The immune checkpoint inhibitor pembrolizumab, which targets programmed death-1 (PD-1) receptor, has recently emerged as the standard-of-care treatment for patients with advanced melanoma, with a response rate of 33% (1). Another agent in the realm of immunotherapy used throughout the world, but not currently approved by the Food and Drug Administration in the United States, is the contact sensitizer diphencyprone (DPCP). Topical DPCP has been used in a 50-patient case series of cutaneously metastatic melanoma, with a response rate of 84%. DPCP also led to nodal or visceral metastasis regression in 4 of the patients (2), thus suggesting the potential for an abscopal effect with this topically applied immunotherapy. A recent 2-patient case series showed that topical immunotherapy has limited effect on internal disease, but that combined treatment with the PD-1 inhibitor nivolumab led to pronounced internal metastasis regression (3). Within an ongoing trial treating melanoma patients with topical DPCP formulated in a non-volatile, aqueous solvent as an Investigational New Drug (ClinicalTrials.gov number, NCT01711684), we have observed a remarkable tumor response in one patient on concurrent pembrolizumab therapy.
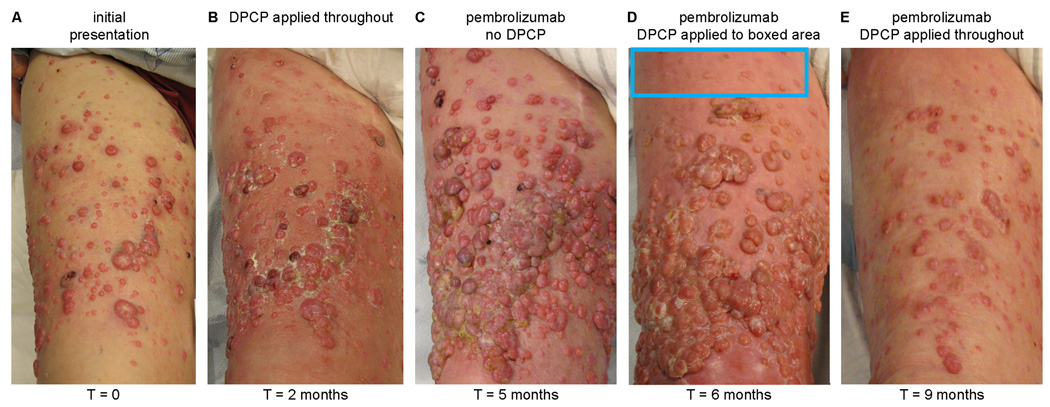
The patient is a 93-year-old man who underwent resection of his primary acral melanoma harboring an exon 13 K642E mutation from his right foot ten years ago. Subsequently, he was treated with ipilimumab as well as imatinib, but ultimately progressed with extensive in-transit metastases throughout his right lower extremity, which is when he presented to our institution, not having been on any medical therapy for 6 months (Figure 1A). The patient received two months of twice weekly topical DPCP applications (0.4% and 0.04% concentrations were alternately used, with the goal being to maintain a tolerable level of inflammation and change concentration as necessary) while on no other therapies for his melanoma, and inflammation was induced as expected, but tumor response was incomplete, with generally only smaller lesions (<1 cm) responding, and larger lesions expanding (Figure 1B). At this point, the patient discontinued DPCP applications to begin treatment with pembrolizumab (2 mg/kg as intravenous infusion every 3 weeks), but during the three months on pembrolizumab (the median time to response for this agent), his disease rapidly progressed (Figure 1C). We then elected to restart DPCP applications (again twice weekly) concurrently with pembrolizumab (again every 3 weeks), first only to a region of skin that selectively responded (Figure 1D), suggesting a synergistic reaction, but these data do not fully exclude the possibility of a delayed response to pembrolizumab. Then applications were extended throughout the skin areas involved with melanoma, with substantial metastasis regression observed (Figure 1E). In comparison to single-agent DPCP, combination therapy required a lower concentration of DPCP to induce the same level of inflammation, further supporting a synergistic reaction. Concurrently, we observed the development of vitiligo on the contralateral leg (not treated with topical DPCP), suggesting a systemic immune response against melanocyte lineage cells.
Figure 1.
Responses of cutaneous melanoma metastases to topical DPCP, pembrolizumab, and a combination of the two. A pretreatment photograph of the patient’s right anterior thigh (the advancing front of his metastatic disease) shows numerous amelanotic cutaneous melanoma metastases (Panel A). Two months of topical DPCP applications (Panel B) led to an incomplete tumor response, while three months of pembrolizumab therapy without DPCP (Panel C) resulted in rapid progression of disease. Topical DPCP added to background pembrolizumab therapy, first to a select area for one month (Panel D), and then throughout the area for an additional three months (Panel E), led to dramatic regression of cutaneous melanoma metastases.
This patient had dramatic reduction in cutaneous metastases upon combined pembrolizumab and DPCP therapy, even though treatment with either of these agents alone (as well as with ipilimumab administered before our trial) resulted in mixed responses or progression of disease. Favorable responses to pembrolizumab require CD8+ T cells and expression of PD-1/PD-L1 (4), which we have previously demonstrated to be increased by topical DPCP (5). DPCP therefore may synergize with pembrolizumab therapy. Since this contact sensitizer is inexpensive with a favorable safety profile established over decades (6), it could have great clinical utility to improve the responses of cutaneous metastases seen with the current standard-of-care pembrolizumab treatment. In our patient, combination therapy was well-tolerated, without any significant systemic toxicity observed. Since DPCP is topically applied, it is relatively simple to change the concentration used, or elect not to perform certain applications, should the induced inflammation become intolerable for the patient.
In addition to suggesting a novel therapeutic approach for melanoma treatment, this case report opens up the possibility of a translational research program designed around the understanding of immune responses that may successfully mediate tumor regression. As the skin is an ideally accessible organ to study by biopsy, samples can be taken of cutaneous metastases that are subject to background pembrolizumab treatment, under direct topical application of DPCP, or a combination of the two. These biopsy tissues can then be examined by immunohistochemistry and gene expression studies in order to obtain a comprehensive cellular and molecular profile of immune reactions. In this way, the different immune responses generated by these two immunotherapeutic approaches can be rigorously studied. The responses generated by other locally applied immunotherapies that activate the immune system, such as IL-2 and interferon-alpha, can be examined in a similar manner, and may lead to similar effects. When this bench research is combined with photographic and histologic demonstration of metastases that successfully regress or not upon treatment (bedside aspect, as demonstrated in this report), there is the potential to greatly enhance understanding of how the immune system can be used to treat cancer.
Acknowledgments
This work was funded by National Institutes of Health (NIH) grant UL1RR024143 from the National Center for Research Resources and the Milstein Medical Program. NG was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH under award number T32GM07739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources played no role in study design, writing, or decision to submit the paper for publication. We thank Patricia Gilleaudeau and Mary Sullivan-Whalen for outstanding patient care.
NG and JGK developed the study design. All authors wrote the manuscript.
ABBREVIATIONS USED
- DPCP
diphencyprone
- PD-1
programmed death-1
Footnotes
CONFLICT OF INTEREST:
We declare no competing interests.
ETHICS COMMITTEE APPROVAL:
This study was approved by The Rockefeller University’s Institutional Review Board. Written, informed consent was obtained, and the study adhered to the Declaration of Helsinki Principles.
REFERENCES
- 1.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 2.Damian DL, Saw RPM, Thompson JF. Topical immunotherapy with diphencyprone for in transit and cutaneously metastatic melanoma. J Surg Oncol. 2014;109:308–313. doi: 10.1002/jso.23506. [DOI] [PubMed] [Google Scholar]
- 3.Fujimura T, Furudate S, Kakizaki A, et al. Contact immunotherapy enhances the therapeutic effects of nivolumab in treating in-transit melanoma: Two cases reports. J Dermatol. 2015;42:1–4. doi: 10.1111/1346-8138.13229. [DOI] [PubMed] [Google Scholar]
- 4.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulati N, Suárez-Fariñas M, Fuentes-Duculan J, et al. Molecular characterization of human skin response to diphencyprone at peak and resolution phases: therapeutic insights. J Invest Dermatol. 2014;134:2531–2540. doi: 10.1038/jid.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durdu M, Özcan D, Baba M, et al. Efficacy and safety of diphenylcyclopropenone alone or in combination with anthralin in the treatment of chronic extensive alopecia areata: a retrospective case series. J Am Acad Dermatol. 2015;72:640–650. doi: 10.1016/j.jaad.2015.01.008. [DOI] [PubMed] [Google Scholar]