Abstract
Objective
The dopamine D2/3 receptor subtypes (DRD2/3) are the most widely studied neurotransmitter biomarker in research on obesity, but results to date have been inconsistent, have typically involved small samples, and have rarely accounted for subjects’ ages despite the large impact of age on DRD2/3 levels. We aimed to clarify the relation between DRD2/3 availability and BMI by examining this association in a large sample of subjects with BMI spanning the continuum from underweight to extremely obese.
Subjects
130 healthy subjects between 18 and 81 years old underwent PET with [18F]falllypride, a high affinity DRD2/3 ligand.
Results
As expected, DRD2/3 availability declined with age. Critically, age significantly interacted with DRD2/3 availability in predicting BMI in the midbrain and striatal regions (caudate, putamen, and ventral striatum). Among subjects under 30 years old, BMI was not associated with DRD2/3 availability. By contrast, among subjects over 30 years old, BMI was positively associated with DRD2/3 availability in the midbrain, putamen, and ventral striatum.
Conclusion
The present results are incompatible with the prominent dopaminergic hypofunction hypothesis that proposes that a reduction in DRD2/3 availability is associated with increased BMI, and highlights the importance of age in assessing correlates of DRD2/3 function.
Keywords: BMI, obesity, dopamine D2 receptor, striatum, midbrain, aging
1. Introduction
Obesity and its complications are the leading causes of preventable death in the U.S. (1). With over one-third of adults and nearly one-fifth of children meeting criteria for obesity, the need to understand the causes and consequences of obesity has never been greater (2). Research exploring the brain’s contributions to obesity have suggested the possible importance of dopamine functioning, which has been associated with body mass index (BMI), food intake, anticipatory response to reward, and responses to food restriction and other weight loss measures (3–7). Within the dopamine system, the dopamine D2/3 receptor subtypes (DRD2/3) have been the most widely studied biomarker in relation to obesity. The Taq1A minor (A1) allele of the DRD2/3 gene is associated with lower DRD2/3 density and has been found to exist in higher frequencies in obese subjects (8–11). A landmark study in 2001 reported that striatal DRD2/3 availability was reduced in extremely obese subjects relative to control subjects and that striatal DRD2/3 availability correlated negatively with BMI in obese subjects (3). These findings gave rise to the prominent dopaminergic hypofunction hypothesis of obesity wherein it is speculated that reduced DRD2/3 availability plays a central role in a reduced response to the hedonic value of food that leads to compensatory overconsumption (3, 12).
Since the initial proposal of the dopaminergic hypofunction account of obesity, several findings suggest that the relation between DRD2/3 and obesity might not reflect a simple reduction in DRD2/3 availability. A few studies with large sample sizes have reported no association between Taq1A and markers of obesity such as BMI (13–15). As a genetic marker, Taq1A polymorphism offers only an indirect assessment of DRD2/3 expression and explains only part of the variance of DRD2/3 availability. In vivo assessment with PET provides a more direct index of DRD2/3 availability, but to date reports of associations between BMI and DRD2/3 availability measured with PET have also been inconsistent (3, 5, 16–21). Although a few PET studies supported the initial finding that lean subjects had greater DRD2/3 availability than higher BMI subjects (5, 18, 20–23), several studies observed no relation (5, 17, 24–26) or a correlation in the opposite direction (16, 18, 19, 26, 27) (Table 1). Several factors may have contributed to these inconsistencies in the current literature. Most of the studies observing lower binding in obesity compared extremely obese subjects (BMI > 40) with normal weight subjects, and these findings may not generalize to other BMI ranges, especially in light of evidence that extreme obesity may reflect a state of aberrance distinct from other BMI categories (28). Additionally, the sample sizes of most PET studies were small, with most having less than two dozen subjects. Because of their wide confidence intervals in estimating correlations (29), these small sample sizes have likely limited the potential of past PET studies to provide clarity on this association.
Table 1.
Previous PET studies reporting associations between BMI and DRD2/3 availability
| Authors | Age (mean ± SD years) |
BMI (mean ± SD) |
Ligand | Findings | Age effects ? |
|---|---|---|---|---|---|
| Positive BMI-BPnd associations | |||||
| Caravaggi o et al. 2015 |
26 healthy normal: 30 ± 7, range 20–45 |
24 ± 3 | PET- [11C]PHNO |
Ventral striatal BPnd positively related to BMI. |
No effect of age on BMI or BPnd. |
| Cosgrove et al. 2015 |
12 healthy normal: 28 ± 6, range 20–37 |
28 ± 5 | PET- [11C]PHNO |
Caudate BPnd positively related to BMI. |
No effect of age on BMI or BPnd. |
| Dunn et al. 2010 |
14 obese: 40 ± 8 | 40 ± 5 | PET- [18F]fallypri de |
Caudate BPnd positively related to BMI. |
na |
| 8 control: 40 ± 9 | 23 ± 2 | ||||
| Guo et al. 2014 |
20 obese: 35 ± 2, range 18–45 |
36 ± 1 | PET- [18F]fallypri de |
Dorsal/lateral striatal BPnd positively related to BMI. |
No effect of age on BMI or BPnd. Age used as nuisan ce covaria te. |
| 23 control: 28 ± 1, range 18–45 |
22 ± 1 | ||||
| Yasuno et al. 2001 |
16 healthy normal: 26 ± 5, range 21–35 |
range 20–26 |
PET- [11C]FLB 457 |
Amygdala BPnd positively related to BMI. |
na |
| Negative BMI-BPnd associations | |||||
| de Weijer et al. 2011 |
15 obese: 38 ± 7, range 26–49 |
47 ± 7 | SPECT- [123I]IBZM |
Striatal BPnd lower in obese than control. |
No effect of age on BPnd. Age used as nuisan ce covaria te. |
| 15 control: 28 ± 10, range 20–60 |
22 ± 2 | ||||
| Frank et al. 2005 |
10 recovered anorexics: 24 ± 5 |
22 ± 3 | PET- [11C]raclopr ide |
Antero-ventral striatal BPnd higher in recovered anorexics than control. |
No effect of age on BPnd. |
| 12 control: 27 ± 6 | 23 ± 2 | ||||
| Guo et al. 2014 |
20 obese: 35 ± 2, range 18–45 |
36 ± 1 | PET- [18F]fallypri de |
Ventromedial striatal BPnd negatively associated with BMI |
No effect of age on BMI or BPnd. Age used as nuisan ce covaria te. |
| 23 control: 28 ± 1, range 18–45 |
22 ± 1 | ||||
| Haltia et al. 2007 |
12 obese: 25 ± 2.5 |
33 ± 5 | PET- [11C]raclopr ide |
Striatal and thalamus BPnd lower in obese than control |
na |
| 12 control: 26 ± 4.5 |
22 ± 1 | ||||
| Steele et al. 2010 |
5 gastric bypass patients: 20–38 (mean 32) |
40–53 (mean 45) |
PET- [11C]raclopr ide |
BPnd increased after gastric bypass surgery. |
na |
| 5 control: mean 22 |
mean 21 |
||||
| Volkow et al. 2008* |
10 obese: 36 ± 10, range 20–55 |
51 ± 5 | PET- [11C]raclopr ide |
Striatal BPnd lower in obese than control. |
na |
| 12 control: 33 ± 8, range 20–55 |
25 ± 3 | ||||
| Wang et al. 2001 |
10 obese: 39 ± 7, range 26–54 |
51 ± 5 | PET- [11C]raclopr ide |
Striatal BPnd lower in obese than control. BMI correlated negatively with BPnd in obese. |
Age negativ ely correlat ed with BPnd in control. Age used as nuisan ce variabl e. |
| 10 control: 38 ± 6, range 25–45 |
25 ± 3 | ||||
| No BMI-BPnd associations | |||||
| Caravaggi o et al. 2015 |
35 healthy normal: 31 ± 9, range 20–47 |
23 ± 3 | PET- [11C]raclopr ide |
No correlation between ventral striatal BPnd and BMI. |
No effect of age on BMI or BPnd |
| Eisenstein et al. 2013 |
15 obese: 33 ± 6, range 25–41 |
40 ± 5 | PET- [11C]NMB |
No striatal BPnd diff. between obese and control. Striatal BPnd not correlated with BMI. |
Putam en BPnd negativ ely correlat ed with age |
| 15 control: 30 ± 6, range 22–40 |
23 ± 2 | ||||
| Karlsson et al. 2015 |
13 obese: 39 ± 11 |
42 ± 4 | PET- [11C]raclopr ide |
No BPnd diff. between obese and control in any brain region. |
na |
| 14 control: 45 ± 13 |
23 ± 3 | ||||
| Steele et al. 2010 |
5 gastric bypass patients: 20–38 (mean 32) |
40–53 (mean 45) |
PET- [11C]raclopr ide |
No striatal BPnd diff. between patients and control. |
na |
| 5 control: mean 22 |
mean 21 |
||||
reanalysis of data from Wang et al. 2001
The present study aimed to clarify the relation between DRD2/3 availability and BMI by examining this often-cited association in a large sample of subjects. To ensure adequate statistical power, we assessed 130 subjects, which is more than three times the sample size of the previous largest PET study examining this question. Instead of contrasting an extremely obese group with a normal weight group, we included subjects spanning the range from mildly underweight to extremely obese in order to examine how DRD2/3 availability relates to the whole BMI spectrum. Lastly, in contrast to the majority of previous PET studies that utilized the tracer [11C]raclopride, here we used [18F]fallypride, which has higher affinity for DRD2/3 than [11C]raclopride and yields higher target-to-background signal for DRD2/3 availability (30).
Furthermore, although the relation between DRD2/3 availability and BMI has received considerable attention in recent years, few studies have examined how this relation may change with age, particularly in humans. The dopamine system undergoes significant changes during aging, and some associations between dopamine and reward functions differ at different life stages (31, 32). Given that both BMI and DRD2/3 availability change across the lifespan, age represents a major potential confound in this literature. As obesity occurs in all stages of life, knowledge of how age influences the link between DRD2/3 availability and BMI may impact the development of effective prevention or treatment for obesity. Indeed, if the dopamine hypofunction hypothesis is correct, one might expect the negative relation between BMI and DRD2/3 receptors to increase with age, given a greater time span for the influence of receptors on BMI and the natural decline in DRD2/3 receptors that occurs with aging. We therefore examined the interaction of age on DRD2/3 availability and BMI to assess how this association changes across the lifespan.
2. Methods
2.1 Subjects
130 healthy subjects (age: 35.6 ± 18.2 years, 72 females, BMI: 25.5 ± 4.8) participated in this study. Among the 4 major BMI categories, 3 subjects were underweight (BMI<18.5), 63 were in the normal range (BMI=18.5–24.9), 46 were overweight (BMI=25–29.9), and 18 were obese (BMI>30; 3 of 18 reached criteria for extreme obesity with BMI >40). Subjects were part of three separate studies examining different questions in our lab. Two studies involved subjects between 18 and 30 years old. The third study included subjects from 18 to 81 years old. Together there were 73 subjects under 30 years old and 57 subjects over 30 years old. Subjects were excluded if they reported any history of psychiatric illness in a screening interview (a Structured Clinical Interview for DSM-IV Diagnosis (33) was also available for all subjects and confirmed no history of major Axis I disorders). Subjects were also excluded if they had any history of head trauma, any significant medical condition, or any condition that would interfere with MRI (e.g. claustrophobia or metal implants). Subjects with major medical disorders including diabetes and/or abnormalities on a comprehensive metabolic panel or complete blood count were excluded. Subjects were also excluded if they reported a history of substance abuse, current tobacco use, alcohol consumption greater than 8 ounces of whiskey or equivalent per week, use of psychostimulants (excluding caffeine) in the past 6 months, or any psychotropic medication in the last 6 months other than occasional use of benzodiazepines for sleep. Any illicit drug use in the last 2 months was grounds for exclusion, even in subjects who did not otherwise meet criteria for substance abuse. Urine drug tests were administered, and subjects testing positive for the presence of amphetamines, cocaine, marijuana, PCP, opiates, benzodiazepines, or barbiturates were excluded. Written informed consent was obtained from all subjects. This study was approved by the Institutional Review Board at Vanderbilt University and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
2.2 Physical exam
Weight and height were measured by a clinician during each subject’s physical exam, which was conducted to ensure that subjects met eligibility for MRI and PET scanning.
2.3 MRI data acquisition
Structural MRI scans were performed on two identically configured 3 Tesla Phillips Achieva scanners located at the Vanderbilt University Institute for Imaging Science (VUIIS). T1-weighted high-resolution 3D anatomical scans (1×1×1mm resolution) were obtained for each participant to aid coregistration and normalization of PET images.
2.4 PET data acquisition
PET imaging was performed on a GE Discovery STE scanner located at Vanderbilt University Medical Center. The scanner has axial slices of 3.25 mm and in-plane pixel dimensions of 2.3×2.3 mm (with estimated FWHM of 4.5–5.5 mm near the center of the field of view). [18F]fallypride ((S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3[18F]fluoropropyl)-2,3-dimethoxybenzamide) was produced in the radiochemistry laboratory attached to the PET unit, following synthesis and quality control procedures described in US Food and Drug Administration IND 47,245. [18F]fallypride is a substituted benzamide with very high affinity to D2/D3 receptors (34).
3D emission acquisition scans were performed following a 5.0 mCi slow bolus injection of [18F]fallypride (specific activity greater than 3000 Ci/mmol). CT scans were collected for attenuation correction prior to each of the three emission scans, which together lasted approximately 3.5 hours, with two 15-minute breaks for subject comfort. PET images were reconstructed with decay correction, attenuation correction, scatter correction, and calibration.
2.5 [18F]fallypride binding potential (BPND) image calculation
Voxelwise D2/D3 binding potential images were calculated using the simplified reference tissue model, which has been shown to provide stable estimates of [18F]fallypride BPND (35). The cerebellum was the reference region because of its relative lack of D2/D3 receptors (36). The cerebellar reference region was obtained from an atlas provided by the ANSIR laboratory at Wake Forest University. Limitations in PET spatial resolution introduce blurring and cause signal to spill onto neighboring regions. Because the cerebellum is located posterior and adjacent to the midbrain, the location of dopamine neurons, only the posterior 3/4 of the cerebellum was included in the ROI to avoid contamination of [18F]fallypride signal from the midbrain. The cerebellum ROI also excluded voxels within 5mm of the cortex to prevent contamination of cortical signals. The putamen ROI, drawn according to guidelines by Mawlawi et al. (37) on the MNI brain, served as the receptor rich region in the analysis. The putamen, unlike other striatal ROIs, is not adjacent to any ventricle so the putamen ROI is free from ventricle-related partial volume effects. The cerebellum and putamen ROIs were registered to each subject’s T1 image using FSL non-linear registration of the MNI template to individual subject T1. T1 images and their associated cerebellum and putamen ROIs were then coregistered to the mean image of all realigned frames in the PET scan using FSL-FLIRT (http://www.fmrib.ox.ac.uk/fsl/, version 6.00). Emission images from the 3 PET scans were merged temporally into a 4D file. To correct for motion during scanning and misalignment between the 3 PET scans, all PET frames were realigned using SPM8 to the frame acquired 10min post injection (www.fil.ion.ucl.ac.uk/spm/). Model fitting and BPND calculation were performed using the PMOD Biomedical Imaging Quantification software (PMOD Technologies, Switzerland). Binding potential images represent the ratio of specifically bound ligand ([18F]fallypride in this study) to its free concentration.
2.6 [18F]fallypride BPND and BMI correlations
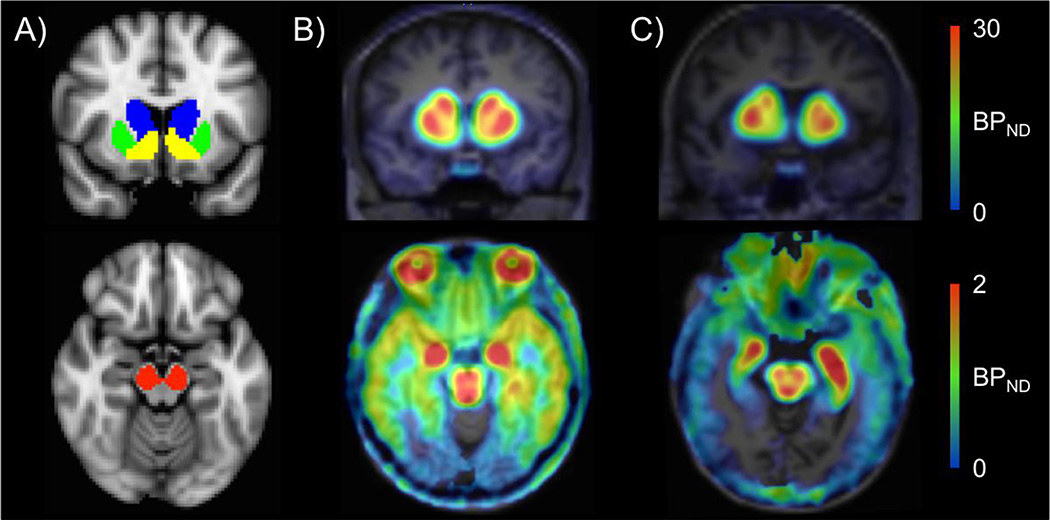
Relations between [18F]fallypride BPND and BMI were assessed with both voxelwise and ROI approaches. In all voxelwise and ROI analyses, gender was entered as a covariate of no-interest. Unless explicitly stated, age was also entered as a covariate of no interest. In voxelwise analyses, implemented in SPM8, BMI, age, and gender were regressed against [18F]fallypride BPND with familywise error correction and small volume correction with a striatal mask consisting of all three striatal ROIs (caudate, putamen, and ventral striatum). We also ran voxelwise analyses without small volume correction to examine associations between BMI and [18F]fallypride BPND in extrastriatal brain areas. In ROI analyses, mean binding potential in the midbrain and 3 striatal ROIs were extracted to regress against BMI with age and gender as covariates of no interest. The midbrain and striatal ROIs were drawn in MNI standard space using previously described guidelines (37–39) and registered to PET images using the same transformations for cerebellum registration to PET images (Fig. 1).
Fig 1.
ROIs and binding potential (BPND) images. A) Striatal (top) and midbrain (bottom) ROIs used for extracting BPND. B) One under 30-year-old subject’s and C) one over 30-year-old subject’s [18F]fallypride BPND images in native PET space. BPND, which declined with age, was highest in the striatum (top) and the midbrain (bottom). Note that the BPND maps use different scales to reflect the large differences in BPND values in striatal and extrastriatal brain regions.
To examine effects of age on the relation between BPND and BMI, we reran the ROI regressions described above with an age by BPND interaction term in the models predicting BMI, with gender as a covariate of no interest. In these analyses age in years was entered as a continuous variable spanning the entire age range of the sample. To more fully characterize the nature of the observed interaction, we further divided subjects into those under and those over 30 years old. We note that the selection of age 30 as a dividing line for grouping subjects is arbitrary, but it was consistent with the preexisting cutoff point for the two studies of young adults included in these analyses. To verify that this grouping captured the interaction and to better understand its spatial representation within the striatum, we performed a follow-up voxelwise analysis contrasting regression slopes for BMI and BPnd between the two age groups (<30 vs. > 30). We then reran regressions between BPND in each ROI and BMI for each age group separately. We converted regression results into Pearson’s r for ease of comparison across the two age groups. Finally, we performed voxelwise analyses regressing BMI on BPND separately for the under and over 30 year old groups to characterize the distribution of associations within the striatum.
3. Results
3.1 BPND and BMI across all ages
Voxelwise analyses (controlling for age and gender) did not identify any brain region showing a significant association between BPND and BMI at the whole brain level (N=130). To confirm this result in DRD2/3 rich areas, we performed ROI analyses regressing BPND from the 3 a priori striatal ROIs and the midbrain ROI against BMI, again controlling for age and gender. We applied Bonferroni correction to counteract the issue of multiple comparisons with 4 ROIs and utilized a corrected significance threshold of p<0.0125. In these more targeted analyses, BMI demonstrated a positive association with putamen BPND at the p-corrected threshold (r=0.27, p=0.002), with putamen BPND explaining 7.5% of the variance in BMI. We note that the observed relation between BMI and putamen BPND was positive, which stands in sharp contrast to the predictions of the dopamine hypofunction hypothesis. No relation was observed between BMI and midbrain, ventral striatum, or caudate BPND across the whole age range (Table 2.A).
Table 2.
Correlations between BPND and BMI across all ages
| A) Model without BPND by age interaction | r | p-value | ||
| midbrain | 0.10 | 0.244 | ||
| putamen | 0.27 | 0.002** | ||
| caudate | 0.16 | 0.063 | ||
| ventral striatum | 0.13 | 0.134 | ||
| B) Model with BPND by age interaction | BPNDby age interaction |
|||
| r | p-value | r | p-value | |
| midbrain | −0.12 | 0.179 | 0.20 | 0.023* |
| putamen | −0.07 | 0.425 | 0.23 | 0.009** |
| caudate | −0.10 | 0.284 | 0.22 | 0.011** |
| ventral striatum | −0.13 | 0.146 | 0.23 | 0.010** |
p-uncorrected,
p-corrected
BMI increased with age (r=0.27, p<0.002) while BPND substantially decreased with age (r<−0.6, p<10−10 for all ROIs). In light of evidence that age was related to both BPND and BMI, we sought to examine whether the relationship between BMI and BPND differs across adulthood. We therefore tested for an interaction of age (in years) and BPND on BMI across all 130 subjects. The interaction of age and BPND on BMI was significant in all 3 striatal ROIs at the p-corrected threshold (r=0.23, p=0.009 for putamen, r=0.22, p=0.011 for caudate, and r=0.23, p=0.010 for ventral striatum) and in the midbrain at the p-uncorrected threshold (r=0.20, p=0.023) (Table 2.B). There was not an additional main effect of putamen BPND on BMI (p=0.425) after including the significant age by BPND interaction in the model. In other words, rather than a consistent relation between putamen BPND and BMI across ages, the relationship (or lack thereof) differed based on the age of the subjects.
The above interaction analyses treated BMI as the dependent variable based on the causal direction implied by the dopaminergic hypofunction hypothesis in which D2/D3 receptor levels influence BMI. However, an alternative hypothesis could be that obesity leads to declines in D2/D3 receptor availability. We tested for this reverse causal direction in a regression model in which BMI and age interact to predict BPnd, These analyses did not reveal significant interactions in any of the ROIs (all p-values >0.5).
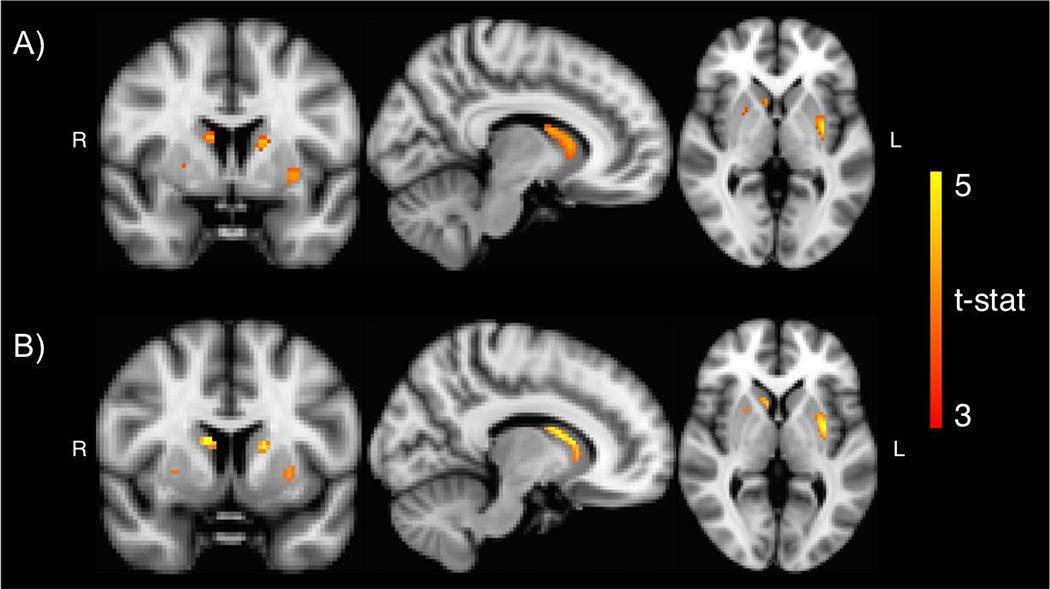
To characterize the interaction, we divided the sample into those above or below age 30. Voxelwise analysis contrasting regression slopes for BMI and BPnd between the under and over 30-year-old groups confirmed that the relation between BPND and BMI differed between those under and those over 30 years old, particularly in the caudate and putamen (Fig. 2A).
Fig 2.
Voxelwise results. A) Age group by BMI interaction analysis showed that the relation between BMI and BPND differed between those under and those over 30 years old in both the caudate and putamen (peak t-stat=5.21, peak coordinate: x=−32, y=−6, x=−2). B) Among subjects over 30 years old, BMI was positively associated with BPND in bilateral caudate and bilateral putamen (peak t-stat=5.39, peak coordinate: x=10, y=6, z=14). Results were small-volumecorrected with a striatal mask.
We further characterized the interactions by examining the relation between BPND and BMI separately for subjects under 30 years old and for subjects over 30 years old. We note that these follow-up analyses were performed not to separately test for relations observed in the above analyses, but to clarify the nature and direction of the observed interaction.
We did not observe an interaction of gender and BPnd on BMI (all p-values > 0.3), suggesting that although the Dunn et al. study included only females, relations between BPnd and BMI do not vary by gender. Furthermore, we controlled for gender in all our analyses.
3.2 BPND and BMI among subjects under 30 years old
ROI analyses of subjects under age 30 (with gender and age entered as covariates of no interest) revealed no significant associations between BMI (range: 17.5–36.3, mean=24.1) and BPND in the midbrain and all striatal ROIs (Table 3). Similarly, voxelwise analysis did not identify any brain region showing a significant (small-volume-corrected) association between BMI and BPND in this young adult age group.
Table 3.
Correlations between BPND and BMI for under and over 30 year old groups
| all BMI values | BMI < 40 | |||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Under 30 years old (N=73) | ||||
| midbrain | −0.10 | 0.393 | na | na |
| putamen | −0.09 | 0.446 | na | na |
| caudate | −0.15 | 0.224 | na | na |
| ventral striatum | 0.00 | 0.978 | na | na |
| Over 30 years old (N=57) | ||||
| midbrain | 0.35 | 0.010** | 0.26 | 0.058 |
| putamen | 0.48 | 0.000** | 0.35 | 0.011** |
| caudate | 0.33 | 0.015* | 0.22 | 0.125 |
| ventral striatum | 0.4 | 0.002** | 0.31 | 0.028* |
p-uncorrected,
p-corrected
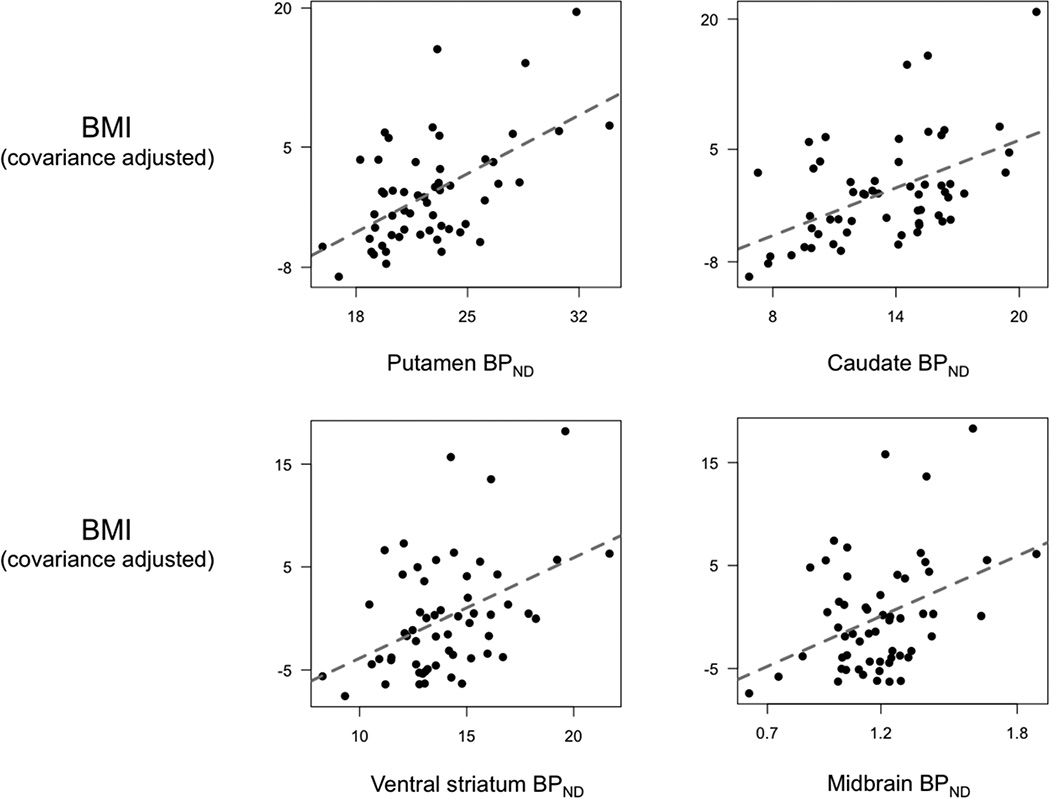
3.3 BPND and BMI among subjects over 30 years old
Age did not correlate with BMI (range: 19.9–44.5, mean=27.4) in this age group (r=−0.07, p=0.603). By contrast, BPND decreased with age in all ROIs (r=− 0.46, p<0.001 for midbrain, r=−0.57, p<0.001 for putamen, r=−0.81, p<0.001 for caudate, r=−0.41, p=0.002 for ventral striatum). ROI analyses (again controlling for age and gender) confirmed that BMI was significantly positively associated with BPND in the midbrain and all 3 striatal ROIs, although only at the p-uncorrected threshold for the caudate (Fig. 3 and Table 3). Three subjects in this age group were in the extremely obese category (BMI>40) and may have magnified the correlations between BMI and BPND. To verify that these subjects did not unduly influence the results, we excluded these 3 subjects and reran the analyses. Without subjects having BMI over 40, the positive correlations between BMI and BPND remained significant in the putamen at the p-corrected threshold and ventral striatum at the p-uncorrected threshold (3). Finally, to better understand the spatial distribution of the observed association, we performed voxelwise analysis examining the association between BMI and BPND controlling for age and gender. This analysis revealed that BPND was positively associated with BMI in the caudate and putamen (Fig. 2B) in an area that largely overlapped with the area identified in the voxelwise analysis testing for the interaction with age group and BMI (Fig. 2A).
Fig 3.
BMI and BPND among subjects over 30 years old. ROI analyses confirmed that BMI correlated positively with BPND in the midbrain, putamen, and ventral striatum at the p-corrected threshold, and the caudate at the p-uncorrected threshold.
Concerning a path of influence between age, BPnd, and BMI, since age was not correlated with BMI, age did not have a direct effect on BMI and can only affect BMI via BPnd, which did correlate with BMI. Therefore, among these variables, the path of influence is likely of age affecting BPnd which affects BMI.
3.4 Cutoff point
To confirm that the differential relationship between BMI and BPnd among the two age groups was not an artifact of the 30 year old cutoff point (which was the age cutoff in two of the studies from which data were drawn), we also analyzed the data using a median split (which corresponded to a 26 year old cutoff point) and a mean split (which corresponded to a 36 year old cutoff point). The pattern of results did not change. With a median split, there was again no significant relation between BMI and BPnd among subjects in the age group below the median split point (n=65) (all p-values > 0.20). Among subjects in the age group above the median split point (n=65), BMI again positively correlated with BPnd significantly in all striatal ROIs (r=0.48, p<0.0001 for putamen, r=0.27, p=0.030 for caudate, r=0.33, p=0.009 for ventral striatum) and at trend level in the midbrain (r=0.24, p=0.060). With a 36 year old cutoff point, we also did not observe any significant relationship between BMI and BPnd in the under 36-year old group (n=83, all p-values>0.10). Among subjects over 36 years old, putamen BPnd remained positively associated with BMI (r=0.33, p-value=0.023) at the smaller sample size (n=47). These additional analyses further confirmed the findings that relations between BMI and BPnd differed across age groups.
4. Discussion
In the largest study to date to assess DRD2/3 availability in vivo in obesity and weight research, we observed relations between DRD2/3 availability and BMI that were dependent upon the age of the subjects studied. The interaction between age and DRD2/3 availability in predicting BMI was significant in the midbrain and all three striatal ROIs. Among subjects under 30 years old, BMI was not associated with DRD2/3 availability in the striatum or the midbrain. However, among subjects over 30 years old, BMI was positively associated with DRD2/3 availability in both the midbrain and the striatum.
The dopaminergic hypofunction hypothesis of obesity states that lower dopamine function leads to deficits in neural reward responses, resulting in compulsive eating and consequently obesity (6, 40). Early reports of lower DRD2/3 density, indexed with the Taq1A gene, in obese subjects and negative associations between DRD2/3 availability and BMI led to the proposal that reduced DRD2/3 availability plays a causal role in altered reward processing in obesity (3, 8, 22). The present results are incompatible with the dopaminergic hypofunction hypothesis, at least as typically formulated in regards to DRD2/3. In young adults, when dopamine function is presumably optimal, individual differences in DRD2/3 availability showed no relation to BMI. Our observation of a positive association between DRD2/3 availability and BMI in adults over age 30 runs directly counter to the predictions of the dopaminergic receptor hypofunction model.
The causal factors leading to the positive association between BMI and DRD2/3 in the older age range but not the younger age range are not immediately clear. If the associations were driven by DRD2/3 receptor availability influencing food consumption, one would predict that this would already have an impact on BMI in young adults. One study using both PET tracers [11C]-(+)-PHNO and [11C]raclopride observed a relationship between BMI and [11C]-(+)-PHNO BPnd but not [11C]raclopride BPnd. Citing evidence that [11C]-(+)-PHNO is more sensitive to dopamine D2 receptor affinity state, the authors proposed that the relation between BMI and [11C]-(+)-PHNO BPnd reflects increasing D2 receptor affinity with higher BMI. Normal aging is associated with numerous changes in dopamine function (31). It may be that beyond a certain age, changes in DRD2 affinity state cause the positive relation between BPnd and BMI to be more prominent and observable with high affinity DRD2/3 ligands, such as 11C]-(+)-PHNO and [18F]fallypride (16, 19, 26). Future studies specifically examining DRD2 affinity and BMI across the lifespan would provide insight into this possibility.
It is possible that methodological issues contribute to some of the inconsistencies that have emerged across studies. In contrast with previous PET studies that examined associations between DRD2/3 availability and BMI, we used the high affinity ligand [18F]fallypride to assess DRD2/3 availability. Previous PET studies that examined the role of DRD2/3 availability in obesity often used the lower affinity ligand [11C]raclopride (3, 5, 21). [11C]raclopride binding is more likely to be displaced by endogenous dopamine release (30, 41), so [11C]raclopride binding potential reflects the combined effects of DRD2/3 availability and dopamine release to a greater degree than [18F]fallypride (42), possibly complicating the interpretation of correlations with [11C]raclopride binding potential especially since dopamine release has been associated with obesity in rodents (43, 44). It is noteworthy that the landmark PET study in 2001 that reported a negative relationship between BMI and DRD2/3 availability used [11C]raclopride (3), whereas PET studies using the higher affinity DRD2/3 ligand [18F]fallypride either reported positive associations between BMI and DRD2/3 availability in the striatum (19) or both positive and negative associations in the striatum, suggesting regional specificity within the striatum. Future studies assessing dopamine release independent of DRD2/3 availability and DRD2 affinity will be necessary to determine whether previous reports of negative associations between [11C]raclopride binding potential and BMI stemmed from a link between BMI and dopamine release and/or DRD2 affinity.
Dopamine plays a critical role in reward processes, including responses to food cues and food intake. Obese individuals habituate to food reward at a slower rate than lean individuals, and high reward sensitivity contributes to overeating (45, 46). If DRD2 availability has a causal influence on reward sensitivity, our findings of a positive association between DRD2/3 availability and BMI suggest that across much of adulthood, higher DRD2/3 availability may affect bodyweight by increasing or maintaining reward sensitivity for food.
Our study is different from some of the previous studies in that we did not focus on extremely obese subjects but instead included individuals along a broad BMI continuum (20). It could be that extreme obesity represents a condition that is distinct from other BMI categories and has a different relationship with DRD2/3 availability; several studies have reported that relations with DRD2/3 availability were different for obese subjects and non-obese subjects (22, 23, 26). Indeed, it has been proposed that, rather than dopaminergic hypofunction influencing BMI, increased dopamine release associated with overeating downregulates dopamine receptor function, leading to the previously observed negative correlation between DRD2/3 availability and BMI in extremely obese subjects but not normal weight subjects (3, 46). There are not enough subjects meeting criteria for extreme obesity in the present study to examine this possibility. However, in our study, positive associations between BMI and DRD2/3 availability among adults over age 30 were as high or higher with extremely obese subjects in the analyses than without. One would have expected the positive association to be reduced when extremely obese participants are included in the analysis if there was a negative association among extremely obese subjects or if there was an inflection point above which the relation between BMI and DRD2/3 reverses. It is notable in this regard that Dunn et al. (2012) found similar positive associations with BMI in an independent sample that included 14 obese women (mean BMI = 40). Taken together, these findings fail to provide evidence for a differential relationship between BMI and DRD2/3 in obese participants. Future clinical studies of the dopamine system exploring differences between extreme obesity and other BMI categories would help answer this question.
The present results suggest the need to play close attention to age when considering relations between dopamine, weight and obesity. While past studies often provide evidence that their obese and nonobese groups do not significantly differ in age, given the robustness of the association between age and DRD2/3 BPND, even relatively modest differences in age could substantially impact results. It is also of note that studies in this area (including the present one) generally pay little attention to the representativeness of samples. To qualify for the studies conducted in our lab, participants had to have no major medical problems (other than obesity), and had to pass a physical exam and blood work (complete blood count and comprehensive metabolic panel). With aging, fewer and fewer potential participants are likely to meet such criteria, and therefore there is a potential bias when selecting healthy subjects in older age groups. This problem is not unique to this study, but may nevertheless influence findings from any study with strict exclusion criteria.
Our findings of a differential relationship between DRD2/3 availability and BMI in different age groups were observed with three different cutoff points for dividing subjects into a younger group and an older group. However, we have not attempted to determine a specific inflection point, whether it is at age 30 vs. age 26 or age 36. The age group categorization was primarily used here to characterize the nature of the observed statistical interaction using the continuous variables. It may prove valuable in future studies to determine if there is a specific age or age range after which the link between BMI and DRD2/3 availability changes. A final caveat is that, like the other PET studies in the field, the present study utilized a cross-sectional design and thus cannot speak to the causality of the current findings. The present study examined the dopaminergic hypofunction hypothesis, which postulates that lower dopamine function as reflected in DRD2 receptors leads to compensatory food intake and consequently obesity. However, it may be that obesity changes dopamine function. Longitudinal data are needed to address this question of causality.
5. Conclusions
The present findings in a large sample of adults demonstrate the importance of age in the relationship between DRD2/3 availability and BMI. Although there was no relation between DRD2/3 and BMI in young adults, a positive relationship emerged later in adulthood. These data provide no support for the idea that lower DRD2/3 plays a casual role in weight gain, and indicate the strong need to incorporate age into the analysis and interpretation of data in the field.
Highlights.
Age interacted with DRD2 availability in predicting BMI.
Among subjects under 30 years old, BMI was not associated with DRD2 availability.
Among subjects over 30 years old, BMI positively associated with DRD2 availability.
Present results are incompatible with the dopaminergic hypofunction hypothesis.
Results highlight the importance of age in assessing correlates of DRD2 function.
Acknowledgments
This study was funded by grants from the National Institute of Aging (R01AG043458 and R01AG044838 to D.H.Z.). L.C.D was funded by F32DA036979. G.R.S-L. was supported by R00AG042596.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010;38(2):138–144. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 4.South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res. 2008;33(3):598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- 5.Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2010;20(3):369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28(52):14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, et al. D2 dopamine receptor gene and obesity. Int J Eat Disord. 1994;15(3):205–217. doi: 10.1002/1098-108x(199404)15:3<205::aid-eat2260150303>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Blum K, Braverman ER, Wood RC, Gill J, Li C, Chen TJ, et al. Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report. Pharmacogenetics. 1996;6(4):297–305. doi: 10.1097/00008571-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Spitz MR, Detry MA, Pillow P, Hu YH, Amos CI, Hong WK, et al. Variant alleles of the D2 dopamine receptor gene and obesity. Nutr Res. 2000;20(3):371–380. [Google Scholar]
- 11.Barnard ND, Noble EP, Ritchie T, Cohen J, Jenkins DJ, Turner-McGrievy G, et al. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition. 2009;25(1):58–65. doi: 10.1016/j.nut.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13(4):279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- 13.Fang YJ, Thomas GN, Xu ZL, Fang JQ, Critchley JA, Tomlinson B. An affected pedigree member analysis of linkage between the dopamine D2 receptor gene TaqI polymorphism and obesity and hypertension. Int J Cardiol. 2005;102(1):111–116. doi: 10.1016/j.ijcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Nisoli E, Brunani A, Borgomainerio E, Tonello C, Dioni L, Briscini L, et al. D2 dopamine receptor (DRD2) gene Taq1A polymorphism and the eating-related psychological traits in eating disorders (anorexia nervosa and bulimia) and obesity. Eat Weight Disord. 2007;12(2):91–96. doi: 10.1007/BF03327583. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson CP, Hanson R, Cray K, Wiedrich C, Knowler WC, Bogardus C, et al. Association of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA with obesity or type 2 diabetes mellitus in Pima Indians. Int J Obes Relat Metab Disord. 2000;24(10):1233–1238. doi: 10.1038/sj.ijo.0801381. [DOI] [PubMed] [Google Scholar]
- 16.Cosgrove KP, Veldhuizen MG, Sandiego CM, Morris ED, Small DM. Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse. 2015;69(4):195–202. doi: 10.1002/syn.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35(9):3959–3965. doi: 10.1523/JNEUROSCI.4744-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry. 2014;19(10):1078–1084. doi: 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, et al. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care. 2012;35(5):1105–1111. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1(1):37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58(11):908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haltia LT, Rinne JO, Merisaari H, Maguire RP, Savontaus E, Helin S, et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse. 2007;61(9):748–756. doi: 10.1002/syn.20418. [DOI] [PubMed] [Google Scholar]
- 24.Eisenstein SA, Gredysa DM, Antenor-Dorsey JA, Green L, Arbelaez AM, Koller JM, et al. Insulin, Central Dopamine D2 Receptors, and Monetary Reward Discounting in Obesity. PLoS One. 2015;10(7):e0133621. doi: 10.1371/journal.pone.0133621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenstein SA, Antenor-Dorsey JAV, Gredysa DM, Koller JM, Bihun EC, Ranck SA, et al. A Comparison of D2 Receptor Specific Binding in Obese and Normal-Weight Individuals Using PET With (N-[C-11]methyl)benperidol. Synapse. 2013;67(11):748–756. doi: 10.1002/syn.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff-Guerrero A. Ventral striatum binding of a dopamine D2/3 receptor agonist but not antagonist predicts normal body mass index. Biol Psychiatry. 2015;77(2):196–202. doi: 10.1016/j.biopsych.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuno F, Suhara T, Sudo Y, Yamamoto M, Inoue M, Okubo Y, et al. Relation among dopamine D-2 receptor binding, obesity and personality in normal human subjects. Neuroscience Letters. 2001;300(1):59–61. doi: 10.1016/s0304-3940(01)01552-x. [DOI] [PubMed] [Google Scholar]
- 28.Mariman ECM, Bouwman FG, Aller EEJG, van Baak MA, Wang P. Extreme obesity is associated with variation in genes related to the circadian rhythm of food intake and hypothalamic signaling. Physiological Genomics. 2015;47(6):225–231. doi: 10.1152/physiolgenomics.00006.2015. [DOI] [PubMed] [Google Scholar]
- 29.Yarkoni T. Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power-Commentary on Vul et al. (2009) Perspect Psychol Sci. 2009;4(3):294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 30.Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64(5):350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav R. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A. 2008;105(39):15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Washington, D.C.: American Psychiatric Publishing, Inc.; 1997. [1/1/1997]. p. 88. [Google Scholar]
- 34.Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol. 1995;22(3):283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 35.Siessmeier T, Zhou Y, Buchholz HG, Landvogt C, Vernaleken I, Piel M, et al. Parametric mapping of binding in human brain of D2 receptor ligands of different affinities. J Nucl Med. 2005;46(6):964–972. [PubMed] [Google Scholar]
- 36.Camps M, Cortes R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience. 1989;28(2):275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- 37.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Dang LC, O'Neil JP, Jagust WJ. Dopamine supports coupling of attention-related networks. J Neurosci. 2012;32(28):9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang LC, O'Neil JP, Jagust WJ. Genetic effects on behavior are mediated by neurotransmitters and large-scale neural networks. Neuroimage. 2012;66C:203–214. doi: 10.1016/j.neuroimage.2012.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinholz J, Skopp O, Breitenstein C, Bohr I, Winterhoff H, Knecht S. Compensatory weight gain due to dopaminergic hypofunction: new evidence and own incidental observations. Nutr Metab. 2008;5 doi: 10.1186/1743-7075-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slifstein M, Hwang DR, Huang Y, Guo N, Sudo Y, Narendran R, et al. In vivo affinity of [18F]fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman primates. Psychopharmacology (Berl) 2004;175(3):274–286. doi: 10.1007/s00213-004-1830-x. [DOI] [PubMed] [Google Scholar]
- 42.Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62(6):399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- 43.Anderzhanova E, Covasa M, Hajnal A. Altered basal and stimulated accumbens dopamine release in obese OLETF rats as a function of age and diabetic status. Am J Physiol-Reg I. 2007;293(2):R603–R611. doi: 10.1152/ajpregu.00301.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang ZJ, Meguid MM. LHA dopaminergic activity in obese and lean Zucker rats. Neuroreport. 1995;6(8):1191–1194. doi: 10.1097/00001756-199505300-00029. [DOI] [PubMed] [Google Scholar]
- 45.Pepino MY, Mennella JA. Habituation to the pleasure elicited by sweetness in lean and obese women. Appetite. 2012;58(3):800–805. doi: 10.1016/j.appet.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbeken S, Braet C, Lammertyn J, Goossens L, Moens E. How is reward sensitivity related to bodyweight in children? Appetite. 2012;58(2):478–483. doi: 10.1016/j.appet.2011.11.018. [DOI] [PubMed] [Google Scholar]