Abstract
Objective
To evaluate the effect of early integrated, late integrated and delayed antiretroviral therapy (ART) initiation during tuberculosis (TB) treatment on incidence rates of loss to follow-up (LTFU) and to evaluate the effect of ART initiation on LTFU rates within trial arms in patients co-infected with TB and HIV.
Methods
A sub-study within a three-armed open label, randomised, controlled trial. Patients were randomised to initiate ART either early or late during TB treatment or after the TB treatment completion. We reported incidence and predictors of LTFU from TB treatment initiation during the 24 months of follow-up. LTFU was defined as having missed 4 consecutive monthly visits with inability to make contact.
Results
Of the 642 patients randomised, a total of 96 (15.0%) were LTFU at a median of 6.0 (interquartile range (IQR): 1.1 to 11.3) months after TB treatment initiation. Incidence rates of LTFU was 7.5 per 100 person-years (py) (95% confidence interval (CI), 4.9 to 11), 10.9 per 100 py (95% CI, 7.6 to 15.1) and 11.0 per 100 py (95% CI, 7.6 to 15.4) in the early integrated, late integrated and delayed treatment arm (p=0.313). Incidence rate of LTFU before and after ART initiation was 31.7 per 100 py (95% CI, 11.6 to 69.0) vs. 6.1 per 100 py (95% CI, 3.7 to 9.4), incidence rate ratio (IRR): 5.2 (95% CI, 2.1 to 13.0), p<0.001 in the early integrated arm; 31.9 per 100 py (95% CI, 20.4 to 47.5) vs. 4.7 per 10 py (95% CI: 2.4 to 8.2), IRR: 6.8 (95% CI, 3.4 to 13.6), p<0.0001 in the late integrated arm and 21.9 per 100 py (95% CI, 14.6 to 31.5) vs. 2.8 per 100 py (95% CI, 0.9 to 6.6), IRR: 7.7 (95% CI, 3.0 to 19.9), p<0.0001 in the sequential arm.
Conclusion
LTFU rates were not significantly different between the three trials arms. However, ART initiation within each trial arm resulted in a significant reduction in LTFU rates among TB patients.
Keywords: TB, HIV, ART, Integration, Loss to follow-up, RCT, South Africa
INTRODUCTION
Tuberculosis (TB) remains a major cause of morbidity and mortality among people living with human immunodeficiency virus (HIV). 1 Almost 78% of TB-HIV co-infected patients reside in sub-Saharan Africa. 2 Loss to follow-up (LTFU) in both TB and antiretroviral therapy (ART) programmes remains a challenge globally, and more especially in sub-Saharan Africa, where patients present late for care with advanced clinical diseases 3,4 and very low CD4+ counts. 5,6 LTFU often masks adverse clinical outcomes such as death and hospitalisation due to poor access to health care, but may also reflect the fact that the local patient population is highly mobile, as they often move between urban and rural homes. 7
While TB therapy and ART saves lives, 8 continuous success depends on regular clinic visits to ensure that viral suppression is sustained and monitor TB adherence until cure or therapy success is achieved. Published data show higher LTFU rates compared to mortality rates in ART programmes in sub-Saharan Africa where the largest contributor of attrition in ART programmes was LTFU at 56% followed by death at 40%. 9 In sub-Saharan Africa, we find retention rates of 49% – 90.3% in the first year of ART and 46% – 85.4% after 2 years. 9 It is important to note that the proportion of ART naïve patients who are LTFU during the first year of ART initiation is 3 fold higher in low income settings than in rich countries. 10 On the other hand, literature shows that LTFU rates among patients on TB treatment eligible for ART are around 11.0% in South Africa.11 These patients were LTFU approximately 2 months after commencing TB treatment. 11 Furthermore, not presenting for ART assessment and not being initiated on ART were risk factors associated with LTFU among TB patients 11. Studies and systematic reviews have described LTFU and mortality among patients attending HIV care services. 9,12,13 However, data on patients co-treated for TB and HIV is still limited.
The aim of the study was to evaluate the effect of early integrated, late integrated and delayed ART initiation during TB treatment on incidence rates of LTFU and to evaluate the effect of ART initiation on LTFU rates within trial arms in patients co-infected with TB and HIV. We report incidence and predictors of LTFU at different follow-up time points in a randomised controlled clinical trial.
METHODS
Study population
The study design and the results of the Starting Antiretroviral Therapy at Three Points in Tuberculosis (SAPiT) trial have been published in detail elsewhere. 8,14,15 Briefly, the study was a three-armed open label, randomised, controlled trial conducted between June 2005 and July 2010 to determine the optimal timing of ART initiation in newly diagnosed patients with HIV and smear positive pulmonary tuberculosis. At TB treatment initiation, ART naïve patients were randomly assigned to initiate ART within 4 weeks of tuberculosis treatment initiation (early integrated arm), within 4 weeks after completion of the intensive phase of tuberculosis treatment (late integrated arm) or within 4 weeks after completion of tuberculosis therapy (sequential arm). Patients accessed routine TB services from the adjacent large outpatient government supported TB facility. Patients accessed HIV care at the CAPRISA treatment research clinic, where counselling, adherence assessments and clinical toxicity monitoring for both TB and HIV treatment also occurred.
Patients who missed scheduled monthly visits were tracked telephonically and if that failed then they were physically tracked. LTFU was defined as having missed 4 consecutive monthly visits with the inability to establish any contact with the patient. Post study exit, the South African Department of Home Affairs system was used to ascertain whether LTFU patients were currently dead or alive.
The SAPIT study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (E107/05) and the Medicines Control Council of South Africa (20060157).
Data analysis
Follow-up time was calculated from TB treatment initiation until the earliest of: last visit date for those who were LTFU, date of death, termination date or administrative censoring at 24 months. In the results published previously, 8,14,15 administrative censoring took place at 18 months post TB treatment initiation but in this analysis administrative censoring was at 24 months. Analysis was conducted at different follow-up times namely: during TB treatment, after the completion of TB treatment, before and after ART initiation as well as at 24 months post TB treatment initiation.
Multivariate proportional hazards regression models were used to identify predictors of LTFU at different follow-up time points. Baseline variables that were included in the multivariate analysis were: trial arm, age, gender, WHO stage of HIV disease, CD4+ cell count, presence or absence of history of TB and employment status. We used Poisson approximations to calculate 95% confidence interval (CI) for incidence rates and incidence rate ratio (IRR). Proportionality was tested by fitting the time dependent covariates created by interacting the baseline variables and a function of survival time. Statistical analyses were done using Statistical Analysis Software version 9.3 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Patients’ characteristics
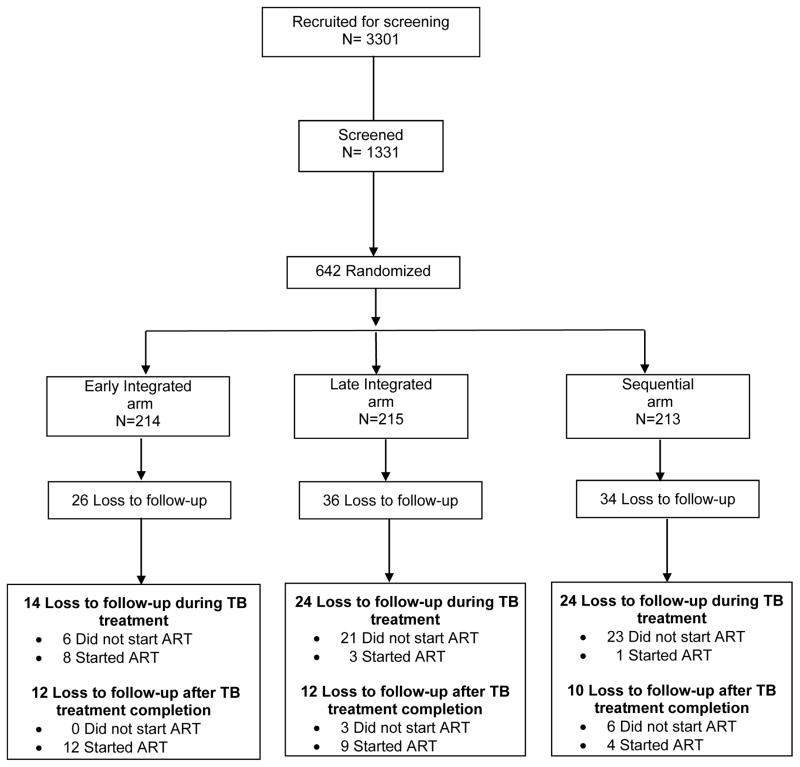
A total of 642 patients were randomised (Figure 1). Patients in the early integrated (n = 214), late integrated (n=215), and sequential (n =213) arms had similar baseline demographic and clinical characteristics. 15 Of the 642, 96 (15.0%) patients were LTFU, 26 (12.1%) in the early integrated, 36 (16.7%) in the late integrated and 34 (16.0%) in the sequential arm. Notably, the proportion of patients who were LTFU in this study was higher than the overall mortality of 10.7% (69/642). The proportion of patients LTFU before and after ART initiation was 9.2% (59/642) and 5.8% (37/642) respectively. LTFU among the 96 patients occurred at a median of 6.0 (interquartile range (IQR): 1.1 to 11.3) months after TB treatment initiation. LTFU before and after ART initiation occurred 2.8 (IQR: 0.5 to 6.7) and 10.4 (IQR: 6.2 to 15.7) months after TB treatment initiation. The mean duration on TB treatment was 7.1 months. The mean duration by trial arm was 7.3, 7.1 and 6.9 months in the early integrated, late integrated and sequential arm respectively. Of the 96 LTFU, 64.6% (62/96) were LTFU during TB treatment while 35.4% (34/96) were LTFU after completing TB treatment (Figure 1).
Figure 1.
SAPiT trial: Screening, randomization, and follow-up of study patients demonstrating loss to follow-up
Vital status for 88 of the 96 patients that were LTFU was obtained, 8 could not be traced (three in the early integrated arm, four in the late integrated arm and one in the sequential arm) due to missing identity numbers. 31.8% (28/88) were confirmed dead (four in the early integrated arm, 10 in the late integrated arm and 14 in the sequential arm), 68.2% (60/88) were still alive (19 in the early integrated arm, 22 in the late integrated arm and 19 in the sequential arm).
Incidence of LTFU
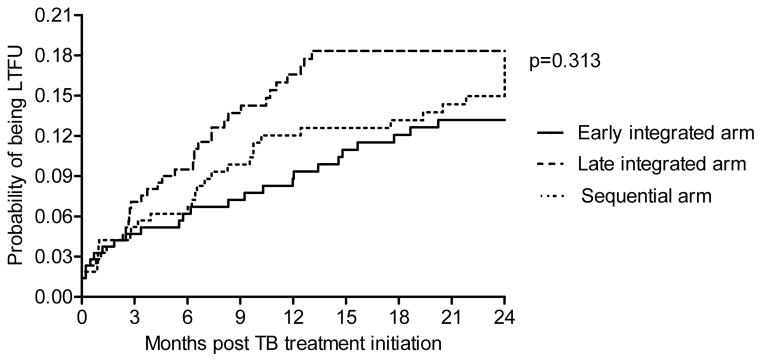
Incidence rates of LTFU was 7.5 per 100 person-years (py) (95% confidence interval (CI), 4.9 to 11), 10.9 per 100 py (95% CI, 7.6 to 15.1) and 11.0 per 100 py (95% CI, 7.6 to 15.4) in the early integrated, late integrated and sequential arm (p=0.313) (Table 1, Figure 2).
Table 1.
Incidence rate of LTFU at different follow-up time points
| Time post TB treatment initiation | Early integrated arm | Late integrated arm | Sequential arm | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Person years | Incidence rate (95%CI) | Events | Person years | Incidence rate (95%CI) | Events | Person years | Incidence rate (95%CI) | ||
| During TB treatment | 14 | 128.7 | 10.9 (5.9 to 18.3) | 24 | 126.6 | 19.0 (12.1 to 28.2) | 24 | 125.9 | 19.1 (12.2 to 28.4) | 0.182 |
| After TB treatment completion | 12 | 217.2 | 5.5 (2.9 to 9.7) | 12 | 203.4 | 5.9 (3.0 to 10.3) | 10 | 182.7 | 5.5 (2.6 to 10.1) | 0.982 |
|
| ||||||||||
| Comparing during and after TB treatment completion | IRR:2.0 (95% CI:0.9 to 4.3) p=0.085 |
IRR: 3.2 (95% CI:1.6 to 6.4) P=0.001 |
IRR: 3.5 (1.7 to 7.3) P=0.001 |
|||||||
|
| ||||||||||
| End of study (24 months) | 26 | 345.8 | 7.5 (4.9 to 11.0) | 36 | 330.0 | 10.9 (7.6 to 15.1) | 34 | 308.6 | 11.0 (7.6 to 15.4) | 0.313 |
| Before ART initiation | 6 | 18.9 | 31.7 (11.6 to 69.0) | 24 | 75.2 | 31.9 (20.4 to 47.5) | 29 | 132.4 | 21.9 (14.6 to 31.5) | 0.503 |
| After ART initiation | 20 | 326.9 | 6.1 (3.7 to 9.4) | 12 | 254.8 | 4.7 (2.4 to 8.2) | 5 | 176.2 | 2.8 (0.9 to 6.6) | 0.085 |
|
| ||||||||||
| Comparing before and after ART initiation | IRR:5.2 (95% CI:2.1 to 13.0) p<0.001 |
IRR: 6.8 (95% CI:3.4 to 13.6) p<0.0001 |
IRR: 7.7 (3.0 to 19.9) p<0.0001 |
|||||||
LTFU: lost to follow-up, TB: tuberculosis, ART: antiretroviral therapy, IRR: incidence rate ratio, CI: Confidence Interval
Figure 2.
Kaplan–Meier estimates of cumulative probability of being LTFU.
Incidence rate of LTFU before and after ART initiation was 31.7 per 100 py (95% CI, 11.6 to 69.0) vs. 6.1 per 100 py (95% CI, 3.7 to 9.4), IRR: 5.2 (95% CI, 2.1 to 13.0), p<0.001 in the early integrated arm; 31.9 per 100 py (95% CI, 20.4 to 47.5) vs. 4.7 per 100 py (95% CI, 2.4 to 8.2), IRR: 6.8 (95% CI, 3.4 to 13.6), p<0.0001 in the late integrated arm and 21.9 per 100 py (95% CI, 14.6 to 31.5) vs. 2.8 (95% CI: 0.9 to 6.6) per 100 py, IRR: 7.7 (95% CI: 3.0 to 19.9), p<0.0001 in the sequential arm (Table 1).
The overall incidence rate of LTFU at 24 months post TB treatment initiation was 9.8 per 100 py (95% CI, 7.9 to 11.9).
The incidence rates for all other follow-up time points are shown in Table 1. Combining the 3 trial arms together, the incidence rate of LTFU was higher during TB treatment and declined thereafter: from 16.3 per 100 py (95%CI, 12.5 to 20.9) to 5.6 per 100 py (95% CI, 3.9 to 7.9) (p < 0.001). The same trend was observed within each trial arm. Incidence rate of LTFU during and after TB treatment completion was 10.9 per 100 py (95% CI, 5.9 to 18.3) vs. 5.5 per 100 py (95% CI, 2.9 to 9.7), IRR: 2.0 (95% CI, 0.9 to 4.3), p=0.085 in the early integrated arm; 19.0 per 100 py (95% CI, 12.1 to 28.2) vs. 5.9 per 100 py (95% CI: 3.0 to 10.3), IRR: 3.2 (95% CI, 1.6 to 6.4), p=0.001 in the late integrated arm and 19.1 per 100 py (95% CI, 12.2 to 28.4) vs. 5.5 per 100 py (95% CI, 2.6 to 10.1), IRR: 3.5 (95% CI, 1.7 to 7.3), p=0.001 in the sequential arm (Table 1).
Risk factors associated with LTFU
In an adjusted analysis, gender (male vs. female) was a significant predictor of LTFU during TB treatment (HR: 1.8; 95% CI: 1.03 to 3.1), after TB treatment (HR: 2.4; 95% CI: 1.1 to 5.0), before ART initiation (HR: 1.9; 95% CI: 1.1 to 3.3) and at 24 months post TB treatment initiation (HR: 1.9; 95% CI: 1.2 to 3.0).
Age (per 5 year increase) was a significant predictor of LTFU during TB treatment (HR: 0.8; 95% CI: 0.6 to 0.9), before ART initiation (HR: 0.7; 95% CI: 0.6 to 0.9), and at 24 months post TB treatment initiation (HR: 0.8; 95% CI: 0.7 to 0.9). Employment status (employed vs. unemployed) was also associated with LTFU after TB treatment (HR: 0.3; 95% CI: 0.1 to 0.6) whereas CD4+ count at baseline (<200 vs. >350 cells/mm3) was a significant predictor of LTFU at 24 months post TB treatment initiation (HR: 0.6; 95% CI: 0.3 to 0.99) (Table 2). In addition, patients who were LTFU had higher mean CD4+ count over time than those who were not LTFU (Figure S1).
Table 2.
Risk factors associated with LTFU
| Variable | Overall: number LTFU/Number of patients | During TB treatment | After TB treatment | Before ART initiation | After ART initiation | Overall (at 24 months) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| aHR (95%CI) | p-value | aHR (95%CI) | p-value | aHR (95%CI) | p-value | aHR (95%CI) | p-value | aHR (95%CI) | p-value | ||
|
| |||||||||||
| a Trial arm | |||||||||||
| Late integrated arm | 36/215 | 1.8 (0.9–3.5) | 0.080 | 0.8 (0.4–1.9) | 0.695 | 1.6 (0.6–4) | 0.348 | 0.6 (0.3–1.3) | 0.218 | 1.4 (0.8–2.3) | 0.222 |
| Sequential arm | 34/213 | 1.8 (0.9–3.5) | 0.080 | 0.8 (0.4–1.9) | 0.662 | 1.2 (0.5–3.2) | 0.659 | 0.3† (0.1–0.8) | 0.024 | 1.4 (0.8–2.3) | 0.230 |
| Age (per 5 year increase) | 0.8† (0.6–0.9) | 0.004 | 0.8 (0.7–1.0) | 0.098 | 0.7† (0.6–0.9) | 0.003 | 0.9 (0.8–1.1) | 0.456 | 0.8† (0.7–0.9) | 0.002 | |
| b Gender | |||||||||||
| Male | 57/319 | 1.8† (1.03–3.1) | 0.038 | 2.4† (1.1–5.0) | 0.024 | 1.9† (1.1–3.3) | 0.032 | 1.8 (0.9–3.8) | 0.098 | 1.9† (1.2–3.0) | 0.004 |
| c CD4+ cell count (cells/mm3) | |||||||||||
| <200 | 51/408 | 0.6 (0.3–1.2) | 0.158 | 0.5 (0.2–1.4) | 0.194 | 0.8 (0.4–1.7) | 0.576 | 0.5 (0.2–1.4) | 0.211 | 0.6† (0.3–0.99) | 0.049 |
| 200–350 | 28/160 | 0.9 (0.4–1.8) | 0.700 | 0.6 (0.2–1.6) | 0.269 | 0.9 (0.4–2.0) | 0.800 | 0.9 (0.3–2.5) | 0.774 | 0.7 (0.4–1.3) | 0.314 |
| d Past history of TB | |||||||||||
| Yes | 29/214 | 1.2 (0.7–2.1) | 0.534 | 0.6 (0.3–1.4) | 0.264 | 1.2 (0.7–2.2) | 0.437 | 0.5 (0.2–1.2) | 0.116 | 1.0 (0.6–1.5) | 0.867 |
| e WHO stage | |||||||||||
| Stage 4 | 4/38 | 0.3 (0–1.9) | 0.189 | 1.4 (0.4–4.8) | 0.557 | No estimate | 1.9 (0.7–5.6) | 0.230 | 0.7 (0.2–1.8) | 0.433 | |
| f Employment status | |||||||||||
| Employed | 55/369 | 1.7 (0.99–3.0) | 0.061 | 0.3† (0.1–0.6) | 0.002 | 1.4 (0.8–2.4) | 0.227 | 0.6 (0.3–1.2) | 0.135 | 0.9 (0.6–1.4) | 0.710 |
aHR: Adjusted hazard ratio, LTFU: lost to follow-up, TB: tuberculosis, ART: antiretroviral therapy, CI: Confidence Interval
Reference categories for each predictor variable:
Early integrated arm (26/214),
Female (39/323),
CD4 cell count>350 cells/mm3 (17/74),
No past history of TB (67/428),
WHO stage 3 (92/604),
Unemployed (41/273)
Statistically significant at a 5% level of significance
DISCUSSION
The incidence rates of LTFU was not significantly different across the three trial arms. However, we report dramatically reduced LTFU rates among TB patients initiating ART compared to those not initiating ART within each trial arm (Table 1). In this paper we provide evidence demonstrating further benefit of initiating ART during TB treatment at both an individual patient level and at a TB control programme level. We demonstrated 5.2; 6.8 and 7.7 fold higher rates of LTFU before ART initiation compared to after ART initiation in the early integrated, late integrated and sequential arms respectively. The median time to LTFU among patients not initiated on ART was 2.8 (IQR: 0.5 to 6.7) months. This increases the strength of the World Health Organisation (WHO) recommendation of initiating ART within the first eight weeks of TB treatment, 16 as this not only provides a survival benefit it also confers a programmatic benefit of reducing LTFU. Data to date have suggested that integrating TB and HIV may undermine the TB control program through issues of increase pill burden and programmatic challenges of co-managing both diseases. 17 However, the risk of increased mortality from not initiating ART timeously in TB patients has been shown. 8
Despite being enrolled for TB services, we found that the majority of retention losses occurred among TB patients that had not yet initiated ART. Furthermore, an observational study conducted on ART eligible patients in South Africa showed LTFU rates of approximately 10%, among newly-diagnosed TB patients and in those already on TB treatment at study entry. The pre-ART LTFU seen in our study is very similar to rates seen elsewhere in sub-Saharan Africa, 13 where up to 69% of patients did not return to the clinic within 1 year of enrolment. 18 The TB programmes in Africa are also affected with higher pre-TB treatment LTFU, with rates ranging from 6% to 38% among smear or culture positive tuberculosis. 19 Our data highlights the wider benefit of ART initiation during TB treatment and underscores the need for implementing retention strategies in pre-ART care especially in TB and HIV endemic settings.
While ART scale up in South Africa has successfully reduced AIDS related mortality, LTFU within the ART programme has increased substantially. 20 These data add to the pool of data suggesting that LTFU masks mortality. We found almost a third (29.2%) of the 96 patients initially deemed LTFU, were later confirmed dead. This and several other studies have showed that the mortality rate among LTFU patients is much higher than among patients remaining in care. 21 While we are unsure whether these deaths occurred early or late in the TB program, it highlights the need for effective retention strategies and seamless linkage to services especially for TB HIV co-infected patients and for those with advanced immunosuppression who are at higher risk for mortality.
Published studies evaluating LTFU cite patient transfer to nearby clinics, financial difficulty, improved or deteriorating health21–24, and mortality in about 20% to 60% of patients as the most common reasons for LTFU. 21,24–26 This study identified several factors that were associated with LTFU in Table 2. Trial arm, age, gender, CD4+ count and employment status were significant predictors of LTFU at different follow-up time points. Males and younger patients were consistently at high risk of being LTFU in most follow-up time points. Consistent with previous publications,20,27–30 females and older patients were less likely to be LTFU. Unemployment has been associated with higher risk of LTFU during TB treatment. 27
However, we found that unemployment was associated with higher risk of LTFU after TB treatment completion. Unemployed patients possibly relocated to other cities to look for employment. Clinic operating hours and finding new employment were cited as the reason for defaulting, 31 therefore it might be difficult for patients to ask the new employer for time off work every month to go to the clinic or hospital. Therefore, Saturday clinics or adjusted clinic hours might be a solution to provide support for such patients.
The risk of being LTFU in our study was higher among patients with high CD4+ cell count. We showed that patients with baseline CD4+ cell count <200 cells/mm3 were less likely to be LTFU than those with CD4+ cell count >350 cells/mm3. Also, LTFU patients had higher CD4+ count over time compared to those who were not LTFU (Figure S1). In agreement, studies conducted in South Africa 6,18 and another in France 32 showed patients who were LTFU either in the pre-ART care or care after ART had high baseline CD4+ count.
In contrast, other studies of patients on ART have shown that patients with low baseline CD4+ count are more likely to be LTFU than those with high CD4+ cell count 20,30,33. As the widespread use of ART increases as well as an increased in CD4+ count threshold for ART initiation, more patients are likely to initiate ART at higher CD4+ counts and be managed in ART programmes for longer.34–36 Within a context of high risk of mortality irrespective of pre-ART CD4+ counts in TB-HIV co-infected patients, a stringent tracing system should be implemented to address LTFU in both TB and HIV programmes among all patients irrespective of CD4+ cell count. Moreover, digital systems that enable patient referral, transfer between facilities and linkage to a national death registry is essential to programmatically address and manage LTFU in TB and ART programs.
Limitations
We acknowledge several limitations. The study was not powered for the LTFU outcome. The reasons for LTFU could not be ascertained due to patients providing unreliable addresses, prepaid mobile phone numbers which are changed repeatedly and some patients migrated to other cities after feeling better. We might have overestimated or underestimated the impact of mortality in patients that were LTFU because eight patients had missing vital status. Follow-up in this study was 24 months, we have no data beyond 24 months to report on long term LTFU rates within programmes. Therefore our study findings are not generalizable beyond 24 months post TB treatment initiation. In addition, these findings may also not be generalizable to patients with multidrug resistant TB and extra pulmonary TB who are likely to be sicker, take longer and more complex treatment regimen.
CONCLUSION
LTFU rates were not significantly different between the three trials arms. However, ART initiation within each trial arm resulted in a significant reduction in LTFU rates among TB and HIV co-infected patients.
The incidence rate of LTFU was higher among TB patients not initiated on ART and also higher during the course of TB treatment. Trial arm, age, gender, CD4+ cell count and employment status were associated with LTFU at different follow-up time points.
Supplementary Material
Acknowledgments
Sources of funding: Nonhlanhla Yende-Zuma and Kogieleum are supported by CAPRISA which was established as part of the Comprehensive International Program of Research on AIDS (CIPRA) (grant # AI51794) from the US National Institutes of Health. The US President’s Emergency Plan for AIDS Relief (PEPfAR) funded the care of all the participants in the trial. The Global Fund to fight AIDS, Tuberculosis and Malaria funded the cost of the drugs used in the trial. The research infrastructure to conduct this trial, including the data management, laboratory and pharmacy cores were established through the CIPRA grant. KN was supported by the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, grant number D43 TW000231).
Nonhlanhla Yende-Zuma and Kogieleum Naidoo are also supported by the South African Medical Research Council (SAMRC).
The authors thank the patients for their participation in this study; Ms Joyce Thembela who was responsible for the tracking of LTFU patients; Dr Anneke Grobler for statistical support; Dr. Tanuja Gengiah and Ms. Anushka Naidoo for pharmacy support; Dr Sheila Bamber, Dr Niraksha Jithoo and Dr Munira Khan for additional clinical support; Dr Nesri Padayatchi for assistance with study oversight, Mr. Faldie Burton for data management; nurses; counsellors and all other members of the SAPiT study team.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.World Health Organisation. [Accessed in May 2011 at];TB/HIV Facts. 2011 http://www.who.int/tb/challenges/hiv/factsheet hivtb 2011.pdf.
- 2.World Health Organisation. [Accessed on October 31, 2014];TB/HIV fact sheets. 2014 at http://www.who.int/tb/challenges/hiv/tbhiv_factsheet_2014.pdf.
- 3.Kigozi IM, Dobkin LM, Martin JN, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. Journal of acquired immune deficiency syndromes. 2009;52(2):280–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Myer L, Orrell C, et al. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids. 2005;19(18):2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 5.Fairall LR, Bachmann MO, Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Archives of internal medicine. 2008;168(1):86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 6.Boyles TH, Wilkinson LS, Leisegang R, et al. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PloS one. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solarsh G, Benzler J, Hosegood V, et al. Population and health in developing countries. Volume 1. Population, health, and survival at INDEPTH sites. INDEPTH Network. 2002;2002:213–250. [Google Scholar]
- 8.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS medicine. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 11.Pepper DJ, Marais S, Bhaijee F, et al. Assessment at antiretroviral clinics during TB treatment reduces loss to follow-up among HIV-infected patients. PloS one. 2012;7(6):e37634. doi: 10.1371/journal.pone.0037634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng EH, Glidden DV, Emenyonu N, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Tropical medicine & international health : TM & IH. 2010;15(Suppl 1):63–69. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidoo K, Yende-Zuma N, Padayatchi N, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Annals of internal medicine. 2012;157(5):313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organisation. [Accessed on 19 May 2015];Consolidated guidelines on the use of antirtroviral drugs for treating and preventing HIV infection. 2013 at www.who.int/hiv/pub/guidelines/arv2013/ [PubMed]
- 17.Abdool-Karim SS, Abdool-Karim Q, Friedland G, et al. Implementing antiretroviral therapy in resource-constrained settings: opportunities and challenges in integrating HIV and tuberculosis care. Aids. 2004;18(7):975–979. doi: 10.1097/00002030-200404300-00004. [DOI] [PubMed] [Google Scholar]
- 18.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Tropical medicine & international health : TM & IH. 2010;15(Suppl 1):43–47. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacPherson P, Houben RM, Glynn JR, et al. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2014;92(2):126–138. doi: 10.2471/BLT.13.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. Aids. 2010;24(14):2263–2270. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PloS one. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalal RP, Macphail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in johannesburg, South Africa. Journal of acquired immune deficiency syndromes. 2008;47(1):101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 23.Maskew M, MacPhail P, Menezes C, et al. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2007;97(9):853–857. [PubMed] [Google Scholar]
- 24.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bulletin of the World Health Organization. 2007;85(7):550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wubshet M, Berhane Y, Worku A, et al. Death and seeking alternative therapy largely accounted for lost to follow-up of patients on ART in northwest Ethiopia: a community tracking survey. PloS one. 2013;8(3):e59197. doi: 10.1371/journal.pone.0059197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bulletin of the World Health Organization. 2008;86(7):559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kizito KW, Dunkley S, Kingori M, et al. Lost to follow up from tuberculosis treatment in an urban informal settlement (Kibera), Nairobi, Kenya: what are the rates and determinants? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105(1):52–57. doi: 10.1016/j.trstmh.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Weigel R, Estill J, Egger M, et al. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. Aids. 2012;26(3):365–373. doi: 10.1097/QAD.0b013e32834ed814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bulletin of the World Health Organization. 2010;88(9):681–688. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clouse K, Pettifor A, Maskew M, et al. Initiating antiretroviral therapy when presenting with higher CD4 cell counts results in reduced loss to follow-up in a resource-limited setting. Aids. 2013;27(4):645–650. doi: 10.1097/QAD.0b013e32835c12f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller CM, Ketlhapile M, Rybasack-Smith H, et al. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Tropical medicine & international health : TM & IH. 2010;15(Suppl 1):48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndiaye B, Ould-Kaci K, Salleron J, et al. Incidence rate and risk factors for loss to follow-up in HIV-infected patients from five French clinical centres in Northern France - January 1997 to December 2006. Antiviral therapy. 2009;14(4):567–575. [PubMed] [Google Scholar]
- 33.Schoni-Affolter F, Keiser O, Mwango A, et al. Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: the effect of the competing risk of death in Zambia and Switzerland. PloS one. 2011;6(12):e27919. doi: 10.1371/journal.pone.0027919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson LF. Access to Antiretroviral Treatment in South Africa, 2004–2011. S Afr J Hiv Med. 2012;(43):22–27. [Google Scholar]
- 35.South African Department of Health. [Accessed 09 July 2015];The South African Antiretroviral Treatment Guidelines 2010. 2010 http://www.uj.ac.za/EN/CorporateServices/ioha/Documentation/Documents/ART%20Guideline.pdf.
- 36.South African Department of Health. [Accessed 09 July 2015];The South African Antiretroviral Treament Guidelines. 2013 http://www.doh.gov.za/docs/policy/2013/ART_Treatment_Guidelines_Final_25March2013.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.