorescence are minimal in the red spectral region, these FPs are particularly important for animal and tissue imaging. Current FP development is focused on the discovery and creation of improved red and far-red FPs.
On-line methods
The methods used in this work all published protocols as described in the main text using previously published fluorescent proteins (FPs)4,6,12,14,15,17,18,36,46–68. Detailed protocols are all presented in Supplementary File 1. These protocols cover the expression and purification of fluorescent proteins, including the use of SDS-Page gels, protein dialysis, and the BCA protein assays needed to verify and quantify DNA and protein levels. The protocols developed to assess purified FPs for their photophysical properties (extinction coefficient, quantum yield, and fluorescence pKa) are also described. FP photobleaching assays also follow published protocols and these are described for embedding the purified FPs polyacrylamide, and measuring their photobleaching in widefield and confocal microscopy. Finally, the protocols for use of the established cytoplasmic end of ER signal anchor membrane protein (CytERM) oligomerization assay are given.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants to D.W.P. (R01DK085064, R01DK098659, S10OD010681, and P20GM072048).
Footnotes
Author contributions
D.W.P., A.U. & M.W.D. designed the research; P.J.C., B.R.S., M.A.B., J.R.A., Z.L., H.M.dG., G.J.K. performed experiments and analyzed data; D.W.P. wrote and edited the paper (with comments from all authors).
Competing Financial Interests
The authors have no competing financial interests to report.
References
- 1.Goldman RD, Swedlow J, Spector DL. Live cell imaging: a laboratory manual. 2nd. Cold Spring Harbor Laboratory Press; 2010. [Google Scholar]
- 2.Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 3.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 4.Matz MV, et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol. 1999;17:969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- 5.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 6.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 7.Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys J. 1997;73:2782–2790. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremers GJ, Gilbert SG, Cranfill PJ, Davidson MW, Piston DW. Fluorescent proteins at a glance. J Cell Sci. 2011;124:157–160. doi: 10.1242/jcs.072744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu X, Shaner NC, Yarbrough CA, Tsien RY, Remington SJ. Novel chromophores and buried charges control color in mFruits. Biochemistry. 2006;45:9639–9647. doi: 10.1021/bi060773l. [DOI] [PubMed] [Google Scholar]
- 10.Ormo M, et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 11.Follenius-Wund A, et al. Fluorescent derivatives of the GFP chromophore give a new insight into the GFP fluorescence process. Biophys J. 2003;85:1839–1850. doi: 10.1016/S0006-3495(03)74612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 13.Cubitt AB, et al. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995;20:448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- 14.Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 15.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 16.Yang TT, et al. Improved fluorescence and dual color detection with enhanced blue and green variants of the green fluorescent protein. J Biol Chem. 1998;273:8212–8216. doi: 10.1074/jbc.273.14.8212. [DOI] [PubMed] [Google Scholar]
- 17.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 18.Shaner NC, et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods. 2008;5:545–551. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremers G-J, Piston DW. Photoconversion of Purified Fluorescent Proteins and Dual-probe Optical Highlighting in Live Cells. J. Vis. Exp. 2010:e1995. doi: 10.3791/1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piston DW, Patterson GH, Knobel SM. Quantitative imaging of the green fluorescent protein (GFP) Methods Cell Biol. 1999;58:31–48. doi: 10.1016/s0091-679x(08)61947-0. [DOI] [PubMed] [Google Scholar]
- 21.Patterson GH, Piston DW. Photobleaching in two-photon excitation microscopy. Biophys J. 2000;78:2159–2162. doi: 10.1016/S0006-3495(00)76762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschfeld T. Quantum efficiency independence of the time integrated emission from a fluorescent molecule. Appl Opt. 1976;15:3135–3139. doi: 10.1364/AO.15.003135. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Hennink EJ, Young IT, Tanke HJ. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. Biophys J. 1995;68:2588–2600. doi: 10.1016/S0006-3495(95)80442-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandison DR, Piston DW, Williams RM, Webb WW. Quantitative comparison of background rejection, signal-to-noise ratio, and resolution in confocal and full-field laser scanning microscopes. Appl Opt. 1995;34:3576–3588. doi: 10.1364/AO.34.003576. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, et al. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat Biotechnol. 2013;31:1032–1038. doi: 10.1038/nbt.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdanov AM, et al. Cell culture medium affects GFP photostability: a solution. Nat Methods. 2009;6:859–860. doi: 10.1038/nmeth1209-859. [DOI] [PubMed] [Google Scholar]
- 27.Dempsey WP, et al. In vivo single-cell labeling by confined primed conversion. Nat Methods. 2015;12:645–648. doi: 10.1038/nmeth.3405. [DOI] [PubMed] [Google Scholar]
- 28.Axelrod D. Chapter 7: Total internal reflection fluorescence microscopy. Methods Cell Biol. 2008;89:169–221. doi: 10.1016/S0091-679X(08)00607-9. [DOI] [PubMed] [Google Scholar]
- 29.Swedlow JR, Sedat JW, Agard DA. Multiple chromosomal populations of topoisomerase II detected in vivo by time-lapse, three-dimensional wide-field microscopy. Cell. 1993;73:97–108. doi: 10.1016/0092-8674(93)90163-k. [DOI] [PubMed] [Google Scholar]
- 30.Gustafsson MG, et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophysical Journal. 2008;94:4957–4970. doi: 10.1529/biophysj.107.120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraoka Y, Sedat JW, Agard DA. Determination of three-dimensional imaging properties of a light microscope system. Partial confocal behavior in epifluorescence microscopy. Biophysical Journal. 1990;57:325–333. doi: 10.1016/S0006-3495(90)82534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlton PM, et al. Fast live simultaneous multiwavelength four-dimensional optical microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16016–16022. doi: 10.1073/pnas.1004037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomer R, Khairy K, Keller PJ. Light sheet microscopy in cell biology. Methods Mol Biol. 2013;931:123–137. doi: 10.1007/978-1-62703-056-4_7. [DOI] [PubMed] [Google Scholar]
- 34.Ward WW. Biochemical and physical properties of green fluorescent protein. Methods Biochem Anal. 2006;47:39–65. [PubMed] [Google Scholar]
- 35.Costantini LM, Fossati M, Francolini M, Snapp EL. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic. 2012;13:643–649. doi: 10.1111/j.1600-0854.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagai T, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 37.Li X, et al. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 38.Terskikh A, et al. "Fluorescent timer": protein that changes color with time. Science. 2000;290:1585–1588. doi: 10.1126/science.290.5496.1585. [DOI] [PubMed] [Google Scholar]
- 39.Valentin G, et al. Photoconversion of YFP into a CFP-like species during acceptor photobleaching FRET experiments. Nat Methods. 2005;2:801. doi: 10.1038/nmeth1105-801. [DOI] [PubMed] [Google Scholar]
- 40.Kremers GJ, Hazelwood KL, Murphy CS, Davidson MW, Piston DW. Photoconversion in orange and red fluorescent proteins. Nat Methods. 2009;6:355–358. doi: 10.1038/nmeth.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SX, et al. Quantification of factors influencing fluorescent protein expression using RMCE to generate an allelic series in the ROSA26 locus in mice. Dis Model Mech. 2011;4:537–547. doi: 10.1242/dmm.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katayama H, Yamamoto A, Mizushima N, Yoshimori T, Miyawaki A. GFP-like proteins stably accumulate in lysosomes. Cell Struct Funct. 2008;33:1–12. doi: 10.1247/csf.07011. [DOI] [PubMed] [Google Scholar]
On-line Methods Only References
- 43.Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry. 2007;46:5904–5910. doi: 10.1021/bi700199g. [DOI] [PubMed] [Google Scholar]
- 44.Subach OM, Cranfill PJ, Davidson MW, Verkhusha VV. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS One. 2011;6:e28674. doi: 10.1371/journal.pone.0028674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goedhart J, et al. Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat Methods. 2010;7:137–139. doi: 10.1038/nmeth.1415. [DOI] [PubMed] [Google Scholar]
- 46.Goedhart J, et al. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93% Nat Commun. 2012;3:751. doi: 10.1038/ncomms1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 48.Markwardt ML, et al. An improved cerulean fluorescent protein with enhanced brightness and reduced reversible photoswitching. PLoS One. 2011;6:e17896. doi: 10.1371/journal.pone.0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ai HW, Henderson JN, Remington SJ, Campbell RE. Directed evolution of a monomeric, bright and photostable version of Clavularia cyan fluorescent protein: structural characterization and applications in fluorescence imaging. Biochem J. 2006;400:531–540. doi: 10.1042/BJ20060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zapata-Hommer O, Griesbeck O. Efficiently folding and circularly permuted variants of the Sapphire mutant of GFP. BMC Biotechnol. 2003;3:5. doi: 10.1186/1472-6750-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cubitt AB, Woollenweber LA, Heim R. Understanding structure-function relationships in the Aequorea victoria green fluorescent protein. Methods Cell Biol. 1999;58:19–30. doi: 10.1016/s0091-679x(08)61946-9. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat Biotechnol. 2005;23:355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- 53.Lam AJ, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9:1005–1012. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaner NC, et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat Methods. 2013;10:407–409. doi: 10.1038/nmeth.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karasawa S, Araki T, Nagai T, Mizuno H, Miyawaki A. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer. Biochem J. 2004;381:307–312. doi: 10.1042/BJ20040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakaue-Sawano A, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 57.Merzlyak EM, et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
- 58.Haas J, Park EC, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996;6:315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- 59.Kredel S, et al. mRuby, a bright monomeric red fluorescent protein for labeling of subcellular structures. PLoS One. 2009;4:e4391. doi: 10.1371/journal.pone.0004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell RE, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shemiakina II, et al. A monomeric red fluorescent protein with low cytotoxicity. Nat Commun. 2012;3:1204. doi: 10.1038/ncomms2208. [DOI] [PubMed] [Google Scholar]
- 62.Shcherbo D, et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem J. 2009;418:567–574. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin MZ, et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem Biol. 2009;16:1169–1179. doi: 10.1016/j.chembiol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu J, et al. Non-invasive intravital imaging of cellular differentiation with a bright red-excitable fluorescent protein. Nat Methods. 2014;11:572–578. doi: 10.1038/nmeth.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Jackson WC, Steinbach PA, Tsien RY. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc Natl Acad Sci U S A. 2004;101:16745–16749. doi: 10.1073/pnas.0407752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.