Abstract
Background
Cryptococcus is the most common cause of adult meningitis in Africa. We evaluated the activity of adjunctive sertraline, previously demonstrated to have in vitro and in vivo activity against Cryptococcus.
Methods
We enrolled 172 HIV-infected Ugandans with cryptococcal meningitis from August 2013 through August 2014 into an open-label dose-finding study to assess safety and microbiologic efficacy. Sertraline 100–400mg/day was added to standard therapy of amphotericin + fluconazole 800mg/day. We evaluated early fungicidal activity via Cryptococcus cerebrospinal fluid (CSF) clearance rate, sertraline pharmacokinetics, and in vitro susceptibility.
Findings
Participants receiving any sertraline dose averaged a CSF clearance rate of −0·37 (95%CI: −0·41, −0·33) colony forming units (CFU)/mL/day. Incidence of paradoxical immune reconstitution inflammatory syndrome (IRIS) was 5% (2/43) and relapse was 0% through 12-weeks. Sertraline reached steady state concentrations in plasma by day 7, with median steady-state concentrations of 201 ng/mL (IQR, 90–300; n=49) with 200mg/day and 399 ng/mL (IQR, 279–560; n=30) with 400mg/day. Plasma concentrations reached 83% of steady state levels by day 3. The median projected steady state brain tissue concentration at 200mg/day was 3·7 (IQR, 2·0–5·7) mcg/mL and 6·8 (IQR, 4·6–9·7) mcg/mL at 400mg/day. Minimum inhibitory concentrations were ≤2 mcg/mL for 27% (35/128), ≤4 mcg/mL for 84% (108/128), ≤6 mcg/mL for 91% (117/128), and ≤8 mcg/mL for 100% of 128 Cryptococcus isolates.
Interpretation
Sertraline had faster cryptococcal CSF clearance, decreased IRIS, and decreased relapse compared with historical experiences. Sertraline reaches therapeutic levels in a clinical setting. This inexpensive and off-patent oral medication is a promising adjunctive antifungal therapy.
Funding
National Institutes of Health, Grand Challenges Canada.
Keywords: Cryptococcus, cryptococcal meningitis, HIV, sertraline, antifungal
Introduction
Cryptococcal meningitis has emerged as the most frequent cause of meningitis among adults in Africa, accounting for 15–20% of AIDS-related deaths.1,2 Ten week mortality from cryptococcal meningitis remains unacceptably high (≥35%),3–5 in large part due to the high cost, toxicity, and relatively limited repertoire of effective antifungal agents. Furthermore, the rate of fungal clearance of Cryptococcus from cerebrospinal fluid (CSF) remains suboptimal, with 60–70% of patients attaining CSF sterilization after 2 weeks of amphotericin B combination therapy.3–5 For these reasons, a critical need exists for new effective antifungals that are readily accessible, especially in resource-limited settings.
Recent evidence suggests that sertraline, the commonly used selective serotonin reuptake inhibitor (SSRI) antidepressant, provides potent in vitro and in vivo fungicidal activity against Cryptococcus through dose-dependent inhibition of protein synthesis via interaction with eukaryotic translation initiation factor (Tif3).6–8 Sertraline concentration in blood is subtherapeutic, yet sertraline is concentrated into brain tissue at a median of 16·5-fold higher levels than in plasma.9 In vitro, sertraline inhibited C. neoformans growth with minimum inhibitory concentrations (MIC) between 2–6 mcg/mL;6–8 and unlike fluconazole, sertraline was fungicidal, with killing independent of cell proliferation.6 The inhibitory effect of sertraline, when pretreated for 7 days, was particularly potent in the brain of infected mice, with efficacy similar to fluconazole.6 The combination of sertraline and fluconazole was either additive or synergistic in vitro, and in animal models led to accelerated fungal clearance at a greater rate than either drug alone.6,10,11 Taken together, these data suggest that sertraline may offer a promising therapeutic option for cryptococcal meningitis.
We hypothesized that sertraline, when added to standard amphotericin plus fluconazole induction therapy, would result in faster rates of fungal clearance from cerebrospinal fluid (CSF) and better clinical outcomes. To evaluate this hypothesis among HIV-infected Ugandans with cryptococcal meningitis, we conducted a prospective, open-label, dose-finding pilot study, which assessed the safety, microbiologic efficacy, and pharmacokinetics of adjunctive sertraline.
Methods
We prospectively enrolled 172 HIV-infected adults with cryptococcal meningitis, who presented to Mulago Hospital in Kampala, Uganda from August 2013 through August 2014. Cryptococcal diagnosis was made via CSF cryptococcal antigen lateral flow assay (Immy Inc., Norman, Oklahoma), and confirmed by quantitative CSF fungal culture. Uganda and Minnesota institutional review boards approved the protocol.
Study Design
Participants received standard antifungal therapy plus adjunctive sertraline at doses of 100–400 mg/day. Standard therapy included amphotericin B (0·7–1·0 mg/kg/day) for up to 14 days and fluconazole (800 mg/day) for 4 weeks, followed by fluconazole 400 mg/day for 8 weeks of consolidation therapy. Fluconazole dose was increased by 50% when receiving rifampin. Amphotericin could be discontinued after 7 days if baseline CSF culture was sterile at 7 days post-collection, with continuation of fluconazole and sertraline. For ART-naïve persons or those on a failing regimen, ART was initiated or changed at 4–6 weeks. At week 9, a 3-week sertraline taper was started, so that participants discontinued sertraline one week prior to study termination at 12 weeks. After 12 weeks, participants were passively followed and instructed to contact study personnel if they had recurrent central nervous system symptoms.
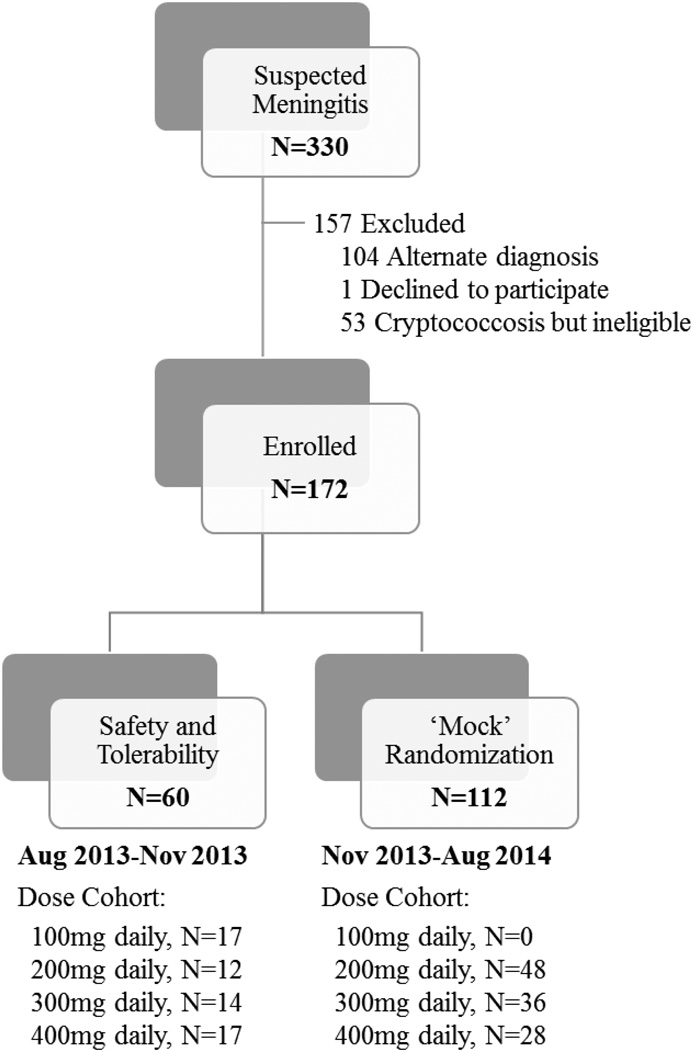
To evaluate safety and tolerability, the first 60 participants were given sertraline at escalating doses of 100 (N=17), 200 (N=12), 300 (N=14), or 400mg/day (N=17) as induction therapy for 2 weeks, followed by consolidation therapy with 200mg/day through 8 weeks (Figure 1). Dose escalation occurred only after five ART-naïve persons with first episode of cryptococcosis completed 2-weeks of induction therapy. Each dose escalation cohort varied in size according to differences in enrollment pace, 2-week survival, and proportion of persons receiving ART. Subjects receiving ART at time of enrollment, with prior history of cryptococcosis, or who died prior to receiving 14 doses of amphotericin were included in the overall analysis, but did not count toward the N=5 needed to complete each dose cohort.
Figure 1. Flowchart of study participation.
A total of 172 individuals with HIV-associated cryptococcal meningitis were enrolled in the study. This reflects N=60 participants being evaluated for safety and tolerability of adjunctive sertraline and additional N=112 participants that either underwent a ‘mock’ randomization of open-labeled sertraline (N=96; participants with first-episode of cryptococcal meningitis) or received 200mg daily of adjunctive sertraline for second episode of cryptococcal meningitis (N=16).
Due to delay in procuring a suitable matched placebo for the follow-up phase III trial, this open-label pilot study was extended to include a ‘mock’ randomization of sertraline doses for purposes of: 1) accruing additional pharmacokinetic and safety data and 2) to provide procedural experience for anticipated phase III randomized controlled trial. Beginning in November 2013, participants with first episode of cryptococcal meningitis were assigned to open-label sertraline at predetermined doses of 200, 300, or 400mg/day as induction therapy for 2 weeks, thereafter following the above dosing schedule. Dose assignment was made via permutated block randomization stratified by ART status for persons with first episode of meningitis. Persons with second episode of cryptococcal meningitis (e.g. relapse or paradoxical IRIS) received 200mg/day beginning in November 2013. This extension continued until the matched placebo became available in August 2014.
Therapeutic lumbar punctures (LPs) were routinely performed using manometers at diagnosis, and on days 3, 7, 10, and 14. Quantitative CSF cultures were performed with five 1:10 serial dilutions of 100µL CSF.12 Details of the methodology used for determining quantitative CSF cultures are previously described.13 CSF culture sterility was defined as no growth of Cryptococcus, with a limit of detection of 10 colony forming units (CFU)/mL. Cryptococcus isolates were stored in glycerol at −80°C, and shipped to the University of Minnesota on dry ice (−20°C). Susceptibility testing was performed by broth microdilution in RPMI1640 media per protocol.14 Sertraline minimum inhibitory concentration (MIC) was defined as the concentration at which no growth was observed based on photometric absorbance at 600nm (OD600), as further described.7
Plasma sertraline concentrations were quantified using reversed-phase liquid chromatography with Agilent 1200 series HPLC pump, auto sampler, and column oven with triple quadrupole mass spectrometer (Appendix 2). We attempted to measure sertraline concentrations directly in CSF, but this was abandoned after discovering very low levels in CSF compared to plasma. As sertraline is a highly lipophilic molecule, we hypothesize that sertraline diffuses from CSF into brain tissue, rendering it undetectable in CSF. Indeed, human CSF sertraline concentrations have never been published. We thus estimated sertraline brain concentrations based on published postmortem tissue levels, which observed a median 16·5-fold higher concentration in brain tissue compared to plasma (Interquartile range (IQR), 13·0 to 21·3).9
Outcome Measures
The primary outcome was the 2-week CSF clearance rate of Cryptococcus, termed early fungicidal activity (EFA). Secondary clinical endpoints included incidence of CSF culture sterility at two weeks, paradoxical immune reconstitution inflammatory syndrome (IRIS) per consensus criteria,15 culture-positive relapse, and safety through 12 weeks. Participants with a prior history of cryptococcosis were only included in the analyses for survival, safety, and relapse. We calculated paradoxical IRIS incidence among persons with a first episode of cryptococcal meningitis, who were ART-naïve at baseline and survived to initiate ART, and/or those who switched to second line ART after hospitalization. We used the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS toxicity scale, version 2009, to evaluate adverse events in all participants. Due to an expected 80% incidence of Grade 3–5 adverse events with amphotericin B deoxycholate, we only captured Grade 4–5 adverse events, which had an expected incidence of 35–40%, dominated by amphotericin-related toxicities.3,5 Additional protocol-specified secondary outcomes of neurocognitive performance, depression, and cost-effectiveness analysis are the focus of future publications. Secondary laboratory endpoints included plasma sertraline concentrations and in vitro sertraline susceptibility of Cryptococcus isolates. Prior genotyping of Cryptococcus clinical isolates in Uganda during 2006–2012 have determined >99% of clinical isolates are Cryptococcus neoformans var. grubii strains.7,16
Statistical Analysis
Baseline characteristics were evaluated across sertraline doses using χ2 or Kruskal-Wallis tests as appropriate. Overall and dose-specific CSF clearance rate was calculated over 2-weeks (EFA) among participants with culture positive, first episode of cryptococcosis and at least 2 quantitative CSF cultures via longitudinal mixed effect models, which included participant-specific random intercept and random slope. Models used restricted maximum likelihood estimation and an unstructured covariance matrix. The Satterthwaite method was used to determine denominator degrees of freedom for p-value estimation. As multiple cohorts have reported mean EFA by patient-specific linear regression,4,17,18 EFA was also estimated by linear regression.
Secondary outcomes were compared across sertraline doses with χ2 or Kruskal-Wallis tests. To evaluate efficacy of consolidation therapy with sertraline added, Cox proportional hazard regression evaluated 12-week all-cause mortality among those who survived 14 days post-enrollment between participants who achieved CSF sterility by 2 weeks versus those who did not. Age and sex adjusted competing-risks regression evaluated Grade 4 or 5 adverse event incidence between current FDA dosing guidelines of 100–200mg/day and higher doses of 300–400mg/day, using the Fine and Grey method where non-adverse event all-cause mortality was a competing risk. We also calculated risk differences between FDA-approved dose groups for Grade 4, Grade 5 cryptococcal related and non-cryptococcal adverse events separately. For other safety outcomes, risk differences were calculated for cryptococcal and non-cryptococcal related deaths, ≥1 occurrence of nausea, vomiting and/or diarrhea, premature sertraline dose reduction, premature sertraline discontinuation, and loss to follow-up between dosing of 100–200mg/day versus and higher doses 300–400mg/day sertraline groups. Analyses were conducted using SAS version 9·3 (SAS Institute, Cary, NC) and evaluated against type I error <0·05.
Pharmacokinetic analyses used nonlinear mixed-effects models to simultaneously estimate parameters of a 1-compartment pharmacokinetic model by including all available sertraline concentrations from all doses. A likelihood ratio test was used to determine the significance of including ART in the model (χ2 test, α = 0·05, df =1). A Monte Carlo simulation for 50,000 simulated subjects determined the proportion with projected therapeutic steady state sertraline brain concentrations, based on the distributions of: steady state plasma concentrations, published fold-change concentration into the brain,9 and Cryptococcus population MICs (Appendix 2d). Pharmacokinetic analyses to determine ART effect were performed in NONMEM version 7·2 (ICON Development Solutions, Dublin, Ireland).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The senior author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Cohort Demographics and Outcomes
Among 330 individuals presenting with suspected meningitis, 172 HIV-infected adults with a positive CSF cryptococcal antigen were consented and enrolled, of which 22 had previous history of cryptococcal meningitis (Supplemental Figure 1). The demographic and baseline clinical characteristics of the study participants are presented in Table 1. In contrast to previous large cohorts of HIV-associated cryptococcal meningitis,3–5 49% of participants were receiving ART prior to hospitalization (29% receiving efavirenz-containing ART), a greater proportion (35%) presented with altered mental status (Glasgow coma scale <15), and fewer patients were women (34%). High baseline fungal burdens (median 4·6 log10CFU/mL; IQR: 3·8–5·4 log10CFU/mL) and elevated CSF opening pressure (median 300 mm H2O; IQR, 190 to 480 mm H2O) were common. Among those with a first episode of cryptococcal meningitis, 8% (12/150) had sterile CSF with a positive CSF CRAG. Demographics did not differ significantly between dosing groups. Sertraline was administered at 100mg (N=17), 200mg (N=60), 300mg (N=50), or 400mg (N=45) daily for the first 2 weeks, and 200mg/day thereafter (n=134 survivors).
Table 1.
Baseline characteristics and outcomes by daily sertraline dose
| Sertraline Dose Cohort | Sertraline | Sertraline | ||||
|---|---|---|---|---|---|---|
| 100mg | 200mg | 300mg | 400mg | All | P-value | |
| N per group | 17 | 60 | 50 | 45 | 172 | 172 |
| Demographics | ||||||
| Age, years | 36 (32, 41) | 37 (32, 43) | 38 (33, 42) | 33 (29, 38) | 36 (32, 42) | 0·08 |
| Male sex | 11 (65%) | 41 (68%) | 31 (62%) | 30 (67%) | 113 (66%) | 0·92 |
| Prior Cryptococcal meningitis | 1 (6%) | 16 (27%) | 3 (6%) | 2 (4%) | 22 (13%) | <0·01 |
| Receiving ART | 9 (53%) | 33 (55%) | 21 (42%) | 21 (48%) | 84 (49%) | 0·58 |
| Receiving TB Therapy | 0 (0%) | 2 (3%) | 7 (14%) | 3 (7%) | 12 (7%) | 0·14 |
| Baseline Clinical Parameters | ||||||
| Glasgow Coma Scale Score <15 | 6 (35%) | 25 (42%) | 16 (32%) | 13 (30%) | 60 (35%) | 0·58 |
| Weight, kg | 52 (49, 57) | 52 (44, 61) | 50 (47, 55) | 52 (45, 56) | 52 (47, 57) | 0·91 |
| CD4 count, cells/µL | 19 (7, 114) | 25 (9, 54) | 16 (6, 50) | 20 (9, 59) | 19 (7, 57) | 0·57 |
| CSF Opening Pressure, >250 mmH2O | 8 (53%) | 36 (69%) | 24 (53%) | 25 (61%) | 93 (61%) | 0·40 |
| CSF Quantitative Culture, log10CFU/mL* | 4·8 (4·1, 5·4) | 4·4 (3·1, 5·4) | 4·9 (4·0, 5·5) | 4·3 (3·3, 5·5) | 4·6 (3·8, 5·4) | 0·46 |
| CSF WBC ≥5 cells/µL | 9 (53%) | 15 (27%) | 19 (40%) | 12 (28%) | 55 (34%) | 0·14 |
| Outcomes | ||||||
| 14-day CSF Sterility† | 6 (43%) | 25 (61%) | 22 (51%) | 20 (50%) | 73 (53%) | 0·61 |
| Paradoxical IRIS‡ | 0 (0%) | 1 (7%) | 0 (0%) | 1 (8%) | 2 (5%) | 0·58 |
| Culture-positive Relapse§ | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | -- |
| 2 week mortality | 5 (29%) | 8 (13%) | 12 (24%) | 13 (29%) | 38 (22%) | 0·21 |
| 12 week mortality | 10 (59%) | 20 (33%) | 21 (42%) | 18 (40%) | 69 (40%) | 0·30 |
Data are median (P25, P75) or N (%). P-values comparing across the four sertraline dosing groups.
Excludes those with sterile culture at diagnosis (n=19). Four persons’ cultures were unable to be quantified.
Excludes those who started with sterile CSF culture (n=12) and/or prior history of cryptococcal meningitis (n=22). Includes all quantitative culture data collected within 14 days of enrollment among 138 sertraline participants (N=14 for 100mg/day; N=41 for 200mg/day; N=43 for 300mg/day; N=40 for 400mg/day).
IRIS incidence among persons with first episode of cryptococcal meningitis, who were ART-naïve at baseline and who survived to initiate ART, and/or those who switched to second line ART after hospitalization (N=3 for 100mg/day; N=14 for 200mg/day; N=15 for 300mg/day; N=11 for 400mg/day). Includes possible IRIS cases.
No other paradoxical IRIS cases occurred among those excluded (e.g. second episodes of cryptococcosis, those already receiving effective ART).
Two culture-positive relapse cases occurred beyond the 12 week study follow-up period, one in the 100mg dose group and another with fluconazole non-compliance.
Early Fungicidal Activity (EFA)
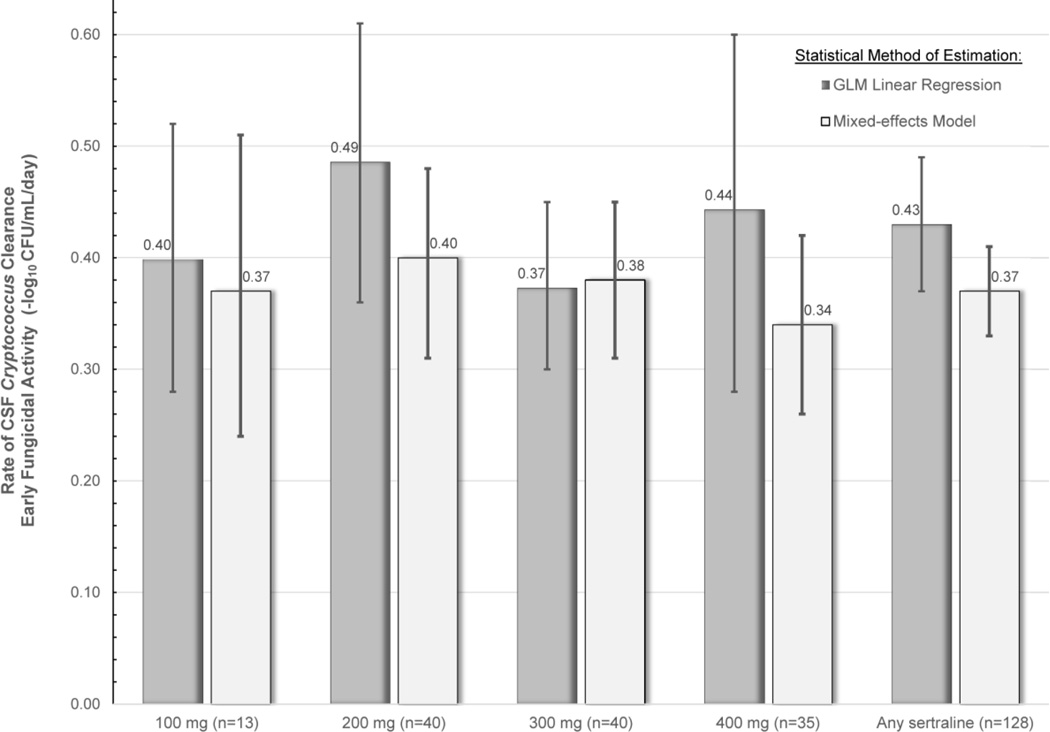
Of the 172 individuals who received sertraline, 44 participants were excluded from EFA analysis due to prior cryptococcal meningitis (N=22), sterile diagnostic CSF cultures (N=12), or <2 CSF cultures performed (N=10). With sertraline, the overall mixed-effect EFA was −0·37 log10CFU/mL CSF/day (95%CI: −0·41, −0·33). There were no statistical differences in EFA observed by sertraline dose (Figure 2).
Figure 2. Rate of CSF Clearance of Cryptococcus.
The figure displays the rate of CSF clearance by sertraline dose. There was no statistical difference in the early fungicidal activity (EFA) between doses of sertraline. Due to the relatively small sample sizes of the dose groups and inherent differences in statistical methods of estimating EFA (i.e. linear regression vs. mixed-model using longitudinal repeated measures), the 95% CI of each sertraline dose groups overlap, and the 95% CI should be appreciated instead of any exact point estimate. Supplemental Figure 2 provides EFA plots by sertraline dose.
Clinical Outcomes
Overall 2-week mortality was 22%, and 12-week mortality was 40%, and did not differ by sertraline dose group (Table 1). There were no significant differences in hospitalization duration, 2 week CSF sterility, or symptomatic recurrence (e.g. paradoxical IRIS, culture-positive relapse) across dosing groups. The paradoxical IRIS incidence was 5% (2/43) among participants with a first-episode of cryptococcosis who were ART-naïve and survived to initiate ART or those switched to second-line therapy due to virologic failure. No cryptococcal relapse occurred during 12 weeks of follow-up. Two participants developed CSF culture-positive relapse after 12 weeks, with one due to fluconazole non-compliance.
Safety and Tolerability
Adverse events and deaths in this critically ill population were common (Table 2). The numbers of individual Grade 4 adverse events occurring were: 6 among 17 participants receiving 100mg, 14 events among 60 participants receiving 200mg, 19 events among 50 participants receiving 300mg, and 8 events among 45 participants receiving 400mg. More broadly, Grade 4 or 5 adverse event risk did not differ between current FDA-approved dosing of 100–200mg/day and higher doses of 300–400mg/day (Hazard Ratio for Grade 4 or 5 adverse events=1·27; 95%CI, 0·69 to 2·32; P=·45). The majority of grade 4 or 5 adverse events were related to amphotericin toxicity or AIDS (53/59) including anemia (N=28), electrolyte abnormalities (N=15), cytopenia (N=4), and acute kidney injury (N=2). Cryptococcal-related deaths occurred in 24–25% of participants through 12 weeks, and non-cryptococcal deaths occurred in 14–17% of enrolled participants. Appendix 1 provides a summary of adverse events by dose group and line listing of the incident Grade 4–5 adverse events.
Table 2.
Adverse Events and Clinical Outcomes with adjunctive Sertraline through 12 weeks
| Clinical Outcomes | Sertraline 100 and 200mg/day |
Sertraline 300 and 400mg/day |
Absolute Risk Difference (95%CI) |
P- value |
|---|---|---|---|---|
| N enrolled | 77 | 95 | ||
| Adverse Events (AEs) | ||||
| Total number Grade 4 AEs | 20 | 27 | ||
| Grade 4 AE, cumulative incidence | 15 (19%) | 22 (23%) | 0·04 (−0·09, 0·16) | 0·56 |
| Total number Grade 5 AEs | 3 | 9 | ||
| Grade 5 AE, cryptococcal related | 1 (1%) | 3 (3%) | 0·02 (−0·02, 0·06) | 0·42 |
| Grade 5 AE, non-cryptococcal | 2 (3%) | 5 (5%) | 0·03 (−0·03, 0·08) | 0·38 |
| Overall 12-week Mortality | ||||
| Cryptococcal-related mortality | 19 (25%) | 23 (24%) | 0·0 (−0·13, 0·12) | 0·94 |
| Non-cryptococcal related mortality | 11 (14%) | 16 (17%) | 0·03 (−0·08, 0·13) | 0·65 |
| Nausea, Vomiting, Diarrhea, ≥1 event | 59 (77%) | 61 (64%) | −0·12 (−0·26, 0·01) | 0·08 |
| Serotonin Syndrome | 0 (0%) | 1 (1%)§ | -- | -- |
| Sertraline dose reduction, all cause | 0 (0%) | 1 (1%)§ | -- | -- |
| Early sertraline discontinuation* | 1 (1%) | 5 (5%) | 0·04 (−0·01, 0·09) | 0·16 |
| Lost to Follow up | 3 (4%) | 2 (2%) | −0·02 (−0·07, 0·03) | 0·49 |
Details by individual dose group are provide in Appendix 1. Hazard ratio and 95% CI from competing risks regression and corresponding p-value.
Excludes those with a previous history of cryptococcal meningitis.
Protocol deviation by a participant taking 800mg/day for three days.
A mild to moderate serotonin syndrome occurred in one participant who unintentionally self-administered 800mg/day of sertraline for three days over a weekend, which was a protocol deviation. Sertraline was held for three days, and then restarted at 200mg/day with an uneventful course thereafter. Overall tolerability was excellent with 4·0% (6/150) early discontinuation among participants with no previous history of cryptococcal meningitis.
Mortality during Consolidation Therapy by CSF Sterility
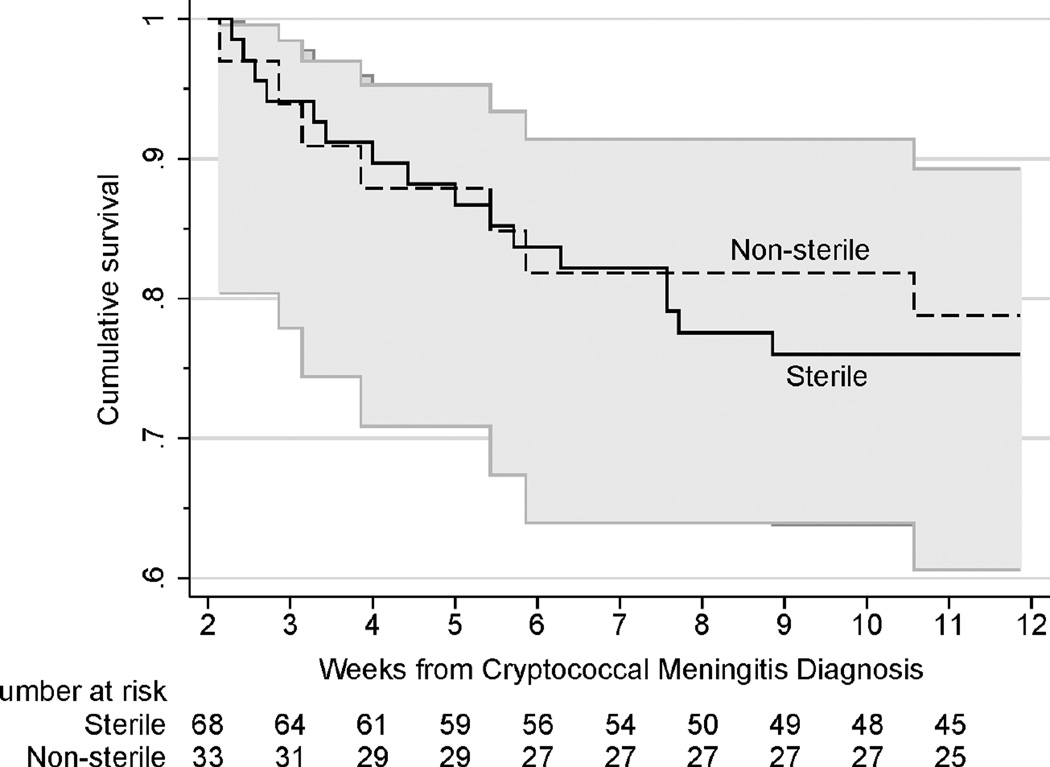
Of 101 participants with no prior history of cryptococcosis and/or sterile diagnostic quantitative cultures who survived 2-weeks post-enrollment, 67% (68/101) achieved CSF sterility by the end of induction therapy. Twenty-three deaths occurred between 2 and 12 weeks, 16 (24%) deaths among those who were sterile and 7 (21%) deaths among those not sterile). Unlike prior studies, CSF culture positivity at the end of induction therapy was not significantly associated with mortality (HR=0·88, 95%CI: 0·36, 2·15; P=·78) (Figure 3).
Figure 3. Kaplan-Meier plot of 12 week survival during consolidation therapy, stratified by 2 week CSF quantitative culture sterility.
Participants received 200mg/day sertraline with fluconazole for the duration of consolidation therapy. Fluconazole was dosed at 800mg for approximately 4 weeks until 2 week cultures were known to be sterile and ART was initiated. Unlike traditional consolidation therapy using only 400mg/day fluconazole,21–23 we did not observe that 2 week CSF culture positivity was associated with excess mortality while receiving sertraline at 200mg/day in combination with fluconazole (P=.78).
Pharmacokinetic Testing
Sertraline concentrations in plasma were quantified among 143 participants. Sertraline reached steady state in plasma by at least day 7, with median levels of 201 ng/mL (IQR, 90–300; n=49 participants) when taking 200mg/day and 399 ng/mL (IQR, 278–560; n=30 participants) when taking 400mg/day during days 7 to 14 of the induction period (Appendix 2). Plasma levels reached 82% of steady state levels by day 3. With 400mg/day, the projected steady state brain tissue concentration was 6·8 (IQR, 4·6–9·7) mcg/mL.
The presence of ART significantly increased sertraline clearance (P<·001), particularly at lower doses. Among 121 participants who had steady state sertraline concentrations measured, 55 (45%) were receiving concurrent efavirenz or nevirapine. Steady state sertraline concentrations were 27% lower among those receiving efavirenz (95%CI, 11–43%, P<·001) and 13% lower among those receiving nevirapine (95%CI, −3–29%, P=·10) when measured between days 7–14. Yet, among patients receiving 400mg/day sertraline, the median concentrations in patients not receiving ART were 397 ng/mL (IQR: 211, 495) versus 431 ng/mL (IQR: 246, 566) receiving ART.
Sertraline Susceptibility
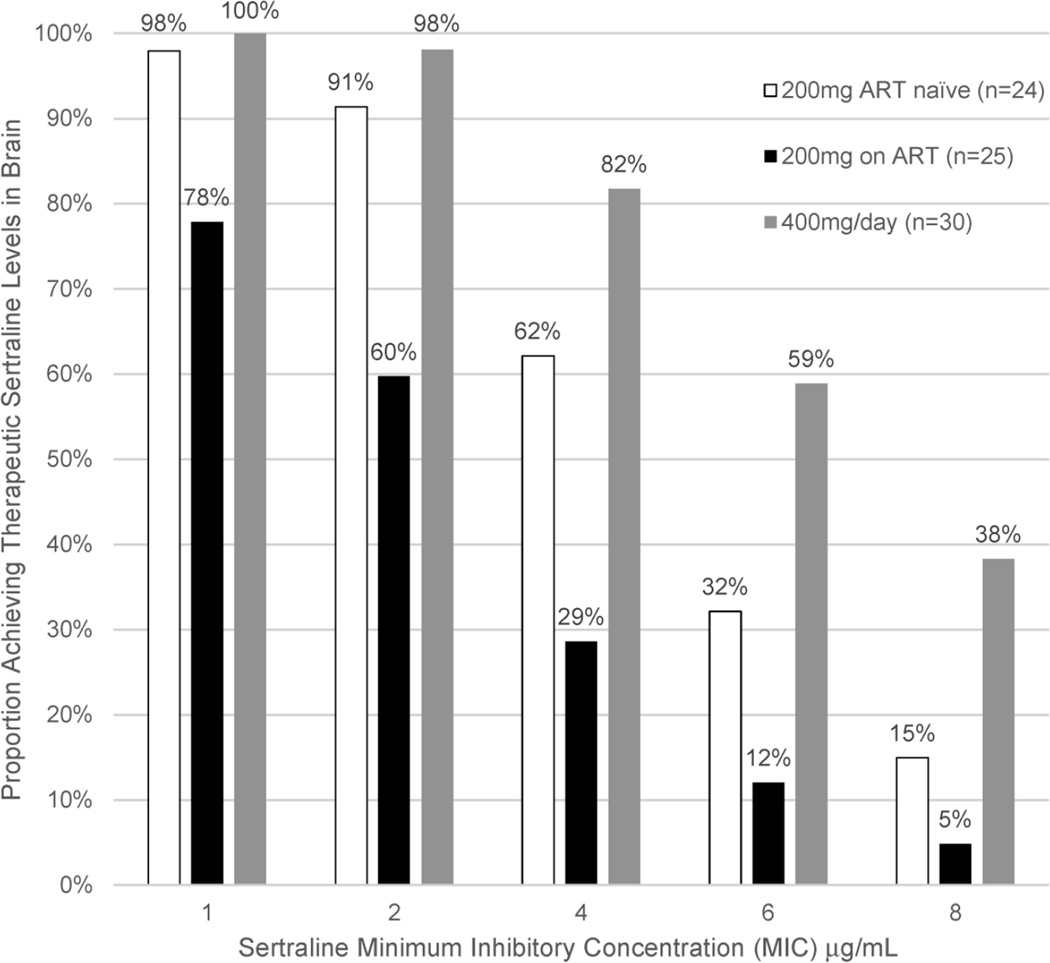
In vitro susceptibility testing was performed on 128 Cryptococcus neoformans isolates obtained from baseline CSF cultures from participants with first episode of cryptococcal meningitis. The MIC for sertraline ranged from 1 to 8 mcg/mL, with 27% of isolates having MICs of ≤2 mcg/mL, 84% ≤4 mcg/mL, 91% ≤ 6 mcg/mL, and 100% ≤8 mcg/mL. The proportion of persons achieving therapeutic brain sertraline levels based on Cryptococcus susceptibility and sertraline dose is presented in Figure 4. With 400mg/day of sertraline, 81% of a population would achieve probable therapeutic sertraline concentrations in the brain, based on the distributions of MICs, drug levels, and variability in brain penetration. With the 2-fold additive/synergistic effects of fluconazole, at sertraline 400mg/day, the projected proportion of persons achieving therapeutic sertraline levels in brain tissue rises from 81% to 97%.
Figure 4. Probability of Achieving Therapeutic Sertraline brain tissue levels based on Cryptococcus Susceptibility and Sertraline Dose in a Population.
Based on the steady state levels achieved between day 7 and 14 as well as the distribution of MICs, in Monte Carlo simulation 81% of a population would likely achieve therapeutic sertraline levels in the brain with 400mg/day, 65% of those ART-naïve receiving 200mg/day, and 35% of those receiving ART with 200mg/day. In the presence of 2-fold additive/synergistic effects of fluconazole, 97% at 400mg/day, 90% at 200mg/day without ART, and 62% at 200mg/day on ART would achieve therapeutic sertraline activity in brain. Overall, 91% (117/128) of Cryptococcus isolates exhibited a MIC of ≤6 µg/mL, 84% (108/128) with ≤4 µg/mL, and 27% (35/128) with MIC ≤2 µg/mL.
Discussion
Sertraline, when added to standard amphotericin-combination therapy for cryptococcal meningitis, may improve CSF fungal clearance. In this pilot dose-finding study, those receiving any sertraline demonstrated an average CSF clearance rate (−0·37 (95%CI: −0·41, −0·33) log10CFU/mL CSF/day. In comparison, the rate of CSF clearance was −0·30 (95%CI: −0·32, − 0·28) log10CFU/mL CSF/day among 208 participants screened for the Cryptococcal Optimal ART Timing (COAT) trial in Uganda and South Africa during 2010–2012. This historical cohort received the same background regimen of amphotericin 0.7–1.0 mg/kg/day and fluconazole 800mg/day without sertraline.5 Similarly, the CSF clearance rate among 99 participants in Vietnam receiving amphotericin 1mg/kg/day and fluconazole 800mg/day was −0·32 (−0·34 to – 0·29) log10 CFU/mL CSF/day.3
The rate of Cryptococcus clearance from the CSF is a powerful method to explore new drug combinations in smaller phase II studies.17 The confidence intervals in the rate of fungal clearance between those receiving any dose of sertraline and historical controls receiving the same standard antifungal therapy at the same site did not overlap.5 The comparison between sertraline dose groups did not reveal any overtly, alarming problems with safety or tolerability of the higher sertraline doses; however, the small sample sizes of the individual dose groups preclude sufficient statistical power to detect possibly important clinical differences.
Although the fungal clearance rate provides a relatively objective outcome measure,17 these results should be interpreted cautiously. Notably, patients receiving ART were excluded in the historical cohort, although neither receipt of ART in this study nor early ART in the COAT trial were statistically associated with EFA.5 The EFA observed with amphotericin and fluconazole 800mg/day in the COAT trial (n=208) was similar as observed by Day et al (n=99) using the identical statistical methodology.3,5 Definitive declarations cannot be given due to the overall small sample size and lack of direct statistical comparison, yet we believe that these observations provide ample justification for a further phase III randomized clinical trial to test the use of adjunctive sertraline.
The current standard therapy for cryptococcal meningitis is based on antifungal regimens that are a half-century old, associated with a range of toxicities, and largely inaccessible in areas of the world where they are needed most. For this reason, the discovery of a widely available, nontoxic, and affordable drug effective against Cryptococcus would represent a significant advance in preventing deaths. Sertraline represents a promising adjunct and deserves further investigation in randomized clinical trials.
The safety and tolerability of sertraline was good. The incidence of Grade 4–5 adverse events and GI side effects, although seemingly high, were similar to or less than historical experience.5 During the COAT trial through 12 weeks,5 there was a 50% cumulative incidence of Grade 4 adverse events and 68% (142/208) incidence of nausea, vomiting, or diarrhea in comparison with higher dose sertraline at 300–400mg/day having 23% cumulative incidence of Grade 4 adverse events and 64% incidence of nausea, vomiting, or diarrhea. Identifying effective and less toxic antifungal induction regimens could lead to less dependence on completing a full, 14-day course of amphotericin. In contrast, fluconazole monotherapy remains a reality worldwide where amphotericin is unavailable19 and leads to suboptimal clearance, resistance, and symptomatic relapse.18,20 Given the widespread availability, low cost ($0·05 per 100mg tablet wholesale), and extensive safety record of sertraline, the combination of sertraline with fluconazole may offer a more efficacious and cost-effective, all-oral option in such settings. Furthermore, unlike amphotericin and flucytosine, the ability to safely administer sertraline over a prolonged duration would allow antifungal benefits to extend through the consolidation and maintenance phases of therapy.
Unlike prior cohorts, we observed no excess mortality over 12 weeks in persons who remained CSF culture positive at 2 weeks,21–23 suggesting the benefit of sertraline may be extended into the consolidation phase due to the additive antimicrobial effects of combination sertraline and fluconazole.6 Depression is common in this population (77%),24 and sertraline would be the obvious antidepressant of choice, if indicated. Additional studies are needed to assess the role of sertraline in consolidation and maintenance phases of therapy, during induction therapy in the absence of amphotericin, and for non-meningitis manifestations of cryptococcosis including asymptomatic cryptococcal antigenemia identified as part of screening programs.25,26
A possible, unanticipated benefit of extending adjunctive sertraline beyond the induction period was the observed low incidence of symptomatic recurrence, including both culture-positive cryptococcal relapse and paradoxical IRIS. Although patients were only actively followed for 12 weeks and passively thereafter, the incidence of ~5% paradoxical IRIS and two late relapse cases appeared to be lower than the 17% IRIS incidence during the COAT trial,5 and lower than the 25–30% paradoxical cryptococcal-IRIS observed in two cohorts using amphotericin alone as induction therapy with fluconazole 400mg/day consolidation therapy.23,27 The lack of achieving eventual CSF culture sterility is a major risk factor for IRIS and relapse.23 Improved overall microbiologic activity on active and quiescent yeasts may be plausible explanations for the low level of recurrence observed. It is also plausible that sertraline may have immunoregulatory effects on immune activation resulting in a decreased incidence of paradoxical IRIS.28 The possible decreased IRIS and relapse with adjunctive sertraline is intriguing and requires further investigation in a randomized trial.
The results of our in vitro susceptibility testing confirm previous studies that provided the impetus for this study, with MICs ≤4 mcg/mL in ~80% of Ugandan isolates, and reported bidirectional synergy with the addition of fluconazole.6,10,11 Synergy may reflect additive mechanisms of antifungal action. Fluconazole targets the enzyme 14α-demethylase, with inhibition of ergosterol synthesis and subsequent membrane disruption. Sertraline, in contrast, inhibits mRNA translation into protein synthesis.6 For these reasons, the addition of sertraline to standard fluconazole-containing regimens could be ideal for treating persistent or recurrent infections with Cryptococcus, factors associated strongly with increased rates of fluconazole resistance.20
SSRIs are among the most prescribed drug classes worldwide, and sertraline remains among the most prescribed medications in the U.S., with approximately 44 million prescriptions in 2014.29 Although inter-person steady-state plasma concentrations vary substantially,30 observed plasma concentrations in our population of Ugandan adults with advanced AIDS were generally in the expected range. Sertraline has minimal inhibitory effects on the major cytochrome P450 enzymes, and few drug-drug interactions of clinical significance have been documented.31 Based on limited observational studies, however, both efavirenz and rifampin are suspected to lower sertraline levels when co-administered.32,33 Our results support these observations, with ~27% lower sertraline plasma concentrations when receiving efavirenz, although the effect of this interaction in the brain is unknown. Only three participants received concurrent rifampin, and sertraline levels were too variable to make firm conclusions. Further analyses of the effect of these medications on sertraline concentrations are needed.
Of particular interest in using sertraline for fungal meningitis, previous pharmacokinetic studies in animals report that sertraline concentrations are 20 to 50-fold higher in the brain than in blood,34 and a human study of 11 fatal air crash victims revealed an average 21-fold and median 16·5-fold higher concentration in human brain tissue compared to blood.9 Although sertraline is concentrated into the brain tissue, we found low levels in CSF, possibly because sertraline is a lipophilic compound concentrated within the brain parenchyma itself, not CSF. As amphotericin penetrates poorly into brain parenchyma,35 the overall benefit of sertraline may be more substantial than the CSF findings suggest. Despite our inability to measure sertraline concentrations in CSF, the inferred pharmacokinetics suggest that brain concentrations of sertraline likely exceed the sertraline MICs reported in vitro when dosed at 400mg/day. In the presence of at least 2-fold additive effect of fluconazole,6 therapeutic levels of sertraline in the brain should be achieved in 97% of persons when dosed at 400mg/day, 90% of persons dosed at 200mg/day without ART, and 62% of persons dosed at 200mg/day on efavirenz. Similar therapeutic levels should be achieved in liver, lung, and spleen tissue.9
In summary, an improved rate of fungal clearance from the CSF, combined with acceptable concentrations and the additive effects of sertraline used in combination with fluconazole in vitro,6 suggest that sertraline may be a useful adjunct for the treatment of cryptococcal meningitis with further prevention of paradoxical cryptococcal IRIS and relapse. Given sertraline’s fungicidal properties, safety profile, lack of relevant drug interactions, likely novel mechanism of action, low cost, and excellent brain penetration, sertraline fulfills many of the characteristics required of a new antifungal against Cryptococcus. Based on the Cryptococcus MICs observed and probable brain concentrations of 400mg/day sertraline, 81% of persons would achieve therapeutic sertraline activity in brain tissue. With the 2-fold additive/synergistic effects of fluconazole, 97% of a population would be predicted to achieve therapeutic sertraline activity in brain tissue. The Adjunctive Sertraline for the Treatment of Cryptococcal Meningitis (ASTRO-CM) randomized clinical trial (Clinicaltrials.gov: NCT01802385) began in March 2015 and is testing if sertraline dosed initially at 400mg/day has a survival benefit compared to placebo when receiving standard induction therapy of amphotericin B deoxycholate and fluconazole 800mg/day.
Supplementary Material
172 participants with cryptococcal meningitis provided informed consented and received at least one dose of sertraline. The primary outcome of the rate of CSF clearance was measured in all participants with first episode of culture-positive cryptococcal meningitis and ≥2 quantitative CSF cultures. Secondary outcomes included in vitro susceptibility testing of Cryptococcus isolates to sertraline and plasma pharmacokinetic concentrations of sertraline.
Acknowledgments
This research was supported by the National Institute of Neurologic Diseases and Stroke (NINDS) and the Fogarty International Center (R01NS086312, R25TW009345), Grand Challenges Canada (S4-0296-01), and National Institute of Allergy and Infectious Diseases (T32AI055433, K24AI096925). This work was supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. Drs. Elissa Butler, Jonathan Dyal, and A. Wendy Fujita are Doris Duke International Clinical Research Fellows.
Appendix
ASTRO-CM Team members: Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Alisat Sadiq, Tadeo Kiiza Kandole, Tony Luggya, Julian Kaboggoza, Eva Laker, Elissa K Butler, Jonathan Dyal, Julie M Neborak, Alexa M King, A. Wendy Fujita, Nathan Yueh, Alice Namudde, Ryan Halupnick, Bilal Jawed, Priya Vedula, Marnie Peterson, Paul R Bohjanen, Andrew Kambugu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: We declare that we have no conflicts of interest.
Contributors:
Study Concept and Design: Boulware, Meya, Rhein
Acquisition of Data: Rhein, Nabeta, Kiggundu, Tugume, Musubire, Akampurira, Williams, Ndyetukira, Ahimbisibwe, Kugonza, Sadiq, Abassi, Bahr, Velamakanni, Butler, Dyal, Yueh, Namudde, Kandole, Luggya, Fisher, Alhadab, Smith, Vedula.
Statistical Analysis: Hullsiek, Morawski, Alhadab, Boulware
Interpretation of data: Boulware, Meya, Rhein, Alhadab, Peterson, Nielsen
Initial Manuscript Drafting: Rhein, Velamakanni, Morawski
Critical revisions for intellectual content: Boulware, Meya, Rhein, Nielsen, Musubire
Obtaining funding: Boulware, Meya, Rhein
Administrative, technical, or material support: Williams, Bohjanen, Kaboggoza, Kambugu, Laker, Peterson, Halupnick
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr. 2013;63(3):e101–e108. doi: 10.1097/QAI.0b013e31828e1e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day JN, Chau TT, Wolbers M, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58(5):736–745. doi: 10.1093/cid/cit794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother. 2012;56(7):3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith KD, Achan B, Hullsiek KH, et al. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother. 2015;59(12):7197–7204. doi: 10.1128/AAC.01299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trevino-Rangel RJ, Villanueva-Lozano H, Hernandez-Rodriguez P, et al. Activity of sertraline against Cryptococcus neoformans: in vitro and in vivo assays. Med Mycol. 2016;54 doi: 10.1093/mmy/myv109. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Lewis RJ, Angier MK, Williamson KS, Johnson RD. Analysis of sertraline in postmortem fluids and tissues in 11 aviation accident victims. J Anal Toxicol. 2013;37(4):208–216. doi: 10.1093/jat/bkt014. [DOI] [PubMed] [Google Scholar]
- 10.Nayak R, Xu J. Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformans and Cryptococcus gattii. Mycology. 2010;1(2):99–105. [Google Scholar]
- 11.Spitzer M, Griffiths E, Blakely KM, et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol. 2011;7:499. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45(1):76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 13.Dyal J, Akampurira A, Rhein J, et al. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol. 2016;54 doi: 10.1093/mmy/myv104. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—third edition. CLSI document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 15.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiesner DL, Moskalenko O, Corcoran JM, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3(5) doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicanic T, Muzoora C, Brouwer AE, et al. Independent association between rate of clearance of infection and clinical outcome of HIV associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49(5):702–709. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47(12):1556–1561. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 19.Loyse A, Thangaraj H, Easterbrook P, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis. 2013;13(7):629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 20.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43(8):1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 21.Robinson PA, Bauer M, Leal MA, et al. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28(1):82–92. doi: 10.1086/515074. [DOI] [PubMed] [Google Scholar]
- 22.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337(1):15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 23.Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27(13):2089–2099. doi: 10.1097/QAD.0b013e3283614a8d. [DOI] [PubMed] [Google Scholar]
- 24.Carlson RD, Rolfes MA, Birkenkamp KE, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis. 2014;29(2):269–279. doi: 10.1007/s11011-013-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count< or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51(4):448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr. 2012;59(5):e85–e91. doi: 10.1097/QAI.0b013e31824c837e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maes M, Song C, Lin AH, et al. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20(4):370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 29.IMS Health. National Prescription Audit. 2015 Jan; https://itunes.apple.com/app/ims-institute/id625347542. [Google Scholar]
- 30.DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41(15):1247–1266. doi: 10.2165/00003088-200241150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Naranjo CA, Sproule BA, Knoke DM. Metabolic interactions of central nervous system medications and selective serotonin reuptake inhibitors. Int Clin Psychopharmacol. 1999;14(Suppl 2):S35–S47. [PubMed] [Google Scholar]
- 32.Ruiz NM, Labriola DF, Fiske WD, Joshi AS, Manion DJ, Villano SA. Efavirenz plasma levels and therapeutic response are affected in patients concomitantly receiving selective serotonin reuptake inhibitors. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; Toronto, Canada. 2000. [Google Scholar]
- 33.Markowitz JS, DeVane CL. Rifampin-induced selective serotonin reuptake inhibitor withdrawal syndrome in a patient treated with sertraline. J Clin Psychopharmacol. 2000;20(1):109–110. doi: 10.1097/00004714-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Tremaine LM, Welch WM, Ronfeld RA. Metabolism and disposition of the 5-hydroxytryptamine uptake blocker sertraline in the rat and dog. Drug Metab Dispos. 1989;17(5):542–550. [PubMed] [Google Scholar]
- 35.Bellmann R. Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol. 2007;2(1):37–58. doi: 10.2174/157488407779422311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
172 participants with cryptococcal meningitis provided informed consented and received at least one dose of sertraline. The primary outcome of the rate of CSF clearance was measured in all participants with first episode of culture-positive cryptococcal meningitis and ≥2 quantitative CSF cultures. Secondary outcomes included in vitro susceptibility testing of Cryptococcus isolates to sertraline and plasma pharmacokinetic concentrations of sertraline.