Abstract
The relationships between age, retrieval-related neural activity, and episodic memory performance were investigated in samples of young (18–29 yrs), middle-aged (43–55 yrs) and older (63–76 yrs) healthy adults. Participants underwent fMRI scanning during an associative recognition test that followed a study task performed on visually presented word pairs. Test items comprised pairs of intact (studied pairs), rearranged (items studied on different trials) and new words. fMRI recollection effects were operationalized as greater activity for studied pairs correctly endorsed as intact than for pairs incorrectly endorsed as rearranged. The reverse contrast was employed to identify retrieval monitoring effects. Robust recollection effects were identified in the core recollection network, comprising the hippocampus, along with parahippocampal and posterior cingulate cortex, left angular gyrus and medial prefrontal cortex. Retrieval monitoring effects were identified in the anterior cingulate and right dorsolateral prefrontal cortex. Neither recollection effects within the core network, nor the monitoring effects differed significantly across the age groups after controlling for individual differences in associative recognition performance. Whole brain analyses did however identify three clusters outside of these regions where recollection effects were greater in the young than in the other age groups. Across-participant regression analyses indicated that the magnitude of hippocampal and medial prefrontal cortex recollection effects, and both of the prefrontal monitoring effects, correlated significantly with memory performance. None of these correlations were moderated by age. The findings suggest that the relationships between memory performance and functional activity in regions consistently implicated in successful recollection and retrieval monitoring are stable across much of the healthy adult lifespan.
Keywords: aging, core recollection network, episodic memory, retrieval, associative recognition, fMRI
1 Introduction
Episodic memory declines over the adult lifespan, even in individuals seemingly free from age-related neurodegenerative disease (Nilsson, 2003; Rönnlund et al., 2005; Old & Naveh-Benjamin, 2008; Koen & Yonelinas, 2014). This observation has motivated numerous studies in which functional neuroimaging was employed to examine the effects of age on the neural correlates of episodic memory (Grady, 2012). As part of this effort, event-related fMRI has frequently been employed to contrast neural activity in healthy young and older adults during either the successful encoding or, the focus of the present paper, the successful retrieval of episodic memories (for reviews see Maillet & Rajah, 2014; Wang & Cabeza, in press).
fMRI studies examining the effects of age on ‘retrieval success effects’ have typically contrasted the neural activity elicited by test items according to the items’ study status and the accuracy of the associated memory judgment. In some of these studies (e.g., Morcom et al., 2007; Duverne et al, 2008; Oedekoven et al., 2015; Angel et al., 2016) the fMRI contrast employed to identify retrieval-related neural activity likely confounded recollection of qualitative information about the study episode with a non-episodic sense of familiarity (Yonelinas, 2002). Recollection and familiarity have distinct neural signatures (Kim, 2010; Johnson et al., 2013) and demonstrate different patterns of age-related decline (Koen & Yonelinas, 2014). Therefore age-related differences in fMRI retrieval-success effects in such studies might reflect the differential mixing of neural activity associated with recollection- and familiarity-based memory judgments, rather than differences specifically in the neural activity supporting recollection of episodic information (Rugg & Morcom, 2005).
Even among studies where this confound is arguably largely absent – for example, where the critical contrast is between correctly recognized test items accorded accurate vs. inaccurate source memory judgments, or items endorsed as ‘remembered’ vs. ‘known’ – the findings are inconsistent, ranging from reports of reductions in retrieval-success effects in older compared with younger individuals, to null findings, through to enhanced effects in older participants (e.g., Duarte et al., 2008; Kukolja et al., 2009; Tsukiura et al., 2010; Dulas & Duarte, 2012; Angel et al., 2013; Cansino et al., 2015; Wang et al., 2016; see Wang & Cabeza, in press, for a review).
Here, we employed fMRI to examine the effects of age on the neural correlates of successful episodic retrieval in a study that, by virtue of the sample size, was substantially more highly powered than its predecessors, and using a memory test widely considered to be heavily dependent on recollection of episodic information. Importantly, although age is a significant source of variance in episodic memory performance, there is substantial variability in performance within groups of similarly aged individuals, and substantial overlap in performance between differently-aged individuals (Nyberg et al., 2012). We took advantage of this variability to assess whether age effects on the neural correlates of episodic retrieval or post-retrieval monitoring can be identified after variance due to individual differences in memory performance is partialled out. The logic of this approach (see also Oedekoven et al., 2015) is similar to that employed in prior studies where performance on a memory test was equated between older and young samples either by experimental manipulation (Morcom et al., 2007; Duverne et al., 2008; Wang et al., 2009; Angel et al., 2016), or by stratifying participants into high- and low-performing sub-groups (e.g., Duarte et al., 2008). If age effects are evident when performance is equated or controlled for, this would suggest that the neural correlates of retrieval-related activity in older individuals differ from those in young adults, potentially providing insight into the causes and moderators of age-related memory decline (Rugg, in press). By contrast, the absence of age effects when performance is experimentally or statistically equated suggests that the patterns of neural activity associated with successful retrieval in older individuals do not differ from those in young individuals performing at the same level. Such a null finding does not, of course, license the conclusion that a given neural region supporting memory retrieval is unaffected by increasing age. Rather, it suggests that increasing age does not modify the relationship between the level of neural activity in the region and memory performance. We return to this issue in the Discussion.
Relatedly, we also examined whether relationships between individual differences in retrieval-related neural activity and memory performance differed across age groups. It has been proposed (e.g., Cabeza et al., 2002; Reuter-Lorenz & Park, 2014), for example, that performance in older adults benefits from age-related neural reorganization (neural ‘compensation’ or ‘scaffolding’). In addition, it is possible that, with increasing age, performance becomes increasingly sensitive to individual differences in the functional integrity of regions vulnerable to aging (de Chastelaine et al., 2011, 2016). In either case, one might anticipate identifying relationships between neural activity and memory performance that are unique to, or stronger in older than in young individuals. We have recently reported such findings from the encoding phase of the present study (de Chastelaine et al., 2015, 2016). To anticipate the present results, in contrast to those findings, here we find no evidence that relationships between individual differences in retrieval-related activity and memory performance are modified by age.
In the present study, we contrasted neural activity elicited during successful and unsuccessful retrieval of associative information. We elected to investigate associative memory because it is strongly dependent on episodic recollection (e.g., Mickes et al., 2010) and thus is a relatively ‘process-pure’ memory test. In addition, associative memory is highly sensitive to age (Old & Naveh-Benjamin, 2008), and the neural correlates of the encoding of associative information are already known to differ according to age (de Chastelaine et al., 2011, 2015, 2016; Miller et al., 2008). Three prior fMRI studies examining the effects of age on the neural correlates of episodic retrieval also employed an associative memory procedure (Oedekoven et al., 2013; Dulas & Duarte, 2016; Wang & Giovanello, 2016). In the study of Oedekoven et al. (2013), the contrast did not allow for an examination of retrieval success effects but, rather, assessed the activity elicited by test items against baseline. In the studies of Dulas & Duarte, 2016 and Wang & Giovanello, 2016 where, like here, neural activity for successful versus unsuccessful retrieval of associative memories was contrasted, age-invariant retrieval effects were identified in the hippocampus. In Wang & Giovanello, 2016 these effects were accompanied by an additional effect in older particpants in a small region of the left posterior hippocampus. In Dulas & Duarte, 2016, enhanced retrieval-related activity was evident in younger participants in several prefrontal regions, but no age effects were reported in cortical regions belonging to the core recollection network (see below).
The present study extends prior research in a second important way. In addition to samples of young and older individuals, we also employed a sample of middle-aged individuals. This age-range has been almost completely neglected in studies examining the neural correlates of episodic memory retrieval (we are aware of only one prior report (Cansino et al., 2015) in which an event-related design was employed to identifiy retrieval-related activity in middle-aged participants (although see Grady et al., 2006, and Kwon et al., 2016, for reports of blocked-design studies that included a middle-aged sample)). The inclusion of a group of middle-aged individuals allows for a more continuous sampling of retrieval effects across the lifespan, and hence a more precise assessment of the profiles of any age-related differences in the effects.
A major focus of the present study was a priori analyses directed at two different components of retrieval processing. The first component comprises processes reflected in greater neural activity elicited by retrieval cues associated with successful rather than unsuccessful recollection (operationalized here as associative hits and misses). Recollection-related enhancement of activity (‘recollection effects’) is consistently observed in what has been termed the ‘core recollection network’, which comprises the hippocampus, parahippocampal cortex, medial prefrontal cortex (mPFC), left angular gyrus, posterior cingulate, and left middle temporal gyrus (Kim, 2010; Rugg & Vilberg, 2013; King et al., 2015). This network – which overlaps substantially with the well-studied ‘default-mode network’ (Buckner et al., 2008) – is held to play a key role in initiating successful retrieval, and in integrating the contents of recollection into a cohesive memory representation. Several of the regions comprising the network have previously been reported to demonstrate age-related reduction in recollection-related activity (e.g., Daselaar et al., 2006; Kukolja et al., 2009; Tsukiura et al., 2010; Angel et al., 2013; Cansino et al., 2015).
The second component of retrieval processing examined here is ‘retrieval monitoring’. This refers to control processes responsible for evaluating the outcome of a retrieval attempt in relation to behavioral goals (Burgess & Shallice, 1996; Rugg, 2004). The neural correlates of monitoring are identified by contrasting retrieval cues eliciting weak versus strong memory signals (e.g., Henson et al., 1999, 2000; Achim & Lepage, 2005; Wang et al., 2016). These prior studies have consistently implicated right dorsolateral prefrontal cortex (rDLPFC) and anterior cingulate cortex (ACC) in monitoring.
A relatively small number of fMRI studies have investigated the effects of age on the neural correlates of monitoring and, echoing findings from studies of recollection success, have yielded inconsistent results (see, for example, Duarte et al., 2010; Giovanello et al., 2010; Dulas & Duarte, 2014; Wang et al., 2016, for reports of null effects of age, and McDonough et al., 2013, and Mitchell et al., 2013, for reports of age-related impairment in monitoring-related activity in rDLPFC). Here, we examined whether fMRI ‘monitoring effects’ are sensitive not only to age, but also to individual differences in memory performance (cf. Wang et al., 2016).
2 Materials and Methods
Data from the encoding phase of this experiment were reported in two prior publications (de Chastelaine et al., 2015, 2016), where additional description of the experimental procedures and methods can be found.
2.1 Participants
Thirty six young (18–29 yrs; M = 22 yrs; SD = 3.0 yrs; 17 female), 36 middle-aged (43–55 yrs; M = 49 yrs; SD = 3.4 yrs; 17 female) and 64 older (63–76 yrs; M = 68 yrs; SD = 3.6 yrs; 35 female) adults participated in the experiment. Participants were recruited from the University of Texas at Dallas and surrounding communities. They gave informed consent in accordance with the UT Dallas and University of Texas Southwestern Institutional Review Boards, and were compensated at the rate of $30 per hour. All participants were right-handed, and were fluent in English before five years of age. No participant had a history of neurological or psychiatric disease, substance abuse, diabetes, or untreated hypertension. None were taking prescription medication that affected the central nervous system.
Twenty members of the present older group, and three members of the young group, also participated in the study of Wang et al., 2016, which employed a quite different experimental design. Data from the young group comprised one of the three data sets (Experiment 2) analyzed in King et al. (2015). However, none of the data from the middle-aged or older age groups, or the outcomes of contrasts between these data and those of the young group, have been reported previously.
2.2 Neuropsychological testing
A standardized neuropsychological test battery was completed by all participants on a separate day prior to the experimental MRI session. The tests assessed a range of cognitive functions known to either decline or be maintained with age. The Mini-Mental State Examination (MMSE) was employed to screen participants for dementia, adopting a nominal cutoff score of 27/30. Other tests included the California Verbal Learning Test-II (CVLT; Delis et al. 2000), the Wechsler Memory Scale (WMS-IV), the Digit/Symbol Coding test of the WAIS-R, Trail Making Tests A and B, letter and category fluency tests, the Digit Span Forward and Backward test of the Wechsler Adult Intelligence Scale Revised (WAIS-R) (Wechsler, 2001), the Wechsler Test of Adult Reading (WTAR; Wechsler 2001) and the Raven’s Progressive Matrices (short version).
Potential participants were excluded if they scored >1.5 standard deviations (SDs) below their age-appropriate norm on either of the long-term memory tests (CVLT or the WMS), scored > 1.5 SDs below the age-appropriate norm on any two of the other neuropsychological tests, or had an estimated full-scale IQ < 100 as indexed by performance on the WTAR. For the analyses of the neuropsychological test data (see Section 3 ‘Results’, Section 3.1 ‘Neuropsychological data’), a composite CVLT recall score was calculated by averaging across the 4 recall tests (immediate and delayed free and cued recall). Similarly, a composite WMS score was computed by averaging scores on the WMS 1 and WMS 2.
2.3 Experimental materials
Experimental items comprised 320 semantically unrelated, visually-presented word pairs, which were randomly divided into four lists of 80 pairs. Across each set of yoked participants (one young, one middle-aged and one or two older participants) lists were rotated so that each list provided the items for each of the critical word-pair categories, namely intact, rearranged, and new pairs (see below). For each set of yoked participants, word pairs from three of the lists were pseudo-randomly ordered to form the study list. The test list included 320 critical word pairs: 160 of these had been presented at study (intact pairs), 80 were studied items that had been re-paired from study (rearranged pairs), and 80 were unstudied pairs (new pairs). For the rearranged test pairs, words were always presented in the same position (i.e., above or below fixation) as they had been at study. Critical test pairs were intermixed with 106 null trials. Two buffer pairs were placed at the start and two in the middle of each of the two study blocks and the three test blocks (see below). The different categories of word pairs in the study and test lists were pseudo-randomly ordered such that the same category did not occur more than three times in succession. A 30s break occurred halfway through each study and test block, while inter-block intervals were approximately 2 mins in duration. Practice study and test lists were formed from items additional to those used to create the experimental lists. In both the study and test phases, the word pairs were presented for 2 s. Inter-item intervals were 1.5 s and 2.5 s at study and test respectively.
2.4 Procedure
Participants were given instructions and practice sessions for both the study task and the memory test prior to scanning. Therefore they were aware that their memory for the study items would be tested. The study and test phases of the experiment took place in separate scanning sessions. The study requirement was to indicate with a button press which of the two objects denoted by the words in each pair was more likely to fit into the other. Study pairs were presented in two consecutive blocks separated by a short rest interval. After completing the study task, participants exited the scanner and rested. Participants re-entered the scanner 15 min later to complete an associative memory test that was presented in three consecutive blocks separated by short rest intervals. The memory test required one of three key press responses to indicate whether each test pair was intact, rearranged or new. An ‘intact’ response was required when participants recognized both words and had a specific memory of the two words having been presented together at study. A ‘rearranged’ response was required when both words were recognized from the study phase but there was no specific memory of the words having been paired together previously. Participants were instructed to make a ‘new’ response when neither word, or only one word, was recognized. They were further instructed to respond ‘intact’ only when they were confident that the words had been studied together. The test phase concluded with the acquisition of diffusion tensor (DTI) and anatomic scans. Study and test instructions emphasized the need for both accuracy and speed.
2.5 MRI data acquisition
Functional and anatomical images were acquired with a Philips Achieva 3T MR scanner (Philips Medical System, Andover, MA USA) equipped with a 32 channel parallel imaging head coil. A T1-weighted anatomical image was acquired with a 3D MP-RAGE pulse sequence (FOV= 256×224, voxel size 1×1×1 mm, 160 slices, sagittal acquisition). Functional scans were acquired with a T2*–weighted EPI with the following parameters: TR 2 s, TE 30 ms, flip angle 70°, field-of-view 240×240, matrix size 80×78). Each EPI volume comprised 33 slices (3 mm thickness, 1 mm inter-slice gap) with an in-plane resolution of 3×3 mm. Slices were acquired in ascending order, oriented parallel to the AC-PC line and positioned for full coverage of the cerebrum and most of the cerebellum. The functional data were acquired using a sensitivity encoding (SENSE) reduction factor of 2. fMRI data were acquired during both study and test phases. The first five volumes of each block were discarded to allow tissue magnetization to achieve a steady state. Test sessions were concatenated to form a single time-series prior to model estimation.
2.6 MRI data analysis
Functional and anatomical images were preprocessed and analyzed with SPM (SPM8, Wellcome Department of Cognitive Neurology, London, UK), run under Matlab R2008a (MathWorks). Functional images were motion and slice-time corrected, realigned, and spatially normalized using a sample-specific template (see de Chastelaline et al., 2015, for further details) based on the MNI reference brain (Cocosco et al. 1997). Images were resampled into 3 mm isotropic voxels and smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel. T1-weighted anatomical images were normalized with a procedure analogous to that applied to the functional images. For each participant, item-elicited neural activity was modeled using a delta function convolved with two haemodynamic response functions (HRFs). These functions comprised a canonical (Friston et al., 1998) and an orthogonalized (Andrade et al., 1999) HRF delayed by one TR from stimulus onset (2 s). The results obtained with the late HRF added little of theoretical significance to the findings obtained with the canonical function, and are not reported here.
The fMRI data were analyzed in two stages. In the first stage, separate GLMs were constructed for each participant. There were two events of interest: correctly endorsed intact pairs (associative hits) and intact pairs incorrectly identified as rearranged (associative misses). The median number (and range) of trials comprising associative hits and associative misses were, respectively, 101 (42–144) and 35 (12–85) for the young adults, 102 (30–134) and 41 (14–78) for the middle-aged adults, and 97 (25–142) and 38 (11–97) for the older adults. Items correctly judged as rearranged (rearranged hits), new pairs correctly judged new (correct rejections), and intact pairs wrongly judged as new were also each separately modeled. All other events, including incorrect responses to rearranged items, false alarms to new pairs, fillers, and trials where a response was not given, were modeled as events of no interest. Three 30s breaks interposed during the test list were also modeled, along with six regressors representing motion-related variance (three for rigid-body translation and three for rotation), and constants representing means across each scan session. Data from volumes showing a transient displacement > 1mm or > 1 degree in any direction were eliminated by their inclusion as covariates of no interest when estimating item-related effects. The time series in each voxel were high-pass filtered to 1/128 Hz to remove low-frequency noise and scaled within session to a grand mean of 100 across voxels and scans.
In the second stage of the analysis, the participant-specic parameter estimates for four events (associative hits, associative misses, rearranged hits and correct rejections) were taken forward to a 3 (age group) × 4 (item type) mixed-design ANOVA model as implemented within SPM8 (and hence employing a single pooled error term). Recollection effects were operationalized as greater BOLD activity for associative hits (studied pairs correctly endorsed as ‘intact’) than for associative misses (studied pairs wrongly judged to be ‘rearranged’). This contrast (also used by Dulas & Duarte, 2016, and Wang & Giovanello, 2016) is assumed to isolate processes associated with successful versus unsuccessful recollection for inter-item associations while holding the familiarity of the individual test items approximately constant (assuming participants conformed to the test instructions, word pairs where the familiarity strength of one or both items was insufficient to support an ‘old’ judgment would have been endorsed ‘new’ rather than ‘rearranged’). The opposite contrast was employed to operationalize retrieval monitoring (associative misses > associative hits). The rationale for this contrast is that monitoring demands covary with the ambiguity of the memory signal elicited by a test item; hence, the weak (and, ultimately, ineffectual) recollection signal elicited by associative misses would be expected to place more demands on monitoring than the stronger recollection signal supporting accurate judgments (see Henson et al., 1999, 2000, for early formulations of this argument; as noted above, we assume however that the word pairs attracting associative misses and associative hits were roughly equivalent in their familiarity). It might be argued that a more appropriate trial type for this contrast would be rearranged hits (rather than associative misses), where a memory signal must be monitored and discounted as being diagnostic of an inter-item association. However, identification of rearranged pairs can be supported by a ‘recall-to-reject’ strategy (Rotello & Heit, 2000; Lepage et al., 2003), and use of this strategy has been reported to be more prevalent in young than in older participants (Cohn et al., 2008). Thus, monitoring contrasts involving rearranged hits might be confounded with age-related differences in the nature of the monitored information.
To identify effects of age outside of the regions of interest (ROIs) described below, we employed an exploratory whole-brain analysis to search for clusters demonstrating age group × item type (associative hits versus associative misses) interaction effects. This analysis was conducted using a height threshold of p < 0.001 (two-sided) and a cluster extent threshold of 21 voxels. The cluster extent threshold was determined by a Monte Carlo simulation implemented in AlphaSim (http://afni.nimh.nih.gov/afni) to give a corrected cluster-wise significance level of p < 0.05. For each cluster identified by this contrast, we extracted mean parameter estimates for the BOLD responses elicited by associative hits and misses across all voxels within a 5 mm radius of the cluster peak in each participant. These data were analyzed with 3 (group) × 2 (item type) ANCOVA models in order to elucidate the interactions and to assess whether they remained after controlling for individual differences in recollection performance.
The second part of the fMRI analyses was aimed at elucidating the relationships between age, recollection performance and fMRI effects associated with recollection and retrieval monitoring within specific ROIs. To avoid possible bias caused by using the same data set to identify and then to contrast ROI measures (Kriegeskorte et al., 2009), we employed independent data sets to define ROIs within the core recollection and retrieval monitoring networks. The data sets were from two previously published experiments examining neural correlates of recollection (employing source memory and Remember/Know test procedures respectively; Elward et al., 2015, and Wang et al., 2016, reported in King et al., 2015, as experiments 1 and 3) and were restricted to young participants1. To localize the peaks of the recollection effects, we took the center of mass of the peaks of the across-experiment conjunction of the effects in each region. This procedure gave the following MNI coordinates: parahippocampal cortex (−21, −37, −17), angular gyrus (−51, −70, 37), middle temporal gyrus (−57, −55, 16), mPFC (−3, 56, 13), posterior cingulate (−6, −46, 37) and bilateral hippocampus (−24, −13, −20 and 27, −16, −10). In the case of the retrieval monitoring effects, we identified the relevant peak from the monitoring contrast of Wang et al., 2016, which was operationalized as greater activity for ‘Know’ than for ‘Remember’ responses to correctly recognized items. Monitoring effects were not previously defined for Elward et al. (2015). In light of prior evidence that source memory judgments place greater demands on monitoring than does the simple rejection of items as unstudied (Rugg et al., 2003), we identified ACC and rDLPFC peaks from the contrast between all items judged as studied (regardless of the accuracy of the ensuing source judgment) versus correctly rejected new items. The center of mass of the respective contrasts across the two studies was 3, 29, 37 for the ACC, and 48, 32, 28 for the rDLPFC.
We extracted parameter estimates for the BOLD responses elicited by associative hits and misses across all voxels within either a 3 mm (hippocampus, parahippocampal cortex) or 5 mm (other regions) radius of the 7 aforementioned peaks within the core recollection network. Mean parameter estimates were also extracted from voxels within a 5 mm radius of the peaks within the rDLPFC and the ACC. The parameter estimates were subjected to 3 (group) × 2 (item type) ANOVAs to assess whether there were group differences in the magnitude of recollection or monitoring effects. For regions showing significant group effects, follow-up ANCOVAs were performed to establish whether these differences remained after controlling for recollection performance.
Using the same parameter estimates, we also conducted multiple regression analyses with associative recognition performance as the dependent variable, and age group, the relevant fMRI effect, and the age group × fMRI effect interaction term as predictor variables. For the models involving the monitoring effects, we added as a predictor variable each participant’s difference in RT between associative hits and associative misses, given evidence that the PFC (particularly the ACC) is implicated in factors that likely co-vary with RT, such as response selection (Rushworth et al., 2007) or time on task (Grinband et al., 2011). For the regression analyses involving monitoring effects, we excluded one older participant who had an outlying effect. Regression analysis of either the recollection or the monitoring data failed to identify any significant age group × fMRI interaction effects (all ps > 0.08). Thus, we report below the outcome of these analyses after dropping the interaction term from the models.
Nonsphericity between levels of repeated measures factors in the ANOVAs and ANCOVAs reported below was corrected using the Greenhouse-Geisser procedure (Greenhouse & Geisser, 1959). The significance levels of t-tests and regression analyses were corrected for multiple comparisons with the Holm-Bonferroni procedure to give a family-wise error rate of p < 0.05 (Holm, 1979). Unless noted otherwise, all results reported as significant remained so after correction. Effect sizes were indexed by eta squared (Cohen, 1988).
3 Results
3.1 Neuropsychological data
The neuropsychological test results are summarized in Table 1. In brief, the older participants showed the typical pattern of age-related reduction and preservation of scores on the neuropsychological test battery (Salthouse, 2010). The three age groups performed equally well on tests typically preserved with age (Digit Span, Letter and Category Fluency, and the WTAR, a measure of crystallized intelligence), while long term memory for word lists (assessed by composite recall on the California Verbal Learning Test) was significantly lower in the older, but not the middle-aged group, compared to the younger participants. Older participants also demonstrated poorer performance on tests of speeded cognition (Trail making and Digit Symbol substitution) relative to both the younger and middle-aged groups, and the middle-aged participants performed more poorly than the young participants on the Digit Symbol substitution test. Performance for both older and middle-aged participants was lower than that for younger participants on a test of fluid intelligence (Ravens Progressive Matrices).
Table 1.
Demographic and neuropsychological data (mean, SD, and range) for young, middle-aged, and older adults.
| Young adults | Middle-aged adults | Older adults | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Age | 22.2 | 3.0 | 18–29 | 49.4 | 3.4 | 43–55 | 68.4 | 3.6 | 63–76 |
| Years of Educationa† | 15.5 | 2.4 | 11–22 | 16.3 | 2.6 | 10–22 | 17.1 | 2.3 | 12–22 |
| Mini Mental State Exam | 29.6 | 0.6 | 28–30 | 29.3 | 0.8 | 28–30 | 29.3 | 0.8 | 27–30 |
| CVLT1 Composite Recalla† | 13.3 | 1.9 | 9.3–16 | 12.4 | 2.0 | 9–16 | 12.0 | 2.5 | 5.5–16 |
| CVLT1 Recognitiona‡, b* | 15.6 | 0.6 | 14–16 | 15.2 | 0.9 | 13–16 | 14.8 | 1.3 | 12–16 |
| CVLT1 False Positivesa†, b* | 0.9 | 1.0 | 0–4 | 1.9 | 2.2 | 0–8 | 1.9 | 2.2 | 0–10 |
| WMS2 Composite Score | 29.0 | 6.6 | 15.5–41 | 27.1 | 6.2 | 14–44.5 | 27.5 | 5.3 | 18.5–39.5 |
| Forward/Backward Digit Span | 18.0 | 3.9 | 11–26 | 18.2 | 3.5 | 13–26 | 18.3 | 4.4 | 12–27 |
| Digit Symbol Substitutiona‡, b†, c† | 61.4 | 10.1 | 39–83 | 55.2 | 7.8 | 41–70 | 49.7 | 8.5 | 31–74 |
| Trail Aa‡, c‡ | 20.9 | 7.3 | 11–47 | 24.1 | 6.7 | 14–39 | 32.5 | 11.4 | 15–88 |
| Trail Ba‡, c‡ | 46.7 | 17.3 | 23–108 | 51.7 | 16.9 | 27–95 | 74.8 | 46.7 | 31–360 |
| Letter Fluency | 43.6 | 11.8 | 23–65 | 47.5 | 12.0 | 28–69 | 45.3 | 12.6 | 21–81 |
| Category Fluency | 24.8 | 5.9 | 16–42 | 24.0 | 5.9 | 13–36 | 22.3 | 5.5 | 12–40 |
| WTAR3 Raw Score | 112.3 | 5.0 | 102–119 | 111.1 | 5.1 | 101–118 | 112.5 | 5.5 | 101–119 |
| Raven’s Prog. Matrices4a‡, b** | 11.2 | 1.0 | 8–12 | 10.4 | 1.5 | 7–12 | 9.7 | 2.0 | 3–12 |
California Verbal Learning Test.
Wechsler Memory Scale (WMS-IV).
Wechsler Test of Adult Reading Full Scale Intellectual Quotient.
Short version of Raven’s Progressive Matrices.
Note: Statistically significant difference between a) young and older adults, b) young and middle-aged adults, c) middle-aged and older adults.
p < 0.05,
p < 0.01,
p < 0.005,
p < 0.001, 2-tailed t-tests.
3.2 Behavioral results
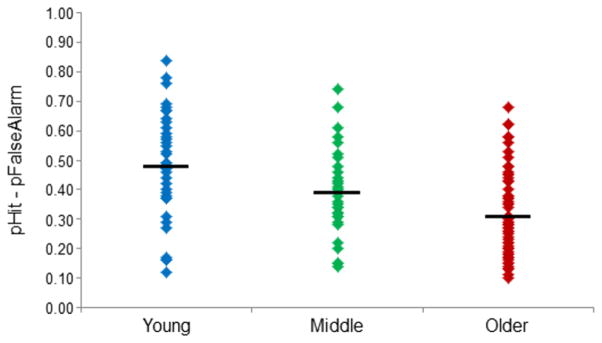
As in our prior reports (de Chastelaine et al., 2011, 2015, 2016; see also King et al., 2015), recollection accuracy was indexed as the difference between the proportion of intact test pairs correctly endorsed as intact (associative hits) and the proportion of rearranged test pairs incorrectly judged intact (associative false alarms). Table 2 provides a summary of associative recognition performance for each age group. Accuracy declined in a graded manner with age (F2, 135 = 12.80, p < 0.001), with means (SDs) of 0.48 (0.19), 0.39 (0.14) and 0.31 (0.15) for the young, middle-aged and older groups, respectively. Follow-up pair-wise contrasts (t-tests, equal variances not assumed) indicated that young participants were more accurate than both middle-aged (t64 = 2.25, p < 0.05) and older participants (t59 = 4.50, p < 0.001), and that middle-aged participants were more accurate than the older participants (t76 = 2.60, p = 0.01). The distribution of scores for each group are illustrated in Figure 1 where it can be seen that, despite the age-related differences in mean recollecton performance, there was considerable overlap between the groups and substantial within-group variability.
Table 2.
Mean proportions (±SD) of intact, rearranged, and new test pairs given intact, rearranged, and new responses in each age group. Correct responses are highlighted in bold.
| Young | Middle-aged | Older | |
|---|---|---|---|
| Intact responses | |||
| Intact pairs | 0.63 (0.17) | 0.63 (0.14) | 0.56 (0.16) |
| Rearranged pairs | 0.15 (0.11) | 0.24 (0.12) | 0.25 (0.13) |
| New pairs | 0.03 (0.06) | 0.06 (0.07) | 0.07 (0.07) |
| Rearranged responses | |||
| Intact pairs | 0.26 (0.12) | 0.27 (0.11) | 0.29 (0.13) |
| Rearranged pairs | 0.64 (0.16) | 0.55 (0.15) | 0.49 (0.16) |
| New pairs | 0.29 (0.15) | 0.30 (0.16) | 0.30 (0.16) |
| New responses | |||
| Intact pairs | 0.11 (0.09) | 0.10 (0.09) | 0.14 (0.09) |
| Rearranged pairs | 0.20 (0.11) | 0.21 (0.13) | 0.26 (0.12) |
| New pairs | 0.68 (0.17) | 0.64 (0.19) | 0.63 (0.18) |
Figure 1.
Plot of the recollection performance of each member of the young, middle-aged and older groups. Mean recollection performance for each age group is denoted by the black horizontal line. [1.5 columns]
Test RTs for associative hits and associative misses are summarized in Table 3. These data were subjected to a 3 (age group) × 2 (item type) mixed design ANOVA which revealed a main effect of item type (F1,133 = 333.69, p < 0.001), indicating faster responses to associative hits than to associative misses. There were no effects of age group, nor was there an age group by item type interaction (Fs > 1.3).
Table 3.
Mean test RTs (±SD) for associative hits and misses in young, middle-aged, and older adults.
| Young | Middle | Older | |
|---|---|---|---|
| Associative hits | 1855 (388) | 1778 (303) | 1912 (303) |
| Associative misses | 2275 (468) | 2161 (339) | 2254 (387) |
3.3 fMRI data
3.3.1 Exploratory whole brain analyses
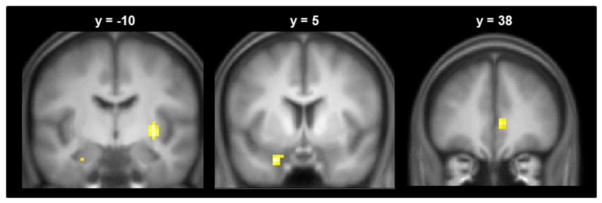
To search at the whole-brain level for clusters demonstrating age-related differences in the fMRI effects, we performed an age group × item type (associative hits versus associative misses) interaction contrast. As is evident in Figure 2, the contrast identified three clusters, with peaks in the putamen (33, −10, −2; Z = 4.30, 52 voxels), right rostral ACC (6, 38, 4; Z = 3.78; 22 voxels) and the left anterior medial temporal lobe (MTL) in the vicinity of the amygdala (−24, 5, −26; Z = 4.14, 44 voxels). ANCOVAs performed on the parameter estimates extracted from each region (see Materials and Methods, Section 2.6 ‘MRI data analysis’) revealed that, in each case, the group × item type interactions remained after controlling for the effects of recollection performance (all ps < 0.005). Follow-up pairwise between-group comparisons for each region were conducted with 2 (age group) × 2 (item type) ANCOVAs, controlling for performance. The effects, which in each case took the form of greater BOLD activity for associative hits relative to misses, were larger in the young group compared to the middle-aged group in the anterior MTL and ACC (ps < 0.005), and larger in the young group compared to the older group in the putamen and ACC (ps < 0.01). There were no significant differences in the magnitude of the effects between middle aged and older groups. Hence, the interactions illustrated in Figure 2 reflected a tendency for recollection effects to be greater in the young group than in the other two groups.
Figure 2.
Clusters demonstrating a group × item type interaction. Effects displayed on coronal sections of the across-groups mean T1-weighted structural image. [1.5 columns]
Using the same parameter estimates, we conducted three multiple regression analyses with associative recognition performance as the dependent variable, and age group, the recollection effect for each of the clusters, and the age group × recollection effect interaction term as predictor variables. These analyses each failed to identify significant age group × recollection effect interactions (all ps > 0.07), and therefore the interaction term was dropped from each of the models. The across-group regression analyses revealed a correlation (after controlling for age group) between fMRI recollection effects and recollection performance in the putamen (r = 0.17, p < 0.05). However, this result did not survive Holm-Bonferroni correction.
3.3.2 Core recollection network – relationships with age group and recollection performance
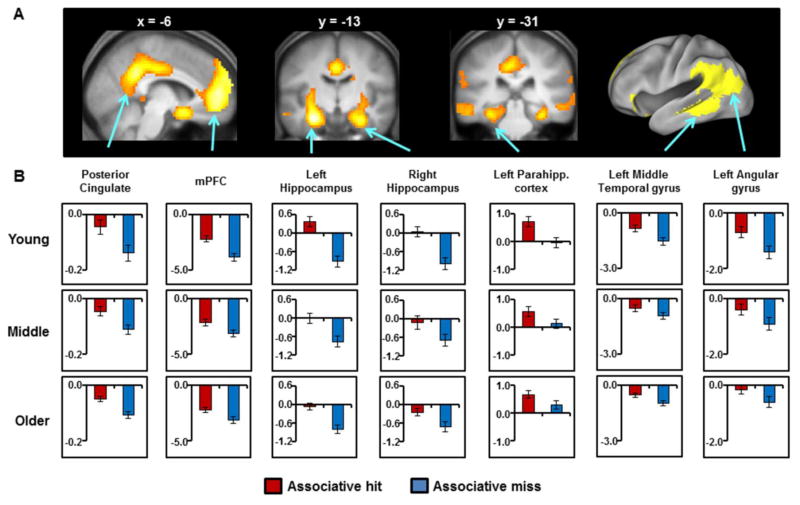
The across-group main effect of recollection (associative hits > associative misses) identified three clusters of effects, one of which was extremely large. To more easily identify individual clusters within the core recollection network, we raised the threshold for the recollection contrast to p < 0.001 with FWE correction. Figure 3A depicts the outcome of this contrast, where it can be seen that recollection effects were evident in all regions of the network. The MNI coordinates corresponding to the peaks of the recollection effects within the network are listed in Table 4.
Figure 3.
(A) Clusters demonstrating the across-group main effect of recollection. Effects are displayed on sagittal and coronal sections of the across-groups mean T1-weighted structural image and the left lateral surface of a standardized brain (PALS-B12) atlas using Caret 5; (B) Mean parameter estimates (arbitrary units) for the three age groups for associative hits and misses extracted from each core recollection ROI. [double column]
Table 4.
Core recollection network regions demonstrating across-participant main effects of recollection.
| Coordinates
|
Region | ||
|---|---|---|---|
| x | y | z | |
| −6 | 53 | 7 | Left mPFC |
| −27 | −13 | −23 | Left hippocampus |
| −30 | −37 | −14 | Left parahippocampal cortex |
| −6 | −46 | 34 | Left posterior cingulate cortex |
| −54 | −64 | 16 | Left middle temporal gyrus |
| 24 | −7 | −23 | Right hippocampus |
| 33 | −31 | −17 | Right parahippocampal cortex |
ROI analyses used data extracted from spheres centered on peak voxels identified in independent data sets (see Materials and Methods, Section 2.6 ‘MRI data analysis’). Mean parameter estimates for associative hits and misses extracted from the core recollection ROIs were subjected to a 3 (age group) × 2 (item type) ANOVA for each of the 7 regions (see Materials and Methods, Section 2.6 ‘MRI data analysis’, for coordinates). Main effects of item type were evident for all ROIs (all ps < 0.001), indicating robust recollection effects for each ROI across the 3 age groups. A main effect of age group was also evident for the angular gyrus (F1,133 = 3.25, p < 0.05). Follow-up pairwise comparisons indicated that item-related activity (i.e., collapsed across associative hits and misses) in this region was greater in the older group relative to the young group (F1,98 = 6.42, p < 0.025). There were no significant differences in item-related activity between the middle-aged and either of the other groups (ps > 0.1). Age group × item type interactions were evident in mPFC, left and right hippocampus, and the left posterior cingulate cortex (see Table 5). In each case, although recollection effects were robust in each group (all ps < 0.005), the interactions reflected smaller recollection effects in the middle-aged and older groups than in the younger participants (although this was only a trend for recollection effects in the posterior cingulate when comparing young and middle-aged participants). No differences were evident between the middle-aged and older participants. Importantly, none of these interactions were significant after the effects of associative recognition performance were partialled out (see Table 5). As can be seen from the table, in all but the posterior cingulate (where the significance and effect size for the age group × item type interactions were already weak in the original ANOVA), controlling for the effects of performance led not only to a non-significant interaction effect, but also to an approximate halving of its effect size.
Table 5.
Statistical significance and effect size (η2) of the age group × item type interaction term for the ANOVAs and ANCOVAs (recollection performance as covariate) of the parameter estimates from each of the core recollection ROIs. ROIs where statistical significance differed across the analyses are highlighted.
| Region | ANOVA | ANCOVA | ||
|---|---|---|---|---|
|
| ||||
| Significance | η2 | Significance | η2 | |
| Left mPFC | p = 0.038 | 0.03 | p = 0.399 | 0.01 |
| Left hippocampus | p = 0.009 | 0.04 | p = 0.074 | 0.02 |
| Left parahippocampal cortex | p = 0.056 | 0.03 | p = 0.140 | 0.02 |
| Left posterior cingulate cortex | p = 0.050 | 0.02 | p = 0.064 | 0.02 |
| Left angular gyrus | p = 0.350 | 0.01 | p = 0.475 | 0.01 |
| Left middle temporal gyrus | p = 0.088 | 0.02 | p = 0.280 | 0.01 |
| Right hippocampus | p = 0.011 | 0.04 | p = 0.249 | 0.01 |
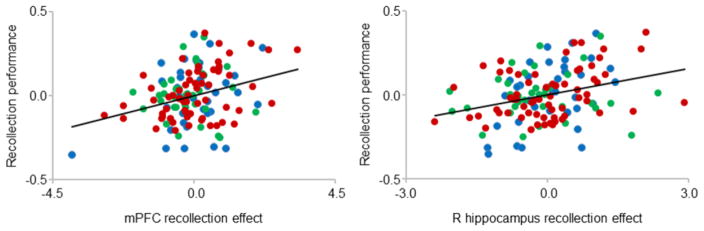
The across-group regression analyses revealed positive correlations (controlling for age group) between fMRI recollection effects and recollection performance in mPFC (r = 0.31, p < 0.001) and right hippocampus (r = 0.30, p < 0.001). Figure 4 depicts the partial plots for these regions. The correlation between recollection performance and mPFC effects remained significant after removing the outlier evident at the bottom left of the relevant plot (r = 0.26, p < 0.005).
Figure 4.
Partial plots of the relationships between recollection performance and recollection effects in the mPFC (left) and the right hippocampus (right). Data points correspond to young (blue), middle-aged (green) and older (red) participants. [1.5 columns]
3.3.3 Retrieval monitoring - relationships with age group and recollection performance
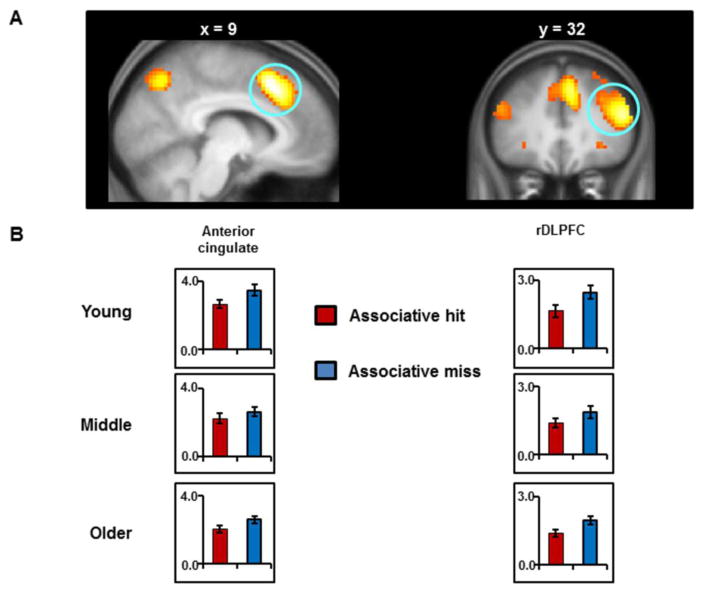
As predicted, the ‘monitoring contrast’ (associative misses > associative hits) identified effects in the rDLPFC (close to where such effects have been identified previously; e.g., Henson et al., 1999; Hayama & Rugg, 2009; Wang et al., 2016) and the ACC, as well as in several other regions (see Figure 5A and Table 6 for more details).
Figure 5.
(A) Clusters demonstrating the across-group main effect of retrieval monitoring. Effects are displayed on sagittal and coronal sections of the across-groups mean T1-weighted structural image; (B) Mean of the parameter estimates (arbitrary units) for associative hits and misses extracted from each monitoring ROI for each age group. [1.5 columns]
Table 6.
Peak voxels of the across-group main effect of the monitoring contrast. The two regions adjacent to those selected for ROI analyses are highlighted.
| Coordinates
|
Peak Z | No. of above-threshold voxels | Region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| −48 | 29 | 25 | 4.69 | 93 | Left middle frontal gyrus |
| −30 | 26 | −2 | 5.31 | 64 | Left anterior insula |
| −42 | 5 | 31 | 3.92 | 26 | Left middle frontal sulcus |
| −27 | −1 | 55 | 3.94 | 48 | Left superior frontal sulcus |
| 30 | 53 | 4 | 5.66 | 121 | Right anterior PFC |
| 9 | 23 | 43 | Infinite | 1732 | Right anterior cingulate |
| 51 | 32 | 22 | 7.68 | sub-peak | Right middle frontal gyrus |
| 36 | 20 | 1 | 6.07 | 143 | Right anterior insula |
| 36 | −61 | 46 | 3.90 | 65 | Right angular gyrus |
| 6 | −67 | 49 | 6.39 | 173 | Right precuneus |
As for the recollection effects, ROI analyses of the monitoring effects used data extracted from spheres centered on peak voxels identified in independent data sets (see Materials and Methods, Section 2.6 ‘MRI data analysis’, for the coordinates). ANOVAs of the mean parameter estimates elicited by associative hits and misses extracted from the ACC and the rDLPFC revealed across-group main effects of monitoring (both ps < 0.001), but failed in each case to demonstrate significant main effects of group (both ps > 0.06) or significant interactions between age group and item type (ACC: p = 0.409, η2 = 0.01; rDLPFC: p = 0.260, η2 = 0.01)2.
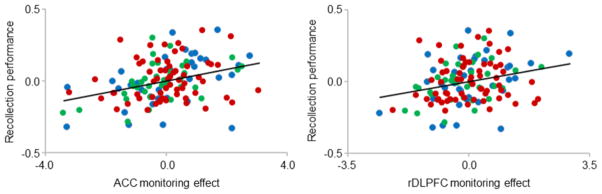
Results of the across-group regression analyses for the ACC and rDLPFC revealed significant positive relationships between associative recollection performance and monitoring effects in both cases (ACC: partial r = 0.34, p < 0.001; rDLPFC: partial r = 0.26, p < 0.005). Figure 6 depicts the partial plots of these relationships.
Figure 6.
Partial plots of the relationships between recollection performance and monitoring effects in the ACC (left) and the rDLPFC (right). Data points correspond to young (blue), middle-aged (green) and older (red) participants. [1.5 columns]
3.3.4 Independence of the relationship between different fMRI effects and recollection performance
The analyses described above indicate that, after controlling for variance due to age, the magnitude of recollection effects in mPFC and the right hippocampus, and monitoring effects in the ACC and rDLPFC, each correlated positively with recollection performance. We employed multiple regression to determine whether these different fMRI effects explained independent fractions of the variance in recollection performance, as well as to assess their aggregate contribution to explaining the variance. The regression model included recollection performance as the dependent variable, and age group, RT differences, mPFC and right hippocampal recollection effects, and ACC and rDLPFC monitoring effects, as predictor variables. The model accounted for a substantial proportion of the variance in recollection performance (F6, 134 = 22.82, p < 0.001, adjusted R2 = 0.494). As is evident from Table 7, recollection effects in the mPFC and right hippocampus, and monitoring effects in the ACC, each accounted for significant proportions of variance in recollection performance independently of age group. Together, the fMRI effects uniquely accounted for 23% of the total variance in performance, over five times the percentage uniquely accounted for by age group.
Table 7.
Results of the across-group regression model investigating variables that predict recollection performance.
| Model | b | SE b | β | P value |
|---|---|---|---|---|
| Age group | −.045 | .013 | −.223 | .001 |
| RT differences | .000 | .000 | −.062 | .373 |
| R hippocampus recollection effect | .029 | .013 | .158 | .024 |
| mPFC recollection effect | .052 | .010 | .405 | .000 |
| ACC monitoring effect | .059 | .012 | .449 | .000 |
| rDLPFC monitoring effect | .017 | .015 | .089 | .285 |
Note: b, unstandardized coefficient; SE b, standard error of the unstandardized coefficient; and β, standardized coefficient.
4 Discussion
Despite the robust differences in associative recognition performance between the age groups, there was considerable overlap between the scores in each group (Figure 1). Thus, the data were well-suited for addressing two of the primary questions motivating the study, namely, whether age-related differences in the neural correlates of episodic retrieval are independent of memory performance, and whether any relationships between retrieval-related neural activity and memory performance are moderated by age.
Starting with the first question, whole brain analyses identified three clusters – in the putamen, rostral ACC and left anterior MTL – where differences between associative hits and misses interacted with age group. In each case, the effects remained after controlling for memory performance, and took the form of larger recollection effects in young participants than in either of the other age groups. The functional significance of these age differences is difficult to assess, not least because they are in regions that are not consistently identified as manifesting recollection-related effects (Kim, 2010; Rugg & Vilberg, 2013; although see Scimeca & Badre, 2012, for discussion of possible striatal contributions to memory retrieval). In short, the relevance of these findings for the understanding of age-related differences in memory performance is uncertain, and will not be elucidated without further research. Nonetheless, the findings are reminiscent of prior reports of age-related reduction in recollection effects (see below), and are consistent with those findings in suggesting that neural correlates of recollection are not wholly impervious to age. The findings further suggest that age effects on neural correlates of recollection can be detected by middle age. Indeed, the recollection effects in this group in the aforementioned regions tended to be smaller than those of the young group, but were statistically equivalent to the effects in the older participants. Thus, if these findings are indicative of age-related neural decline (although see below), they suggest that such decline onsets relatively early in the course of the adult lifespan.
Robust recollection effects were evident in each age group in ROIs within the core recollection network. In three of the ROIs there were no detectable age-related differences in the magnitudes of the effects, whereas in the remaining regions – mPFC, bilateral hippocampus and posterior cingulate – effects were larger in the young group. These findings of age-related reductions in recollection effects are consistent with those reported in some prior studies (e.g., Kukolja et al., 2009; Tsukiura et al., 2010; Angel et al., 2013; Cansino et al., 2015). Unlike the age effects identified by the whole brain analyses discussed above, however, after controlling for memory performance the effects of age in these ROIs were not statistically significant. Thus, the recollection effects identified in these regions in middle-aged and older individuals were approximately the same size as those that would be expected in young adults with equivalent levels of memory performance. In other words, there was little or no evidence that age uniquely accounted for any of the variance in retrieval-related activity within the core recollection network.
Our finding that retrieval effects within the core recollection network were insensitive to age after controlling for differences in performance is consistent with findings from two prior studies where recollection was operationalized using the ‘Remember/Know’ procedure (Duarte at al., 2008; Wang et al., 2016; see also Dulas & Duarte, 2012, for analogous findings using a test of source memory, and Wang & Giovanello, 2016 and Dulas & Duarte, 2016 for additional evidence of age-invariant hippocampal associative retrieval effects). In the case of Duarte et al. (2008), the null findings for age were restricted to a sub-group of older participants in whom recollection performance was equivalent to that of the young group, whereas in Wang & Giovanello, 2016 and Dulas & Duarte, 2016 there were no significant age-related differences in performance. In Wang et al., 2016, however, recollection effects were age-invariant despite a small but reliable reduction in recollection performance in the older group. Together with the present results, these previous findings stand in contrast to studies where recollection-related activity in one or more members of the core recollection network was reported to decline with age (Daselaar et al., 2006; Kukolja et al., 2009; Tsukiura et al., 2010; Angel et al., 2013; Cansino et al., 2015). With the exception of Angel et al. (2013), recollection performance in these studies was superior in the young participants, and it is unclear whether similar findings would have emerged were performance to have been equated or controlled for across age groups. In Angel et al. (2013), however, age-related differences in recollection effects were evident even though performance was experimentally equated. The reason for the discrepancy between the findings of Angel et al. (2013) and the present results (and those of Duarte et al. (2008) and Wang et al., 2016) is unclear. The balance of the current evidence suggests, however, that when memory performance is either comparable between age groups or is controlled for statistically, recollection effects within the core recollection network differ little in their magnitudes across much of the healthy adult lifespan.
As was noted in the Introduction, the finding that an age-related reduction in recollection-related activity in a region of the core recollection network is no longer evident when individual differences in performance are controlled for does not necessarily imply that the region is unaffected by age. One possibility, for example, is that as a result of the depradations of age the region is no longer able to achieve the levels of activation evident in high-performing young individuals, and thus to achieve the levels of behavioral performance demonstrated by these individuals. The findings do however suggest that the relationship between the level of neural activity in the region and performance does not differ with age. According to the CRUNCH model, for example (Reuter-Lorenz & Cappell, 2008; see also Grady, 2012), to achieve levels of performance equivalent to those of young individuals, older individuals must activate task-relevant neural regions to a greater extent than the young to compensate for a decline in neural efficacy. In the present study, evidence in favor of this and similar proposals would have been forthcoming had it turned out that, after controlling for performance, older individuals demonstrated greater recollection effects than young participants. Similarly, had it transpired that an age-related reduction in a recollection effect remained after controlling for performance (as was the case in some other regions; see above), this would also have suggested that the effects of age on recollection-related activity in the region in question were independent of age-related differences in memory performance, prompting, perhaps, a search for regions demonstrating compensatory age-related ‘over-activation’ (cf. Gutchess et al., 2005).
The contrast employed here to identify the neural correlates of retrieval monitoring identified effects in ACC and rDLPFC, two regions previously implicated in monitoring (Henson et al., 1999; 2000; Fletcher & Henson, 2001; Wang et al., 2016). The loci of these ‘monitoring effects’ overlapped the effects reported in prior studies, despite the employment of a very different memory test (associative recognition rather than Remember/Know) and, consequently, a quite different operationalization of retrieval monitoring. As was also reported in three previous studies where PFC monitoring effects were contrasted according to age group (Giovanello et al., 2010; Dulas & Duarte, 2014; Wang et al., 2016; see also Mark & Rugg, 1998, and Dulas & Duarte, 2013, for convergent ERP evidence), the present effects were age-invariant. These findings are seemingly inconsistent with the results of two other studies where fMRI monitoring effects were contrasted according to age (McDonough et al., 2013; Mitchell et al., 2013). In both of these studies, monitoring demands were manipulated by varying the difficulty of the retrieval task, and neural correlates of monitoring were identified by contrasting activity elicited by test items belonging to the hard versus the easy task. In each case, activity in DLPFC and ACC was elevated in the hard condition in young but not in older participants, and the findings were interpreted as evidence of a failure on the part of older individuals to approporiately engage neural resources supporting monitoring. In both studies, however, monitoring demands were (necessarily) confounded with the type of retrieval test. There is evidence that older adults are less adept than young individuals in adopting test-appropriate ‘retrieval orientations’ (Morcom & Rugg, 2004; Jacoby et al., 2005; Duverne et al., 2009). Thus, it is possible that the failure of the older participants in McDonough et al. (2013) and Mitchell et al. (2013) to demonstrate task-dependent monitoring effects in the PFC reflected a more general difficulty in adopting differential task sets. In any case, the present findings converge with those of several prior studies (see above) to indicate that there are circumstances where older (and middle-aged) individuals can control, and seemingly benefit from, engagement of retrieval monitoring to the same extent as young adults.
We identified age-invariant, across-participant relationships between associative recognition performance and recollection effects in the mPFC and right hippocampus, and monitoring effects in the ACC and rDLPFC. The findings for the hippocampus and for the two monitoring effects are strongly reminiscent of the findings of Wang et al., 2016 but, to our knowledge, the present study is the first to identify a relationship between individual differences in retrieval-related activity in the mPFC and recollection performance. Strikingly, the fMRI effects collectively accounted for more of the variance in recollection performance than did age. Together, these findings testify to the stability of the functional neuroanatomy of episodic retrieval in people of differing ages, and suggest that at least some of the neural regions implicated in successful recollection and monitoring support processes whose functional relationship to memory performance is age-invariant.
To our knowledge, there is only one prior event-related fMRI study of retrieval success effects in which a group of middle-aged adults was included and which also employed an experimental task (source memory) that permitted the neural correlates of recollection to be identified with a relatively ‘process pure’ contrast (Cansino et al., 2015). Largely consistent with the present findings, Cansino et al. (2015) failed to identify differences in recollection effects between their young and middle-aged groups, although, as was noted above, differences were identified in Cansino et al.’s (2015) study between the young group and a sample of older adults. In light of the present findings we think it is not coincidental that the fMRI findings of Cansino et al. (2015) were paralleled by performance on the experimental task: whereas source memory accuracy did not significantly differ between their young and middle-aged samples, accuracy in the older group was significantly lower.
We conclude with two caveats to the interpretation of the present (and previous) findings (see also Rugg, in press). First, our conclusion that age has little impact on retrieval-related neural activity rests on the assumption that the transfer function mediating between neural activity and the fMRI BOLD signal is age-invariant. There is evidence however that cerebro-vascular reactivity (CVR) – an important non-neural determinant of BOLD signal magnitude – declines with age (e.g., Lu et al., 2011; Liu et al., 2013). Therefore it will be important to see whether the present findings require qualification in light of further research in which retrieval-related BOLD activity is corrected for individual differences in CVR (cf. Tsvetanov et al., 2015).
The second caveat arises because of the evidence that a significant proportion of the variance across the lifespan in both memory performance and the neural correlates of memory processing is likely attributable to factors such as birth cohort (Rönnlund & Nilsson, 2009; Baxendale, 2010) and sampling bias (Nyberg et al., 2010; Rugg, in press), rather than to age-related changes in brain structure and function. Disentangling the contributions of these different factors to the effects of age on behavioral and neural measures necessitates a longitudinal rather than a cross-sectional approach (Raz & Lindenburger, 2011). Importantly, this caveat does not qualify the most important of the present findings, which bear on the stability, rather than the lability, of retrieval-related neural activity across the healthy adult lifespan. The findings add weight to prior proposals (Mark & Rugg, 1998; Wang et al., 2016) that age-related reduction in episodic memory performance likely owes more to the effects of age on encoding operations (Maillet & Rajah, 2014) and retrieval cue processing (Morcom & Rugg, 2004; Jacoby et al., 2005) than on how mnemonic information is processed after its retrieval has been successfully initiated.
Acknowledgments
This research was supported by the National Institute on Aging (NIH 1R01AG039103). We acknowledge the contributions of Hannah Stanton and Kay Moolenijzer for their assistance with participant recruitment and neuropsychological data collection. We also thank the staff of the UTSW Advanced Imaging Center for their assistance with MRI data collection.
Footnotes
An alternative strategy would have been to identify ROIs from the ‘recollection’ and ‘monitoring’ effects in the aging study of Wang et al., 2016 after collapsing across the young and older groups. Unfortunately, because 20 of the 24 older participants employed by Wang et al., 2016 also belonged to the current older sample, those contrasts are not fully independent of the ones reported here. We therefore elected to use the outcome of the contrasts from the young group only of Wang et al., 2016, and to increase the generality of the recollection contrast by combining it with a theoretically (but not procedurally) analogous contrast from a second, entirely independent, study.
As we noted in the Methods section, we elected to identify the neural correlates of monitoring through the contrast between associative misses and associative hits, rather than that between associative correct rejections and associative hits, so as to minimize possible confounding effects of age-related differences in employment of a recall to reject strategy. At the request of a reviewer, we reanalyzed the monitoring effects employing the second of the two aforementioned contrasts. With one exception, the findings for the ACC and right DLPFC were identical to those reported for our preferred contrast. Thus, for both regions, there were robust, age-invariant correlations with rcollection performance (partial rs of .279 and .272 respectively, ps < .005), and in the case of the ACC, no sign of an age group × monitoring effect interaction (p > .15). There was however a significant age group × monitoring effect interaction for the right DLPFC (p < .001). This remained after controlling for performance (p < .025) and reflected larger effects in the young than the other two groups, where the effects, while significant in each case (ps < .001), did not differ. For the reasons noted previously, interpretation of this interaction is complicated by the possibility of age-related differences in monitoring strategies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition tests. Neuroimage. 2005;24(4):1113–1121. doi: 10.1016/j.neuroimage.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Andrade A, Paradis AL, Rouquette S, Poline JB. Ambiguous results in functional neuroimaging data analysis due to covariate correlation. Neuroimage. 1999;10(4):483–486. doi: 10.1006/nimg.1999.0479. [DOI] [PubMed] [Google Scholar]
- Angel L, Bastin C, Genon S, Balteau E, Phillips C, Luxen A, Maquet P, Salmon E, Collette F. Differential effects of aging on the neural correlates of recollection and familiarity. Cortex. 2013;49(6):1585–1597. doi: 10.1016/j.cortex.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Angel L, Bastin C, Genon S, Salmon E, Fay S, Balteau E, Maquet P, Luxen A, Isingrini M, Collette F. Neural correlates of successful memory retrieval in aging: Do executive functioning and task difficulty matter? Brain Research. 2016;1631:53–71. doi: 10.1016/j.brainres.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Baxendale S. The Flynn effect and memory function. Journal of Clinical and Experimental Neuropsychology. 2010;32(7):699–703. doi: 10.1080/13803390903493515. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4(4):359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cansino S, Trejo-Morales P, Estrada-Manilla C, Pasaye-Alcaraz EH, Aguilar-Castañeda E, Salgado Lujambio P, Sosa-Ortiz AL. Brain activity during source memory retrieval in young, middle-aged and old adults. Brain Research. 2015;1618:168–180. doi: 10.1016/j.brainres.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Pike GB, Evans AC. Brainweb: Online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:425. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, New Jersey: L; 1988. [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychology and Aging. 2008;23(1):93–103. doi: 10.1037/0882-7974.23.1.93. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cerebral Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cerebral Cortex. 2011;21(9):2166–2176. doi: 10.1093/cercor/bhq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. Sensitivity of negative subsequent memory and task-negative effects to age and associative memory performance. Brain Research. 2015;1612:16–29. doi: 10.1016/j.brainres.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. The relationships between age, associative memory performance and the neural correlates of successful associative memory encoding. Neurobiology of Aging. 2016;42:163–176. doi: 10.1016/j.neurobiolaging.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex. 2008;18(9):2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiology of Aging. 2010;31(10):1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cerebral Cortex. 2012;22(1):37–50. doi: 10.1093/cercor/bhr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The influence of directed attention at encoding on source memory retrieval in the young and old: An ERP study. Brain Research. 2013;1500:55–71. doi: 10.1016/j.brainres.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. Aging affects the interaction between attentional control and source memory: an fMRI study. Journal of Cognitive Neuroscience. 2014;26(12):2653–2669. doi: 10.1162/jocn_a_00663. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. Age-related changes in overcoming proactive interference in associative memory: The role of PFC-mediated executive control processes at retrieval. NeuroImage. 2016;132:116–128. doi: 10.1016/j.neuroimage.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29(12):1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. Effects of age on the neural correlates of retrieval cue processing are modulated by task demands. Journal of Cognitive Neuroscience. 2009;21(1):1–17. doi: 10.1162/jocn.2009.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elward RL, Vilberg KL, Rugg MD. Motivated memories: effects of reward and recollection in the core recollection network and beyond. Cerebral Cortex. 2015;25(9):3159–3166. doi: 10.1093/cercor/bhu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory. Brain. 2001;124(5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Kensinger EA, Wong AT, Schacter DL. Age-related neural changes during memory conjunction errors. J Cogn Neurosci. 2010;22:1348–1361. doi: 10.1162/jocn.2009.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature Reviews Neuroscience. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24(2):95–112. [Google Scholar]
- Grinband J, Savitskaya J, Wager TD, Teichert T, Ferrera VP, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage. 2011;57(2):303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess A, Welsh RC, Hedden T, Bangert A, Minear M, Liu L, Park D. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hayama HR, Rugg MD. Right dorsolateral prefrontal cortex is engaged during post-retrieval processing of both episodic and semantic information. Neuropsychologia. 2009;47(12):2409–2416. doi: 10.1016/j.neuropsychologia.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12(6):913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Jacoby LL, Shimizu Y, Velanova K, Rhodes MG. Age differences in depth of retrieval: Memory for foils. Journal of Memory and Language. 2005;52(4):493–504. [Google Scholar]
- Johnson JD, Suzuki M, Rugg MD. Recollection, familiarity, and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study. Frontiers in Human Neuroscience. 2013;7(219):1–15. doi: 10.3389/fnhum.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-Related Increases in Functional Connectivity Predict Individual Differences in Memory Accuracy. The Journal of Neuroscience. 2015;35(4):1763–1772. doi: 10.1523/JNEUROSCI.3219-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: a meta-analytic review. Neuropsychology Review. 2014;24(3):332–354. doi: 10.1007/s11065-014-9266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Wilms M, Mirzazade S, Fink GR. Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiology of Aging. 2009;30(4):630–645. doi: 10.1016/j.neurobiolaging.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Kwon D, Maillet D, Pasvanis S, Ankudowich E, Grady CL, Rajah MN. Context Memory Decline in Middle Aged Adults is Related to Changes in Prefrontal Cortex Function. Cerebral Cortex. 2016;26:2440–2460. doi: 10.1093/cercor/bhv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Brodeur M, Bourgouin P. Prefrontal cortex contribution to associative recognition memory in humans: an event-related functional magnetic resonance imaging study. Neuroscience Letters. 2003;346(1):73–76. doi: 10.1016/s0304-3940(03)00578-0. [DOI] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Section J, Park DC, Lu H. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage. 2013;78:415–425. doi: 10.1016/j.neuroimage.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, … Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cerebral Cortex. 2011;21(6):1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Rajah MN. Age-related differences in brain activity in the subsequent memory paradigm: A meta-analysis. Neuroscience & Biobehavioral Reviews. 2014;45:246–257. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval. An electrophysiological study. Brain. 1998;121:861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- McDonough IM, Wong JT, Gallo DA. Age-related differences in prefrontal cortex activity during retrieval monitoring: Testing the compensation and dysfunction accounts. Cerebral Cortex. 2013;23(5):1049–1060. doi: 10.1093/cercor/bhs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickes L, Johnson EM, Wixted JT. Continuous recollection versus unitized familiarity in associative recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36(4):843. doi: 10.1037/a0019755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BD, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Ankudowich E, Durbin KA, Greene EJ, Johnson MK. Age-related differences in agenda-driven monitoring of format and task information. Neuropsychologia. 2013;51(12):2427–2441. doi: 10.1016/j.neuropsychologia.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Rugg MD. Effects of age on retrieval cue processing as revealed by ERPs. Neuropsychologia. 2004;42(11):1525–1542. doi: 10.1016/j.neuropsychologia.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cerebral Cortex. 2007;17(11):2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal aging. Acta Neurologica Scandinavica. 2003;107(s179):7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends in Cognitive Sciences. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Lind J, Pudas S, Persson J, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proceedings of the National Academy of Sciences. 2010;107(52):22682–22686. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oedekoven CS, Jansen A, Kircher TT, Leube DT. Age-related changes in parietal lobe activation during an episodic memory retrieval task. Journal of Neural Transmission. 2013;120(5):799–806. doi: 10.1007/s00702-012-0904-x. [DOI] [PubMed] [Google Scholar]
- Oedekoven CS, Jansen A, Keidel JL, Kircher T, Leube D. The influence of age and mild cognitive impairment on associative memory performance and underlying brain networks. Brain Imaging and Behavior. 2015;9(4):776–789. doi: 10.1007/s11682-014-9335-7. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychology and Aging. 2008;23(1):104. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Only time will tell: Cross-sectional studies offer no solution to the age – brain – cognition triangle: Comment on Salthouse (2011) Psychological Bulletin. 2011;137(5):790–795. doi: 10.1037/a0024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17(3):177–182. [Google Scholar]
- Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review. 2014;24(3):355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20(1):3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Rönnlund M, Nilsson LG. Flynn effects on sub-factors of episodic and semantic memory: Parallel gains over time and the same set of determining factors. Neuropsychologia. 2009;47(11):2174–2180. doi: 10.1016/j.neuropsychologia.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Heit E. Associative recognition: A case of recall-to-reject processing. Memory & Cognition. 2000;28(6):907–922. doi: 10.3758/bf03209339. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41(1):40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Retrieval processing in human memory: electrophysiological and fMRI evidence. In: Gazzaniga MS, editor. The Cognitive Neurosciences III. Cambridge (MA): MIT Press; 2004. pp. 727–737. [Google Scholar]
- Rugg MD, Morcom AM. The relationship between brain activity, cognitive performance, and aging. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2005. pp. 132–154. [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD. Interpreting age-related differences in memory-related neural activity. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. 4. Oxford University Press; in press. [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Current Opinion in Neurobiology. 2007;17(2):220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of the International Neuropsychological Society. 2010;16(05):754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75(3):380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Sekiguchi A, Yomogida Y, Nakagawa S, Shigemune Y, Kambara T, Akitsuki Y, Taki Y, Kawashima R. Effects of Aging on Hippocampal and Anterior Temporal Activations during Successful Retrieval of Memory for Face–Name Associations. Journal of Cognitive Neuroscience. 2010;23(1):200–213. doi: 10.1162/jocn.2010.21476. [DOI] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RN, Tyler LK, Davis SW, Shafto MA, Taylor JR, Williams N, Cam-CAN &, Rowe JB. The effect of ageing on fMRI: Correction for the confounding effects of vascular reactivity evaluated by joint fMRI and MEG in 335 adults. Human Brain Mapping. 2015;36(6):2248–2269. doi: 10.1002/hbm.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Cabeza R. Episodic memory encoding and retrieval in the aging brain. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. 4. Oxford University Press; in press. [Google Scholar]
- Wang WC, Giovanello KS. The role of medial temporal lobe regions in incidental and intentional retrieval of item and relational information in aging. Hippocampus. 2016;26(6):693–699. doi: 10.1002/hipo.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Johnson JD, de Chastelaine M, Donley BE, Rugg MD. The Effects of Age on the Neural Correlates of Recollection Success, Recollection-Related Cortical Reinstatement, and Post-Retrieval Monitoring. Cerebral Cortex. 2016;26:1698–1714. doi: 10.1093/cercor/bhu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Kruggel F, Rugg MD. Effects of Advanced Aging on the Neural Correlates of Successful Recognition Memory. Neuropsychologia. 2009;47:1352–1361. doi: 10.1016/j.neuropsychologia.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]