Abstract
Background and Purpose
Acute intracranial occlusion can be associated with “in situ thrombo-occlusion” in relation to pre-existing intracranial atherosclerotic disease (ICAD). We aimed to assess residual stenosis at the site of a target arterial lesion (TAL) to determine whether residual stenosis at the TAL is associated with underlying ICAD.
Methods
One hundred and sixty-three patients who underwent endovascular therapy for M1 middle cerebral artery occlusion and achieved angiographic reperfusion were selected for analysis. The presence of residual stenosis at the TAL was classified using the Arterial Occlusive Lesion (AOL) scale at post-procedural angiography, and the severity of stenosis was grouped into none, mild (<50%), moderate (50–69%), severe (70–99%), and occlusion on post-procedural and follow-up angiography. We also recorded the incidence of instant reocclusion occurring during the procedure and delayed reocclusion detected on follow-up angiography.
Results
Seventy-four patients (45.5%) showed target arterial residual stenosis (AOL 2). As to the TAL etiology, 40 patients were classified into in situ thrombo-occlusion (54.1% of AOL 2 and 24.5% of M1 occlusion). The occurrence of instant or delayed reocclusion was independently associated with a low chance of favorable 3-month outcome. Further, the occurrence of delayed reocclusion was associated with excellent pretreatment collateral-flow and early neurological worsening, but not the severity of residual stenosis at the TAL.
Conclusions
In population with a high prevalence of ICAD, residual stenosis may be attributed to in situ thrombo-occlusion with underlying ICAD about 25% of cases, hindering functional recovery via the occurrence of instant or delayed reocclusion.
Keywords: ischemic stroke, reperfusion, intracranial stenosis
INTRODUCTION
In the newly established era of endovascular therapy (EVT) as a standard treatment for acute proximal vessel occlusive anterior circulation stroke,1–5 the intrinsic pathology of a target arterial lesion (TAL) may be a major determinant toward the success of EVT and subsequent clinical outcome.6 However, the etiologic diagnosis of an acute intracranial occlusion using baseline imaging alone is often challenging,7 as the occlusion may be caused by embolism from proximal or undetermined sources or by intrinsic arterial pathology.
Furthermore, in East Asia, there is a high prevalence of intracranial atherosclerotic disease (ICAD).8 Acute intracranial occlusion can be considered as an ‘in situ thrombo-occlusion (IST)’ in relation to pre-existing ICAD at the site of occlusion, if there is any evidence of residual stenosis at the TAL subsequent to EVT or short-term follow-up angiography and no evidence of any definite proximal sources of embolism.7,9 To examine the presence of residual stenosis at the site of a TAL, the Arterial Occlusive Lesion (AOL) scale which measures the degree of recanalization (none, partial, or complete) may be used.10 The presence of any degree of stenosis with distal flow at the site of a TAL denotes AOL grade 2, which may be a key indicator of underlying or intrinsic TAL pathology (e.g., IST in relation to pre-existing ICAD). However, one of the major issues in grading AOL hinges on the difficulty of distinguishing partial recanalization with a residual thrombus versus underlying stenosis or device-related vasospasm.10
In the light of the above and the high prevalence of ICAD among Asians, we hypothesized that most instances of residual stenosis at a TAL may relate to ‘IST with underlying ICAD’. The aim of this study was to evaluate the baseline and follow-up characteristics of residual stenosis at the site of the TAL following EVT in relation to stroke etiology, along with clinical and vascular outcome, to test this hypothesis.
METHODS
Patients
Consecutive patients from a prospectively maintained acute stroke registry between May 2006 and December 2014 at our institution were retrospectively reviewed and selected. The inclusion criteria in this study were as follows: (1) EVT due to acute anterior circulation stroke with middle cerebral artery (MCA) M1 occlusion, (2) sufficient angiographic reperfusion, which was defined by a modified Treatment in Cerebral Ischemia (mTICI) grade of 2 to 3,10 (3) evaluable angiographic imaging for the measurement of the stenosis at the TAL and grading of collateral flow, and (4) evaluable follow-up angiographic imaging (MR angiography/CT angiography) acquired 5 to 7 days after EVT. Cases in which the cause of M1 occlusion was attributed to non-atheroslerotic conditions (e.g., dissection, vasculitis, or moyamoya disease) were excluded from this analysis. The study protocol of retrospective analysis was approved by the local Institutional Review Board.
Endovascular procedures
All selected patients underwent transfemoral cerebral angiography including injection into both carotid arteries and the dominant vertebral artery through the late venous phase under local or general anesthesia to determine the angioarchitecture of the occluded vessel and to assess collateral flow from all possible sources. Then, EVT targeting the MCA M1 occlusion was initiated, and treatment strategies were selected on the basis of the available therapies at the time of angiography by the attending neurointerventionalist, which included intra-arterial thrombolytic (urokinase or rtPA) infusion, mechanical clot disruption, mechanical thrombectomy (MT) including forced arterial suction thrombectomy or retrievable stent thrombectomy, rescue intra- or extracranial stenting, or a combination of these approaches.11–13 Adjuvant low-dose intra-arterial tirofiban infusion after reperfusion was used in the cases of reocclusion during the procedure.14
Evaluation of angiographic images
All angiographic classification was evaluated by two independent raters (YWK and DHK). Inter-observer disagreement was resolved by consensus. The baseline grade of collateral flow was evaluated according to the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology Collateral Flow Grading System on pre-treatment angiography. As per this angiographic scale, collateral flow can be classified into grades 0 to 4 according to the completeness and rapidity of collateral filling in a retrograde fashion.10 Recanalization and reperfusion status was measured using AOL and mTICI scales,10 and if the TAL showed residual stenosis on final post-procedural angiography (AOL grade 2), the degree of stenosis was measured by the Warfarin-Aspirin Symptomatic Intracranial Disease Study (WASID) method.15 The degree of stenosis was classified as mild (<50%), moderate (50–69%), and severe (70–99%). Further, the degree of stenosis on follow-up (5–7 days) angiographic images were categorized as normal, mild (signal reduction <50%), moderate (signal reduction ≥50%), severe (focal signal loss with the presence of distal flow), and occlusion (sudden cutoff without distal flow void) using MR angiography (MRA; n=151;92.6%).16 In patients who underwent CT angiography (CTA) for follow-up angiographic imaging (n=12; 7.4%), the WASID method was applied to determine the degree of stenosis, and the presence of flow gap on CTA was defined as severe stenosis (with the presence of distal flow) or occlusion (without the presence of distal flow). The kappa coefficient for interobserver agreement was 0.794 for each stenosis grading; specifically, 0.889 for ‘≥50% stenosis (moderate to occlusion)’ and 1.000 for occlusion. Moreover, occurrence of reocclusion during the procedure was recorded prospectively and defined as ‘instant reocclusion (IR)’, while reocclusion detected on follow-up angiography was defined as ‘delayed reocclusion (DR)’.
Etiologic stroke classification
Stroke subtypes were operationally defined in this study to identify the etiology of baseline TAL occlusion with reference to identifiable sources of embolism, the degree of stenosis at the TAL, as detected on the final post-procedural angiography and follow-up angiography, and concomitant stenosis (>50%) of the intracranial internal carotid artery (ICA), MCA, vertebral artery (VA), or basilar artery (BA). The detailed flow chart of decision-making is depicted in Figure 1.
Figure 1.
Flow chart describing the classification of the etiologic stroke subtypes in this study.
AOL=arterial occlusive lesion; TAL=target arterial lesion; ICAD=intracranial atherosclerotic disease; IST=in situ thrombo-occlusion; ATA=artery-to-artery embolism; CE=cardioembolism.
Clinical and radiographic evaluation
Information on demographic and clinical characteristics, vascular risk factors, and clinical outcomes were collected at baseline in prospective fashion. The onset of stroke was defined as the time when the patient was last observed to be normal. Stroke severity was assessed using the NIHSS at baseline and around 24-hours post-procedure. The diffusion-weighted imaging (DWI) performed at baseline was assessed using the Alberta Stroke Program Early CT Score (ASPECTS).17 All patients underwent a CT or MRI scan at 24 to 48 hours after the treatment. If there was evidence of hemorrhage, the subtype was classified as hemorrhagic infarction (HI), parenchymal hematoma (PH), subarachnoid hemorrhage (SAH), intraventricular hemorrhage, or mixed.18 Symptomatic intracranial hemorrhage was defined as any type of hemorrhage associated with an increase in the NIHSS score of 4 or more within 24 hours.19 Functional status was assessed using the modified Rankin Scale (mRS) score at 3 months, and a favorable outcome was defined as an mRS score of 2 or less, or equal to the prestroke mRS score if the prestroke mRS was greater than 2.19,20
Statistical analysis
Statistical analysis was performed using the SPSS statistical package (version 20.0, Chicago, IL, USA). Statistical significance for intergroup differences was assessed by chi-squared test for categorical variables, Student t test for continuous variables, and the Mann-Whitney U test for ordinal variables and continuous variables that had skewed distributions. Multivariable regression analysis was performed to identify the factors that could be considered independent predictors of clinical and angiographic outcome. The results were presented as odds ratio (OR) estimates of relative risk with a 95% confidence interval (CI). Probability values less than 0.05 were considered statistically significant.
RESULTS
During the study period, a total of 163 patients were included for analysis, and the details of exclusion are described in Figure I in the online-only Data Supplement. Based on the presence of residual stenosis at the TAL on final post-procedural angiography, 74 patients (45.5%) were assigned to the TAL stenosis group (specifically, AOL grade 2), and 89 patients, to the no-TAL stenosis group (AOL grade 3). The baseline characteristics, imaging, and clinical outcomes of the patients are described in the Table 1. Compared to patients without residual stenosis at the TAL, those with the stenosis had a younger age at stroke onset, different profiles in vascular risk factors (higher prevalence of current smoker, and lower prevalence of atrial fibrillation) and etiologic stroke subtypes (higher prevalence of in situ thrombo-occlusion), longer onset-to-puncture and EVT procedure times, and a higher incidence of reocclusion during EVT (instant reocclusion; IR) and follow-up angiography (delayed reocclusion; DR), which were statistically significant.
Table 1.
Baseline characteristics, imaging, and clinical outcomes based on the presence of target arterial stenosis at final procedural angiography.
| Arterial Occlusive Lesion (AOL) scale | |||
|---|---|---|---|
| AOL grade 2 (n=74) | AOL grade 3 (n=89) | p-value | |
| Age, year | 63.5 (55.0–70.3) | 70.0 (60.0–76.0) | 0.025 |
| Male | 46 (62.2%) | 44 (49.4%) | 0.104 |
| Baseline NIHSS score | 16.0 (11.0–19.0) | 16.0 (12.5–21.0) | 0.122 |
| Baseline DWI ASPECTS | 8.0 (7.0–9.0) | 8.0 (6.0–9.0)* | 0.864 |
| Vascular risk factors | |||
| Hypertension | 48 (64.9%) | 63 (70.8%) | 0.419 |
| Dyslipidemia | 31 (41.9%) | 38 (42.7%) | 0.918 |
| Diabetes mellitus | 18 (24.3%) | 20 (22.5%) | 0.781 |
| Atrial fibrillation | 17 (23.0%) | 61 (68.5%) | 0.000 |
| Current smoking | 35 (47.3%) | 21 (23.6%) | 0.002 |
| Etiologic stroke subtypes | 0.000 | ||
| In situ thrombo-occlusion | 40 (54.1%) | 0 (0%) | |
| Artery-to-artery embolism | 2 (2.7%) | 5 (5.6%) | |
| Cardioembolism | 15 (20.3%) | 69 (77.5%) | |
| Cryptogenic | 8 (10.8%) | 15 (16.9%) | |
| Two or more | 9 (12.2%) | 0 (0%) | |
| Onset-to-puncture time, min | 303.0 (218.8–427.5) | 230.0 (138.5–326.5) | 0.000 |
| EVT procedure time, min | 51.5 (25.0–75.3) | 24.0 (14.0–52.5) | 0.000 |
| Instant reocclusion | 20 (27.0%) | 1 (1.1%) | 0.000 |
|
Degree of TAL stenosis at follow-up MRA/CTA |
0.000 | ||
| None | 13 (17.6%) | 61 (68.5%) | |
| Mild (<50%) | 15 (20.3%) | 23 (25.8%) | |
| Moderate (50–69%) | 22 (29.7%) | 4 (4.5%) | |
| Severe (70–99%) | 16 (21.6%) | 0 (0%) | |
| Occlusion | 8 (10.8%) | 1 (1.1%) | |
| 3-month favorable outcome | 45 (60.8%) | 62 (69.7%) | 0.236 |
| Symptomatic ICH | 1 (1.4%) | 2 (2.2%) | 1.000** |
| Mortality at 3-month | 2 (2.7%) | 4 (4.5%) | 0.545 |
Numbers are median (interquartile range) or number (%).
2 cases were excluded for DWI ASPECTS analysis due to no baseline DWI.
Fisher exact test
NIHSS=National Institutes of Health Stroke Scale; DWI ASPECTS=diffusion-weighted imaging Alberta Stroke Program Early CT Score; EVT=endovascular therapy; TAL=target arterial lesion; MRA=MR angiography; CTA=CT angiography; ICH=intracranial hemorrhage.
A favorable clinical outcome at 3 months was observed in 107 of the 163 patients (65.6%) in our cohort. Further, the rates of favorable outcome between patients with AOL grade 2 and AOL grade 3 did not differ significantly (60.8% vs. 69.7%, p=0.236). On multivariable analysis, the presence of IR (OR 0.188; 95% CI, 0.053–0.669; p=0.010) and DR (OR 0.035; 95% CI, 0.005–0.243; p=0.001) was found to be independently associated with a lower chance of favorable outcome after adjustment for age at onset, baseline NIHSS score, and good or excellent baseline collateral flow (grade 3 to 4; Table 2).
Table 2.
Multivariable regression models for predicting clinical and angiographic outcomes
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Model for predicting favorable outcome | ||
| Age, year | 0.926 (0.888–0.965) | 0.000 |
| NIHSS score | 0.843 (0.771–0.922) | 0.000 |
| Collateral flow (grade 3 to 4) | 7.108 (2.868–17.618) | 0.000 |
| Instant reocclusion | 0.188 (0.053–0.669) | 0.010 |
| Delayed reocclusion | 0.035 (0.005–0.243) | 0.001 |
| Model for predicting delayed reocclusion in patients with AOL 2 | ||
| Collateral flow (grade 4) | 8.477 (1.169–61.464) | 0.034 |
|
Any neurological worsening around 24-hours post-procedure |
10.388 (1.287–83.876) | 0.028 |
| mTICI 2a reperfusion | 3.113 (0.458–21.158) | 0.245 |
| EVT procedure time (each 10 min) | 1.032 (0.845–1.261) | 0.758 |
| TAL stenosis (each severity) | 0.809 (0.274–2.393) | 0.702 |
NIHSS=National Institutes of Health Stroke Scale; mTICI=modified Treatment in Cerebral Ischemia; EVT=endovascular therapy; TAL=target arterial lesion.
Characteristics in relation to in situ thrombo-occlusion (IST)
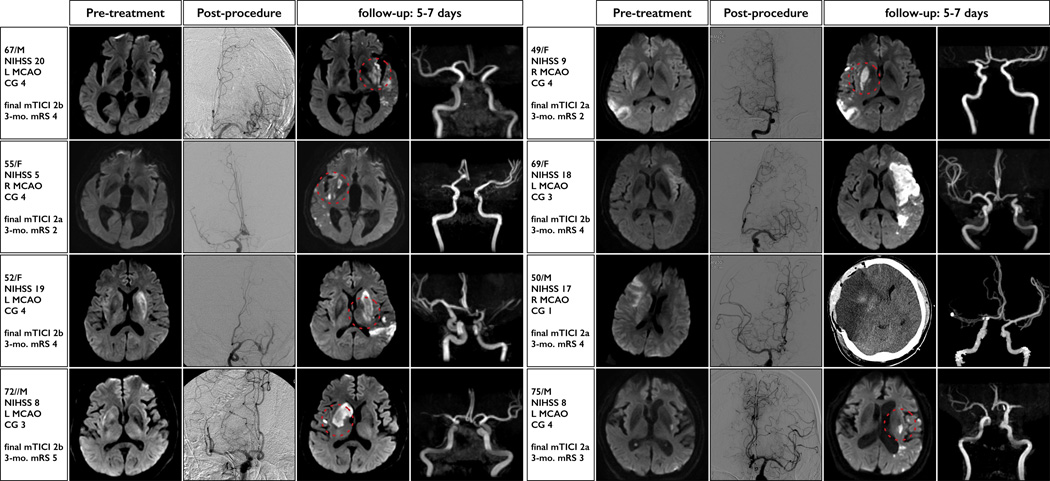
Forty patients (24.5%) in the cohort were found to have IST as the presumed hidden etiology of the TAL according to our newly devised definitions (Figure 1), and all of these patients showed target arterial residual stenosis at the final post-procedural angiography [40/74 (54.1%)]. Further, the severity of residual stenosis at the TAL was highly associated with the etiologic stroke subtypes: specifically, IST was highly prevalent in patients with severe TAL stenosis among those with AOL grade 2 [30/37 (81.1%); Figure 2A]. Compared to patients with non-IST etiologies, those with IST had a lower NIHSS score at baseline, higher prevalence of current smoker and excellent baseline collaterals, longer onset-to-puncture duration and EVT procedure time, and higher rate of IR and DR (Table 3). With regard to the IST subgroup, favorable 3-month outcomes were observed in 2 of 8 (25.0%) patients with DR and in 23 of the 32 (71.9%) patients without DR (p=0.014). The detailed descriptions of the cases with DRs are provided in Figure 3, which shows the extension of the basal ganglionic ischemic lesion in most cases.
Figure 2.
The proportion of (A) etiologic stroke subtypes (p=0.000) and (B) the severity of stenosis on follow-up angiography (p=0.000) based on the degree of target arterial residual stenosis at post-procedural angiography in the AOL grade 2 subgroup.
IST=in situ thrombo-occlusion; CE=cardioembolism; ATA=artery-to-artery embolism.
Table 3.
Baseline characteristics, imaging, and clinical outcomes based on the operationally defined TAL etiologies.
| TAL etiologies | |||
|---|---|---|---|
| IST (n=40) | Non-IST (n=123) | p-value | |
| Age, year | 65.5 (55.0–71.5) | 68.0 (58.0–75.0) | 0.268 |
| Male | 27 (67.5%) | 63 (51.2%) | 0.072 |
| Baseline NIHSS score | 14.5 (9.3–18.0) | 16.0 (12.0–20.0) | 0.006 |
| Baseline DWI ASPECTS | 8.0 (7.0–9.0) | 8.0 (6.0–9.0)* | 0.657** |
| Vascular risk factors | |||
| Hypertension | 32 (80.0%) | 79 (64.2%) | 0.063 |
| Dyslipidemia | 14 (35.0%) | 55 (44.7%) | 0.280 |
| Diabetes mellitus | 10 (25.0%) | 28 (22.8%) | 0.771 |
| Atrial fibrillation | 0 (0%) | 78 (63.4%) | 0.000 |
| Current smoking | 23 (57.5%) | 33 (26.8%) | 0.000 |
|
Excellent collaterals (grade 4) |
15 (37.5%) | 24 (19.5%) | 0.021 |
| Onset-to-puncture time, min | 354.5 (236.3–513.5) | 240.0 (150.0–330.0) | 0.000** |
| EVT procedure time, min | 61.5 (35.5–79.8) | 30.0 (14.0–58.0) | 0.000** |
| Instant reocclusion | 16 (40.0%) | 5 (4.1%) | 0.000 |
|
Degree of TAL stenosis at follow-up MRA/CTA |
0.000 | ||
| None | 1 (2.5%) | 73 (59.3%) | |
| Mild (<50%) | 2 (5.0%) | 36 (29.3%) | |
| Moderate (50–69%) | 15 (37.5%) | 11 (8.9%) | |
| Severe (70–99%) | 14 (35.0%) | 2 (1.6%) | |
| Occlusion | 8 (20.0%) | 1 (0.8%) | |
| 3-month favorable outcome | 25 (62.5%) | 82 (66.7%) | 0.630 |
| Symptomatic ICH | 0 (0%) | 3 (2.4%) | 1.000** |
| Mortality at 3-month | 2 (5.0%) | 4 (3.3%) | 0.636** |
Numbers are median (interquartile range) or number (%).
2 cases were excluded for DWI ASPECTS analysis due to no baseline DWI.
Fisher exact test or Mann-Whitney U test
NIHSS=National Institutes of Health Stroke Scale; DWI ASPECTS=diffusion-weighted imaging Alberta Stroke Program Early CT Score; EVT=endovascular therapy; TAL=target arterial lesion; MRA=MR angiography; CTA=CT angiography; ICH=intracranial hemorrhage.
Figure 3.
Key pre-treatment, post-procedure, and follow-up images in patients who experienced delayed reocclusion (DR) in the IST subgroup. Distinctive enlargement patterns that involve basal ganglionic extensions (dotted red circle) were observed in 6 of 8 patients and 5 of 5 patients who showed excellent baseline collaterals (grade 4).
NIHSS=National Institutes of Health Stroke Scale; MCAO=middle cerebral artery occlusion; CG=collateral-flow grade; mTICI=modified Treatment in Cerebral Ischemia (mTICI); mRS=modified Rankin Scale.
Factors associated with instant reocclusion and delayed reocclusion in AOL grade 2 subgroup
Overall, 20 patients (27.0%) revealed ‘instant reocclusion (IR)’ during the procedure in the AOL grade 2 subgroup (Table 1). Acute management for IR during the procedure includes intra-arterial tirofiban infusion (15 of 20; 75%), rescue stenting (4 of 20; 20%), and repeated mechanical thrombectomy only (3 of 20; 15%). The detailed description of IR cases was shown in Table I in the online-only Data Supplement. The rate of IR was much higher in patients with severe TAL stenosis (15 of 37; 40.5%) than in those with mild (2 of 17; 11.8%) or moderate (3 of 20; 15.0%) stenosis (p=0.032). Further, the procedure time for EVT was considerably longer in cases with IR than in those without IR (p=0.000). The proportion of stenosis severity on follow-up angiography was diverse compared to the stenosis severity on post-procedural angiography (p=0.000; Figure 2B). Also, 8 patients (10.8%) showed ‘delayed reocclusion (DR)’ on follow-up in the AOL grade 2 subgroup (Table 1). Furthermore, the rate of DR showed no intergroup difference in terms of stenosis severity (p=0.673; Table II in online-only Data Supplement). The baseline variables which was related to DR were final mTICI 2a reperfusion, excellent baseline collateral-flow, and any neurological worsening around 24-hours post-procedure (Table II in online-only Data Supplement). On multivariable analysis, the presence of excellent baseline collateral-flow and the presence of any neurological worsening was independently associated with a higher probability of DR after adjustment for the severity of residual stenosis at the TAL, EVT procedure time, and mTICI 2a reperfusion (Table 2).
DISCUSSION
In areas with a high prevalence of ICAD as an etiology of stroke, the presence of intrinsic atherosclerosis can be problematic in terms of revascularization strategies.14,21 However, little is known about the incidence and interval changes of target arterial residual stenosis, its association with ICAD, and its impact on outcome. This study explored “target arterial residual stenosis” after EVT as a marker of IST, and its impact on clinical and angiographic outcome in relation to ICAD. The novel findings of this study establish that: (1) the rate of residual stenosis at the TAL (AOL grade 2) was 45.5%; (2) IST as the underlying TAL pathology accounts for 24.5% of M1 occlusions and 54.1% of AOL grade 2; (3) the presence of IR and DR, which mostly occurred in IST etiology, was independently associated with a lower chance of favorable clinical outcome; and (4) the occurrence of DR was highly associated with excellent baseline collateral-flow and any early neurological worsening, rather than the severity of target arterial residual stenosis in the AOL grade 2 subgroup.
In general, the AOL scale, which measures the degree of arterial patency at the site of an occlusion after EVT, considered as a surrogate marker for clinical outcome.10 However, the distinction between AOL grades 2 and 3 has drawn little attention in the clinical literature. The reasons for using both AOL grades 2 and 3 as a common denominator were as follows: (1) both AOL 2 and 3 recanalization were associated with good clinical outcomes;22 (2) the proportion of patients with AOL grade 2 among those with AOL grades 2 and 3 might has been insignificant in recent endovascular trials [for example, the rate of AOL 2 in the recent Interventional Management of Stroke (IMS) 3 trial was 18 of 111 patients (16.2%) for patients with M1 occlusion and mTICI 2–3 reperfusion following EVT]; and (3) the majority of cases of underlying etiology within a TAL might be attributable to an embolism without any intrinsic pathology, especially in western countries.23
In our cohort, the proportion of AOL grade 2 cases was about 45%. Additionally, more than half of AOL grade 2 cases and about 25% of M1 occlusion cases were linked to ICAD as the underlying pathology. Recently, the etiologic diagnosis of a TAL was explored based on different operational definitions, and about 15–30% of intracranial occlusions were associated with underlying ICAD, which is in concordance with our results.14,21,24 However, the definition of “IST with underlying ICAD” is still arbitrary, and most previous studies have adopted “percent stenosis” criteria to define the presence of ICAD.21,24 However, in our study, the main concept in classifying underlying TAL pathology was based on the interval changes between final post-procedural angiography and 5- to 7-day follow-up angiography to exclude residual stenosis due to procedure-related endothelial damage or a partially-remaining thrombus (Figure 1). Interestingly, about 81% of the cases of severe stenosis and 27% of the cases of mild to moderate TAL stenosis were assigned to IST etiology, which denotes the dynamic nature of target arterial residual stenosis over a short-term period.
As to the EVT, the presence of underlying ICAD can lead to a higher probability of IR, which might increase the procedure time.14,25 However, its impact on clinical recovery is not yet clearly known. In our cohort, the occurrence of IR was closely associated with the IST etiology, the severity of target arterial residual stenosis, and poor clinical recovery. Moreover, the occurrence of DR on follow-up MRA/CTA was associated with IST etiology and poor clinical recovery. Unlike IR, the severity of target arterial residual stenosis was unrelated to the occurrence of DR. Interestingly, the presence of excellent baseline collaterals, partial reperfusion (mTICI 2a), and any early neurological worsening was associated with the occurrence of DR, and after adjustment for mTICI 2a reperfusion, procedure time, and stenosis severity, the presence of excellent collaterals and any early neurological worsening remained as an independent predictor of DR. This finding can be novel, because it suggests that if a patient has well-developed collaterals at baseline, the chance of reocclusion during early post-procedural period can be high despite reperfusion. The reason for this phenomenon might be speculated as follows: (1) the pre-existing excellent collaterals may preclude maintenance of re-established antegrade perfusion; and (2) competing between antegrade and collateral flow may result in increased thrombogenicity at the site of a TAL due to slower flow.26 Despite excellent baseline collaterals, DR resulted in the extension of basal ganglionic ischemic lesions, which might impede functional recovery. Also, any early neurological worsening as a predictor of DR was associated with minute rather than major changes in NIHSS score, which can be explained in virtue of pre-existing excellent collaterals perhaps having a protective role in major neurological worsening. Further, it might prevent clinicians to detect any clinical situations necessitating further rescue treatment for DR. Also, proposed management strategies were depicted in Figure II in the online-only Date Supplement.
This study has some limitations, and accordingly, the results need to be interpreted with caution. EVT in our cohort includes thrombolytic infusion and a variety of endovascular techniques. Therefore, the distinction between AOL grades 2 and 3 may be influenced by the device efficacy, and not only by the underlying ICAD, although the selection of EVT was based on the best available methods at the time of treatment. Further, the exclusion of patients with failed reperfusion despite EVT and exclusion of patients without follow-up angiographic images might under- or over-estimate the actual proportion of patients with underlying ICAD. Additionally, the definition of IST etiology was based mainly on angiographic findings, and not on pathology. Further, the interpretation of MR angiographic data at follow-up has some limitations in relation to flow artifacts, which can overestimate the degree of stenosis despite a kappa coefficient for interobserver agreement that was high enough for ‘≥50% stenosis’ and occlusion classification.
Conclusions
In summary, the novel findings of this study provide some insight into the relationships between target arterial residual stenosis, in situ thrombo-occlusion, instant or delayed reocclusion, and clinical recovery. These findings could be informative in designing EVT trials and performing EVT in populations with the highest prevalence of ICAD. However, further deliberations are required to establish criteria for the etiologic diagnosis of IST and determine the best strategies to prevent instant or delayed reocclusion in this era of EVT.
Supplementary Material
Acknowledgments
We thank Wade Martin of Emareye for his critical English revision.
Potential Conflicts of Interest
Dr. Hwang reports grants from Kyungpook National University, outside the submitted work; Dr. Liebeskind reports grants from NIH-NINDS, other from Medtronic, other from Stryker, outside the submitted work.
Footnotes
Authors’ contributions
Study concept and design by YHH and DSL; Analysis and interpretation of data by YHH, YWK, DHK, and DSL; Drafting of the manuscript by YHH; Critical revision of the manuscript for important intellectual content by YSK and DSL
REFERENCES
- 1.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, et al. REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, et al. ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 6.Matias-Guiu JA, Serna-Candel C, Matias-Guiu J. Stroke etiology determines effectiveness of retrievable stents. J Neurointerv Surg. 2014;6:e11–e11. doi: 10.1136/neurintsurg-2012-010395. [DOI] [PubMed] [Google Scholar]
- 7.Cho A-H, Kwon SU, Kim JS, Kang D-W. Evaluation of early dynamic changes of intracranial arterial occlusion is useful for stroke etiology diagnosis. J Neurol Sci. 2012;312:127–130. doi: 10.1016/j.jns.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Wong LKS. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke. 2014;16:8–17. doi: 10.5853/jos.2014.16.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators, STIR Revascularization working group, STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang Y-H, Kang D-H, Kim Y-W, Kim Y-S, Park S-P, Suh C-K. Outcome of forced-suction thrombectomy in acute intracranial internal carotid occlusion. J Neurointerv Surg. 2013;5:i81–i84. doi: 10.1136/neurintsurg-2012-010277. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Hwang Y-H, Kim YS, Park J, Kwon O, Jung C. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol. 2011;32:283–287. doi: 10.3174/ajnr.A2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang D-H, Kim Y-W, Hwang Y-H, Park J, Hwang J-H, Kim Y-S. Switching strategy for mechanical thrombectomy of acute large vessel occlusion in the anterior circulation. Stroke. 2013;44:3577–3579. doi: 10.1161/STROKEAHA.113.002673. [DOI] [PubMed] [Google Scholar]
- 14.Kang D-H, Kim Y-W, Hwang Y-H, Park S-P, Kim Y-S, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37:350–355. doi: 10.1159/000362435. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher HC, Meyers PM, Higashida RT, Derdeyn CP, Lavine SD, Nesbit GM, et al. Reporting standards for angioplasty and stent-assisted angioplasty for intracranial atherosclerosis. Stroke. 2009;40:e348–e365. doi: 10.1161/STROKEAHA.108.527580. [DOI] [PubMed] [Google Scholar]
- 16.Kwon SU, Hong K-S, Kang D-W, Park J-M, Lee JH, Cho Y-J, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42:2883–2890. doi: 10.1161/STROKEAHA.110.609370. [DOI] [PubMed] [Google Scholar]
- 17.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 18.Hacke W, Kaste M, Fieschi C, Kummer von R, Dávalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 19.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 20.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 21.Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery. 2015;76:680. doi: 10.1227/NEU.0000000000000694. 6– discussion 686. [DOI] [PubMed] [Google Scholar]
- 22.Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, et al. Interventional Management of Stroke II Investigators. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29:582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Hong JM, Lee KS, Suh HI, Demchuk AM, Hwang Y-H, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis. 2015;24:2074–2080. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Heo J-H, Lee K-Y, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology. 2003;60:1684–1687. doi: 10.1212/01.wnl.0000063323.23493.98. [DOI] [PubMed] [Google Scholar]
- 26.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, et al. Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) Investigators. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.