Abstract
Our previous study demonstrated that CD8+ T cells remove cysts of Toxoplasma gondii from the brain through perforin-mediated mechanisms. We here show that a transfer of CD8+ immune T cells primed with a type II or a type III strain of T. gondii both efficiently removed cysts of a type II strain from infected SCID mice, although the former tended to be slightly more efficient than the latter. Similarly, a transfer of type II-primed CD8+ T cells removed cysts of a type III strain. Therefore, CD8+ T cells are capable of removing T. gondii cysts by recognizing epitopes commonly expressed in types II and III strains or cross-reactive between these two genotypes.
Keywords: Toxoplasma gondii, Cyst, Genotype, CD8+ T cells, Perforin
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite present ubiquitously worldwide. Infection in humans occurs by ingestion of undercooked meat containing cysts, or food and water contaminated with oocysts. During the acute stage of infection, tachyzoites proliferate in a variety of organs and can cause various diseases such as lymphadenitis, chorioretinitis, and congenital infection of the fetuses [1]. Interferon-gamma (IFN-γ)-mediated immune responses and, to a lesser extent humoral immune responses, are able to limit the tachyzoite proliferation [2–4], but the parasite establishes a chronic infection by forming tissue cysts, which can contain hundreds to thousands of bradyzoites, preferentially in the brain and skeletal muscle. This chronic infection is one of the most common parasitic infections in humans, and up to a third of the world’s population is infected with this parasite [1].
Chronic infection with T. gondii can reactivate and cause life-threatening toxoplasmic encephalitis in immunocompromised individuals such as those with AIDS, neoplastic diseases, and organ transplants [1,5,6]. This reactivation is caused by rupture of the cysts followed by conversion of released bradyzoites to tachyzoites, and proliferation of tachyzoites. Therefore, the reactivation of infection can be prevented if there is a way to remove tissue cysts of T. gondii from the tissues of infected individuals. However, there is currently no drug treatment available to eliminate the cyst stage of the parasite. It had also generally been considered that the immune system is unable to recognize or remove T. gondii cysts. In this regard, our recent studies however demonstrated that CD8+ immune T cells of chronically infected BALB/c mice, which are genetically resistant to the infection, possess a potent activity to initiate the immune process to remove the tissue cysts from the brain when these T cells are transferred to infected immunodeficient (athymic nude or SCID) mice that have already developed large numbers of cysts [7]. This anti-cyst activity of CD8+ immune T cells is mediated by a perforin-dependent mechanism [7]. Perforin is critical for cytotoxic activity of CD8+ T cells. Therefore, it is most likely that cytotoxic activity of CD8+ T cells initiates the immune process to remove T. gondii cysts. This is in contrast to the protective immune responses to control tachyzoite proliferation, in which IFN-γ production by T cells is required [2–4]. During the T cell-mediated anti-cyst immune process, phagocytes, morphologically microglia and macrophages, accumulate and penetrate into the cysts [7]. Therefore, those phagocytes are likely important effector cells that eliminate the cysts once cytotoxic activity of CD8+ T cells initiates the immune process.
T. gondii cysts are formed within infected host cells [8,9]. In order to elucidate the mechanisms by which CD8+ T cells induce removal of T. gondii cysts, it is essential to understand how CD8+ T cells recognize cyst-containing cells to initiate anti-cyst immune process. However, the information in this regard is lacking. The majority of T. gondii isolates from infected people in North America and Europe are one of three genotypes, types I, II, and III [10]; type II is predominant and type III is also common in the isolates from immunocompromised individuals [11,12], in which reactivation of chronic infection causes serious diseases. Therefore, to begin understanding T. gondii antigens recognized by CD8+ T cells for removing cysts, it is valuable to determine whether the protective T cells recognize epitopes of T. gondii antigens commonly present in type II and type III of the parasite or epitopes specific to each of the genotypes. In the present study, we examined the role of these two genotypes of T. gondii on the activity of CD8+ T cells to induce cyst removal.
2. Materials and methods
2.1. Mice
BALB/c and BALB/c-background SCID mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Female animals were used for all studies and animals were 10–15 weeks old when used. SCID mice were all age-matched within a single experiment. BALB/c mice used as donors of CD8+ immune T cells were also age-matched within a single experiment. There were 4–5 SCID mice in each experimental group. Mouse care and experimental procedures were performed under specific pathogen-free conditions in accordance with established institutional guidance and approved protocols from the Institutional Animal Care and Use Committee.
2.2. Infection with T. gondii
SCID mice were infected with 20 cysts of the ME49 strain (type II) perorally by gavage [7]. The SCID mice were treated with sulfadiazine in the drinking water (400 mg/L) beginning at 9 days after infection for the entire period of experiment to control proliferation of tachyzoites and establish a chronic infection [7,13]. In another experiment, SCID mice were infected intraperitoneally with 1 × 104 tachyzoites of the VEG strain (type III) and treated with sulfadiazine beginning at 8 days after infection. In general, both intraperitoneal infection with tachyzoites and peroral infection with cysts result in development of cysts in the brain and an establishment of a chronic infection in mice. To generate CD8+ immune T cells primed with a type II or a type III strain, BALB/c mice were infected with 1 × 103 tachyzoites of the ME49 or CEP (type III) strains intraperitoneally. The CEP-infected mice received sulfadiazine in drinking water to establish a chronic infection beginning at 16 days after infection. Each of these immune CD8+ T cells primed with the ME49 or CEP strain was transferred into ME49-infected SCID mice. In the experiment with the VEG-infected SCID mice, CD8+ immune T cells were obtained from BALB/c mice infected with 10 cysts of the ME49 strain perorally and transferred to the SCID mice. The experiments with the SCID mice infected with either ME49 or VEG strains were both aimed to examine whether CD8+ immune T cells recognize epitopes of T. gondii antigens commonly present in type II and type III of the parasite by using different approaches. Therefore, one experiment for each of these two approaches was performed. Tachyzoites of the VEG, ME49, and CEP were obtained from cultures of infected human foreskin fibroblasts [14,15].
2.3. Purification and transfer of CD8+ immune T cells
Spleen cells were obtained from 7 to 12 BALB/c mice that had been infected with the ME49 or CEP strain for 7 or 12 weeks in each experiment. Within a single experiment, all mice were infected for an identical period of time before harvesting their spleen cells. The spleen cells were suspended in Hanks’ balanced salt solution (Hyclone, Logan, UT) with 2% fetal bovine serum (Sigma, St. Louis, MO), and CD8+ immune T cells were purified from the spleen cells using magnetic bead-conjugated anti-mouse CD8 (53–6.7) monoclonal antibody (Miltenyi Biotech, Auburn, CA) [7,16]. As a control, CD8+ normal T cells were also purified from the spleens of 8–12 uninfected BALB/c mice in the same manner. Infected SCID mice received the purified CD8+ T cells (3.5 or 7.5 × 106 cells) intravenously at 3 weeks after infection [7].
2.4. Quantifying numbers of T. gondii cysts and amounts of mRNA for bradyzoite-specific BAG1, perforin, and granzyme B in the brains of infected SCID mice
One week after a transfer of CD8+ T cells, the brains of SCID mice were removed and a half of each brain was triturated in 0.5 ml of phosphate-buffered saline [7]. Numbers of cysts in 5–6 aliquots (20 μl each) of the brain suspensions were counted microscopically. RNA was purified from a half of each brain, and amounts of mRNA for BAG1, perforin, and granzyme B were measured by reverse transcription real-time PCR using StepOnePlus real-time PCR system with TaqMan reagents (Applied Biosystems, Branchburg, New Jersey) [7,13]. Primers and probe for BAG1 were as follows: TCACGTGGAGACCCAGAGT (Forward), CTGGCAAGT-CAGCCAAAATAATCAT (reverse), TTTGCTGTCGAAC TCC (probe) [17]. Primers and probes for perforin and granzyme B were from Applied Biosystems.
2.5. Statistical analysis
Levels of difference between experimental groups were determined by Mann–Whitney test (IBM SPSS Statistics, version 22, IBM). Corrected P value (Pc) was calculated by multiplying the P value by the number of comparisons performed within the experiment. Differences which provided Pc < 0.05 were considered significant.
3. Results and discussion
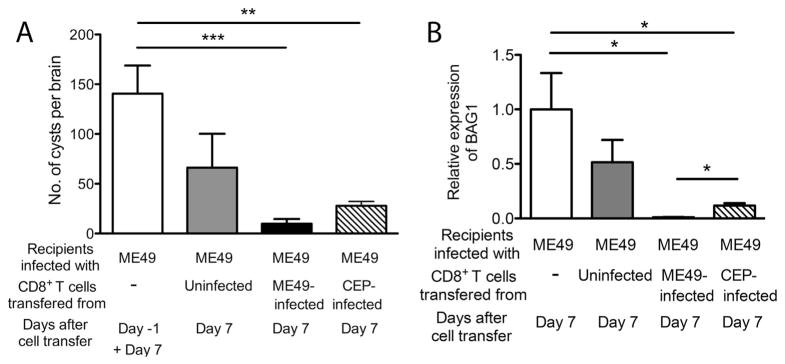
We first examined whether CD8+ immune T cells primed with either a type II or a type III strain of T. gondii are both able to remove tissue cysts of a type II strain of the parasite. SCID mice were infected with the ME49 (type II) strain and treated with sulfadiazine to establish a chronic infection in their brains. Animals received a systemic transfer of CD8+ immune T cells (7.5 × 106 cells) purified from the spleens of BALB/c mice chronically infected with either the ME49 or CEP (type III) strains. As a control, additional groups of mice received no T cells or CD8+ normal T cells from uninfected BALB/c mice. Numbers of cysts in the brains of the control group without T cell transfer were examined on one day before (Day-1) and 7 days after (Day 7) the time of the T cell transfer. The cyst numbers in the control group did not differ between these two time points, indicating that cyst numbers were stable during this time period. Therefore, the data from these two time points in this control group were combined (Fig. 1A).
Fig. 1.
A transfer of CD8+ immune T cells primed with either a type II (ME49) or a type III (CEP) strain of T. gondii are both capable of removing cysts of a type II (ME49) strain from the brains of SCID mice. SCID mice were infected with 20 cysts of the ME49 strain perorally and treated with sulfadiazine beginning at 9 days after infection to establish a chronic infection in their brains. At 3 weeks after infection, the mice received a systemic transfer of CD8+ immune T cells (7.5 × 106 cells) from BALB/c mice chronically infected with either the ME49 or CEP strain. As a control, a group of infected SCID mice received CD8+ normal T cells from uninfected BALB/c mice. Numbers of T. gondii cysts (A) and the amounts of bradyzoite-specific BAG1 mRNA (B) in the brains of the SCID mice were measured at 1 day before (Day-1) and 7 days after (Day 7) the T cell transfer. All data represent mean ± SEM. There were 4–5 mice in each experimental group. *Pc < 0.05, **Pc < 0.01, and ***Pc < 0.005.
Seven days after the T cell transfer, numbers of cysts in the brains of SCID mice that had received normal T cells tended to be fewer than the control animals that had received no T cells, but such differences did not reach statistical significance (Fig. 1A). In contrast, numbers of cysts in the brains of mice that had received CD8+ immune T cells primed with either ME49 or CEP strain were 96 and 84% less than those in the control animals that had received no T cells, respectively (Pc < 0.005 and Pc < 0.01, respectively, Fig. 1A). When compared between the groups that received the ME49-or CEP-primed CD8+ immune T cells, numbers of cysts tended to be less in the former than the latter, although such difference did not reach statistical significance (Pc = 0.064, Fig. 1A). These results indicate that CD8+ immune T cells primed with either a type II (ME49) or a type III (CEP) strain are both able to recognize host cells containing tissue cysts of a type II strain in the brain and initiate the immune process to remove the cysts, although the former tended to function slightly more efficiently than the latter.
Differences in amounts of mRNA for bradyzoite-specific BAG1 in the brains of each group of the SCID mice were consistent with the observations described above on numbers of cysts in their brains. Amounts of BAG1 mRNA in the brains of mice that had received CD8+ immune T cells primed with either ME49-or CEP strain were 99 and 88% less than those in the brains of the control animals without the T cell transfer, respectively (Pc < 0.05 for the both groups, Fig. 1B). BAG1 mRNA levels in the brains of mice that had received CD8+ normal T cells did not differ from those of the control animals without T cell transfer (Fig. 1B). Amounts of BAG1 mRNA in mice that had received the ME49-primed CD8+ T cells were significantly less than those in animals that had received the CEP-primed CD8+ T cells (Pc < 0.05, Fig. 1B). Therefore, CD8+ T cells mostly recognize the epitopes of T. gondii antigens commonly expressed in both type II or III strains of the parasite or cross-reactive between these two genotypes to induce the immune process for removal of the cysts. The epitopes specific to the genotypes may also contribute, to a lesser extent, to the recognition of cyst-containing cells by CD8+ T cells for inducing cyst removal. However, the latter point requires further analyses, since the ME49 strain may form larger numbers of cysts than the CEP strain in the donor BALB/c mice and thereby the former is relatively more efficient at inducing cyst-recognizing CD8+ immune T cells than the latter even if the T cells recognize the same epitope.
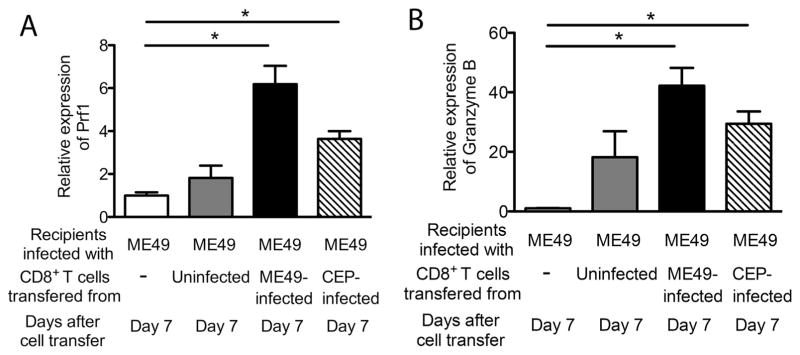
We previously demonstrated that the activity of CD8+ immune T cells to remove cysts from the brain does not require their IFN-γ production, the molecule essential for controlling tachyzoite proliferation, but does require perforin [7]. Therefore, we examined whether perforin expression increases in the brains of ME49-infected SCID mice that received CD8+ immune T cells from the ME49-or CEP-infected BALB/c mice. Perforin mRNA levels in the brains of SCID mice that had received CD8+ normal T cells did not differ from those of the control animals that had received no T cells (Fig. 2A). In contrast, perforin mRNA levels in the brains of SCID mice with the ME49-primed or CEP-primed CD8+ immune T cells were 6.2 and 3.6 times greater than those in the brains of the control animals with no T cells, respectively (Pc < 0.05, Fig. 2A). Consistent with the tendency of greater reduction of cyst numbers in the mice with the ME49-primed T cells than those with the CEP-primed T cells as shown in Fig. 1A, cerebral perforin mRNA levels also tended to be greater in the former than the latter (Pc = 0.064, Fig. 2A).
Fig. 2.
Increased expression levels of mRNA for perforin and granzyme B is associated with removal of type II (ME49) cysts by CD8+ immune T cells primed with a type II (ME49) or a type III (CEP) strain of T. gondii. SCID mice were infected with 20 cysts of the ME49 strain perorally and treated with sulfadiazine beginning at 9 days after infection to establish a chronic infection in their brains. At 3 weeks after infection, the mice received a systemic transfer of CD8+ immune T cells (7.5 × 106 cells) from BALB/c mice infected with the ME49 or CEP strain. As a control, a group of infected SCID mice received CD8+ normal T cells from uninfected BALB/c mice. Amounts of mRNA for perforin (A) and granzyme B (B) were measured at 7 days after the T cell transfer (Day 7). All data represent mean ± SEM. There were 4–5 mice in each experimental group. *Pc < 0.05.
Perforin is critical for cytotoxic activity of CD8+ T cells. Granzyme B is also important for cytotoxic activity of CD8+ T cells. Amounts of mRNA for granzyme B in the brains of SCID mice with the ME49-primed or CEP-primed CD8+ immune T cells were 42 and 29 times greater than those in the brains of the control animals with no T cells, respectively (Pc < 0.05, Fig. 2B). In contrast, granzyme B mRNA levels in the brains of SCID mice that had received CD8+ normal T cells did not differ from those of the control animals that had received no T cells (Fig. 2B). These results further support that CD8+ immune T cells primed with either a type II or a type III strain both are able to recognize host cells containing T. gondii cysts of a type II strain and activate their cytotoxic activity to initiate removal of the cysts from the brain.
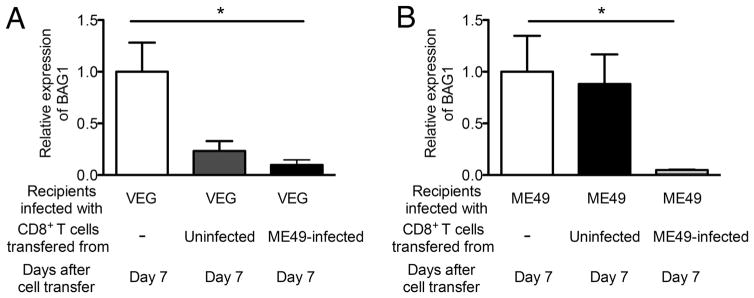
To further confirm the capability of CD8+ T cells to remove T. gondii cysts of different genotypes by recognizing epitopes commonly present in both type II and III of the parasite, we first attempted to infect SCID mice with the CEP strain and treated with sulfadiazine to establish a chronic infection. However, even with the sulfadiazine treatment, the animals were unable to control the parasite and developed progressive severe illness. Therefore, we next infected SCID mice with another type III (VEG) strain and treated with sulfadiazine. These animals were able to control the parasite and established a chronic infection. The VEG-infected SCID mice received type II (ME49)-primed CD8+ immune T cells from infected BALB/c mice. As a positive control, ME49-infected SCID mice received the ME49-primed CD8+ immune T cells. As a negative control, infected SCID mice received no T cells. At 7 days after the T cell transfer, amounts of mRNA for bradyzoite-specific BAG1 were 90% less in the brains of VEG-infected SCID mice that had received the ME49-primed CD8+ immune T cells than the negative control mice that received no T cells (Pc < 0.05, Fig. 3A). Numbers of cysts in the brains of VEG-infected SCID mice were too small to perform microscopic examination of cyst numbers, although a cyst was detected in one mouse from the control group. Amounts of BAG1 mRNA in the brains of mice that had received CD8+ normal T cells tended to be less than the control animals that had received no T cells, but such differences did not reach statistical significance (Fig. 3A). In ME49-infected SCID mice, as expected, amounts of mRNA for bradyzoite-specific BAG1 were markedly (95%) less in the brains of the mice that had received the ME49-primed CD8+ T cells when compared to the negative control mice that did not receive any T cells (Pc < 0.05, Fig. 3B). Therefore, the ME49 (type II)-primed CD8+ immune T cells are able to remove cysts of both ME49 (type II) and VEG (type III) strains. These results further confirm that CD8+ immune T cells are capable of recognizing epitopes of T. gondii antigens commonly present in type II or type III strains or cross-reactive between these two genotypes to initiate the immune process for cyst removal.
Fig. 3.
CD8+ immune T cells primed with a type II (ME49) strain of T. gondii are capable of removing cysts of a type III (VEG) strain from the brains of SCID mice. SCID mice were infected with 20 cysts of the ME49 perorally or 1 × 104 tachyzoites of the VEG strain intraperitoneally, and treated with sulfadiazine beginning at 8 or 9 days after infection to establish a chronic infection in their brains. At 3 weeks after infection, the mice received a systemic transfer of CD8+ immune T cells (3.5 × 106 cells) from ME49-infected BALB/c mice. As a control, a group of infected SCID mice received CD8+ normal T cells from uninfected BALB/c mice. Amounts of BAG1 mRNA were measured at 7 days after the T cell transfer (Day 7). All data represent mean ± SEM. *Pc < 0.05.
Reactivation of chronic infection with T. gondii is an important medical problem in immunocompromised patients as mentioned earlier [1,5,6]. Even in immunocompetent individuals, recent epidemiological studies demonstrated that T. gondii infection is associated with a 1.8-fold increase in the risk of brain cancers [18], and that brain cancer mortality rates increase with seroprevalence of IgG antibodies to T. gondii [19]. Therefore, it is important to develop a method to eliminate T. gondii cysts from chronically infected individuals. However, there is no current drug that can target the cyst stage of the parasite. The present study demonstrated that CD8+ immune T cells are capable of recognizing epitopes commonly expressed by type II and III parasites or cross-reactive between these two genotypes to induce removal of the cysts. This information is valuable to evaluate possible candidates of the epitope for determining dominant CD8+ T cells epitopes for cyst removal. Type II infections have been recognized as predominant in North American and European populations [11,12,20,21], and type III is the second most common among isolates from immunocompromised patients in Europe [12]. Therefore, developing an immunization method to induce CD8+ immune T cells capable of removing T. gondii cysts of both type II and type III strains, and possibly other strains as well, appears to be an important and valuable treatment and preventive approach to eliminate chronic infection with this parasite.
Acknowledgments
The studies are supported in part by NIH grants (AI095032, AI073576, and AI078756) (Y.S.). This work was also supported, in part, by the Intramural Research Program of the National Institutes of Health, NIAID (M.E.G.). M.E.G. is a Scholar of the Canadian Institute for Advanced Research (CIFAR) Integrated Microbial Biodiversity program.
Footnotes
Conflict of interest
All authors declare that there is no conflict of interest.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Sa Q, Gehman M, Ochiai E. Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert Rev Mol Med. 2011;13:e31. doi: 10.1017/S1462399411002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240:269–85. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 5.Israelski DM, Remington JS. Toxoplasmosis in the non-AIDS immunocompromised host. Curr Clin Top Infect Dis. 1993;13:322–56. [PubMed] [Google Scholar]
- 6.Wong SY, Remington JS. Toxoplasmosis in the setting of AIDS. In: Broder S, Mergan TC, Bolognesi D, editors. Text book of AIDS medicine. Baltimore: Williams & Wikins; 1994. pp. 223–57. [Google Scholar]
- 7.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol. 2010;176:1607–13. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73:483–91. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- 9.Ghatak NR, Zimmerman HM. Fine structure of Toxoplasma in the human brain. Arch Pathol. 1973;95:276–83. [PubMed] [Google Scholar]
- 10.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–6. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 11.Howe DK, Honore S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol. 1997;35:1411–4. doi: 10.1128/jcm.35.6.1411-1414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajzenberg D, Yera H, Marty P, Paris L, Dalle F, Menotti J, et al. Genotype of 88 Toxoplasma gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. J Infect Dis. 2009;199:1155–67. doi: 10.1086/597477. [DOI] [PubMed] [Google Scholar]
- 13.Ochiai E, Sa Q, Brogli M, Kudo T, Wang X, Dubey JP, et al. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. Am J Pathol. 2015;185:314–24. doi: 10.1016/j.ajpath.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers L, Wang X, Wen X, Dunford B, Miller R, Suzuki Y. Strains of Toxoplasma gondii used for tachyzoite antigens to stimulate spleen cells of infected mice in vitro affect cytokine responses of the cells in the culture. Parasitol Res. 2005;97:332–5. doi: 10.1007/s00436-005-1416-5. [DOI] [PubMed] [Google Scholar]
- 15.Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science. 2001;294:161–5. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, Suzuki Y. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect Immun. 2001;69:2920–7. doi: 10.1128/IAI.69.5.2920-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sa Q, Ochiai E, Sengoku T, Wilson ME, Brogli M, Crutcher S, et al. VCAM-1/α4β1 integrin interaction is crucial for prompt recruitment of immune T cells into the brain during the early stage of reactivation of chronic infection with Toxoplasma gondii to prevent toxoplasmic encephalitis. Infect Immun. 2014;82:2826–39. doi: 10.1128/IAI.01494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, Misse D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol Lett. 2012;8:101–3. doi: 10.1098/rsbl.2011.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vittecoq M, Elguero E, Lafferty KD, Roche B, Brodeur J, Gauthier-Clerc M, et al. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect Genet Evol. 2012;12:496–8. doi: 10.1016/j.meegid.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Ajzenberg D, Cogne N, Paris L, Bessieres MH, Thulliez P, Filisetti D, et al. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186:684–9. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- 21.Pernas L, Ramirez R, Holmes TH, Montoya JG, Boothroyd JC. Immune profiling of pregnant Toxoplasma-infected US and Colombia patients reveals surprising impacts of infection on peripheral blood cytokines. J Infect Dis. 2014;210:923–31. doi: 10.1093/infdis/jiu189. [DOI] [PMC free article] [PubMed] [Google Scholar]