Abstract
Recent studies have demonstrated that anabolic-androgenic steroids (AAS) modify cognitive processes such as decision making and behavioral flexibility. However, the neural mechanisms underlying these AAS-induced cognitive changes remain poorly understood. The mesocorticolimbic dopamine (DA) system, particularly the nucleus accumbens (Acb), is important for reward, motivated behavior, and higher cognitive processes such as decision making. Therefore, AAS-induced plasticity in the DA system is a potential structural substrate for the observed cognitive alterations. High doses of testosterone (the most commonly-used AAS) increase dendritic spine density in limbic regions including the amygdala and hippocampus. However, effects on Acb are unknown. This was the focus of the present study. Adolescent male Long-Evans rats were treated chronically for 8 weeks with high-dose testosterone (7.5 mg/kg in water with 13% cyclodextrin) or vehicle sc. Brains were stained by Golgi-Cox to analyze neuronal morphology in medium spiny neurons of the shell region of Acb (AcbSh). 8 weeks of testosterone treatment significantly decreased spine density in AcbSh compared to brains of vehicle-treated rats (F1,14 = 5.455, p<0.05). Testosterone did not significantly affect total spine number, dendritic length, or arborization measured by Sholl analysis. These results show that AAS alter neuronal morphology in AcbSh by decreasing spine density throughout the dendritic tree, and provides a potential mechanism for AAS to modify cognition and decision-making behavior.
Keywords: anabolic agents, testosterone, nucleus accumbens, dendritic spine density, medium spiny neurons
Once restricted to elite athletes, anabolic-androgenic steroid (AAS) abuse is increasingly common among adolescents and young adult men. 4–6% of high school boys in the U.S. have used AAS (Johnston et al. 2013), and it is estimated that AAS use among men in their 20s is even higher (Pope et al. 2013). Health risks of AAS include cardiovascular, hepatic and reproductive dysfunction (Pope et al. 2014). AAS are also implicated in maladaptive behavioral changes including increased aggression and risk taking (Middleman et al. 1995; Hall et al. 2005). However, the neural mechanisms underlying AAS-induced alterations in risk taking and decision making are not well understood.
Animal studies have shown that, like other drugs of abuse, AAS are rewarding and reinforcing. Rats will form a conditioned place preference for a chamber paired with AAS (Packard et al. 1997) and will work to self-administer AAS (Wood et al. 2004). Recent studies using operant discounting tasks have also shown that AAS alter decision making in rats (Wood et al. 2013; Cooper et al. 2014; Wallin et al. 2015). The present study explored the neuroanatomical changes potentially underlying cognitive and reinforcing effects of AAS. We tested the effects of AAS on dendritic spine density in the nucleus accumbens (Acb), an important region of the mesocorticolimbic dopamine (DA) system and a key target for drugs of abuse. Dendritic spines on medium spiny neurons (MSNs) in Acb integrate ascending DA projections from the ventral tegmental area (VTA) with excitatory glutamatergic inputs from the prefrontal cortex (PFC), amygdala, and hippocampus, and provide a neural substrate for behavioral changes in addiction (Russo et al. 2010).
Nearly every drug of abuse has been shown to induce structural plasticity in Acb (reviewed in Robinson and Kolb, 2004; Russo et al. 2010). However, different classes of addictive drugs can exert opposite effects on MSN spine density. For instance, stimulants such as cocaine and amphetamine increase the dendritic spine density of Acb MSNs (Robinson et al. 2001; Li et al. 2003). Conversely, opiates like morphine decrease MSN spine density (Robinson and Kolb, 1999). AAS resemble opioids in their physiological effects. Like opioids, high doses of AAS cause profound central autonomic depression, sometimes resulting in death. This effect is blocked by pretreatment with the opioid antagonist naltrexone (Wood, 2008). This suggests that AAS may affect the brain via opioidergic mechanisms. Thus, we hypothesized that AAS would decrease dendritic spine density in Acb, similar to opioids.
Already, we know that pubertal exposure to AAS increases dendritic spine density in the medial amygdala (Me) and in CA1 of the hippocampus (Cunningham et al. 2007). In particular, the increase in CA1 persists weeks after treatment cessation, suggesting long-term effects of AAS on neural anatomy. Although the effects of AAS on dendritic spine density in Acb are unknown, male rats have decreased spine density in Acb compared to females (Forlano and Woolley, 2010; Wissman et al. 2011). This suggests that testosterone at normal physiologic levels reduces Acb spine density.
Acb is important not only for reward and motivation, but also for complex processes such as decision making. In this regard, Acb includes two sub-regions. The core region is involved in motor learning aspects of motivated behavior. The shell region (AcbSh) is involved in the cognitive aspects of motivated behavior: assigning and updating relevance of environmental cues, suppression of behavioral responses to irrelevant stimuli, and use of reward feedback to guide responses during decision making (Floresco, 2015). In the probability discounting task, inactivation of AcbSh decreases selection of the large reward (Stopper and Floresco, 2011). Similarly, AAS treatment also decreases large reward selection in this task (Wallin et al. 2015). Therefore, we focused our analysis of MSN spine density on AcbSh.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
16 male Long-Evans rats (5 weeks of age at the start, Charles River Laboratories, MA) were pair-housed under a reversed 14L:10D photoperiod. Rats were treated for 8 weeks with testosterone (n=8) or vehicle (n=8). To approximate AAS use by humans, rats remained gonad-intact. All rats were food-restricted to maintain a slow rate of growth (3-4 g/day) to facilitate responding for food in an operant discounting test (Wallin et al, 2015). Experimental procedures were approved by USC's Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Ed (National Research Council, National Academies Press, Washington DC; 2011).
2.2 AAS treatment
Beginning at 5 weeks of age, rats received daily injections of testosterone (7.5 mg/kg; Steraloids, RI) or aqueous vehicle [3% ethanol and 13% cyclodextrin (RBI, MA)] sc 5 d/week. Pubertal treatment mirrors patterns of human use, where 4-6% of high school boys in the United States have used AAS (Johnston et al, 2013). Furthermore, AAS have the strongest behavioral effects in rodents when introduced in adolescence (Salas-Ramirez et al, 2008). Testosterone is the prototypical AAS, and is the most common performance-enhancing substance (55.5%) detected in urine tests by World Anti-Doping Agency-accredited laboratories (WADA, 2012). The 7.5 mg/kg dose is equivalent to doses used by humans to enhance performance, and has previously been shown to modulate decision making and cognition in rats (Cooper et al. 2014; Wallin et al. 2015; Wallin and Wood, 2015).
2.3 Golgi-Cox Staining
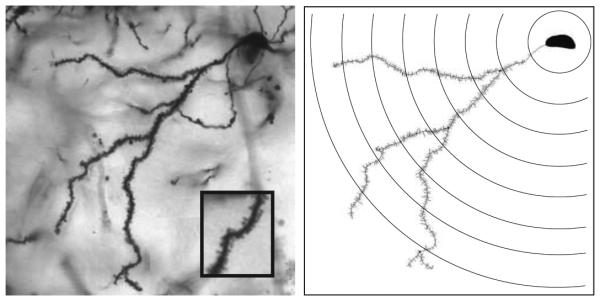
During drug treatment, all rats underwent daily operant behavioral testing for decision making (Wallin and Wood, 2015). 4 days after completion of behavioral testing, rats received a final injection of testosterone or vehicle 1 hour prior to sacrifice. To collect brains for analysis, rats were deeply anesthetized with sodium pentobarbital (150 mg/kg BW) and sacrificed by decapitation. Golgi-Cox staining was performed using reagents and methods of the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Ellicott City, MD). After staining, sections through the rostral-caudal extent of Acb were mounted on gelatin-coated slides. Slides were stored in the dark overnight and developed the following day according to the Rapid GolgiStain Kit protocol. MSN morphology was analyzed on coded slides by an observer blinded to the treatment groups using a Nikon Eclipse 80i microscope (Nikon Instruments, Inc. Melville, NY) with motorized stage and MicroFire camera (Olympus America, Inc. Center Valley, PA). Dendritic morphology was measured using the Neuron Tracing function in NeuroLucida (MicroBrightField, Inc. Williston, VT) (Figure 1).
Figure 1.
Photomicrograph of a representative Golgi-impregnated medium spiny neuron from the shell region of the nucleus accumbens at 40x magnification (left) with corresponding neurolucida tracing of one primary dendrite and concentric Sholl analysis rings every 10 um (right).
Analysis was performed as previously described in Antzoulatos et al. (2011). Briefly, 3-5 neurons from each rat were analyzed, and morphologic data were averaged to provide a single data point for each animal used in statistical comparison. MSNs selected for analysis were located in AcbSh (Plates 11-14 of [Paxinos and Watson, 1998]). Selected MSNs were fully impregnated with Golgi stain, and had clearly-visible spines with minimal or absent obstruction by neighboring Golgi-stained cells or blood vessels. As in previous studies (Ball et al. 2009 and 2010; Antzoulatos et al. 2011), spine density was analyzed on the entire dendritic tree of one primary dendrite from each neuron. All dendrites subject to morphologic analysis had at least 3 branch orders. Dendrites were traced under a 40x lens and morphometric analysis was conducted using NeuroExplorer software (MicroBrightField, Inc.). Briefly, each dendritic segment was assigned a branch order with the dendritic segment proximal to the soma identified as the first branch. Spine density was computed for each branch as spine number divided by dendritic length. In addition, total spine density, highest branch order, and dendritic arborization were calculated for the entire dendritic tree. Dendritic arborization was determined by a Sholl analysis (described in Sholl, 1953). Briefly, concentric rings with increasing radii (10-100 um from the soma, 10 um increments) were centered around the soma and intersections of dendritic branches with rings were quantified at each radius (Figure 1B).
2.4. Statistics
Group differences between testosterone- and vehicle-treated rats for dendritic length, spine number, and spine density were determined by repeated measures analysis of variance (RM- ANOVA), with branch order as the repeated measure. Dendritic arborization was also compared by RM-ANOVA with ring radius as the repeated measure. Average highest branch order was compared by Student’s t-test.
3. RESULTS
3.1 Spine Density
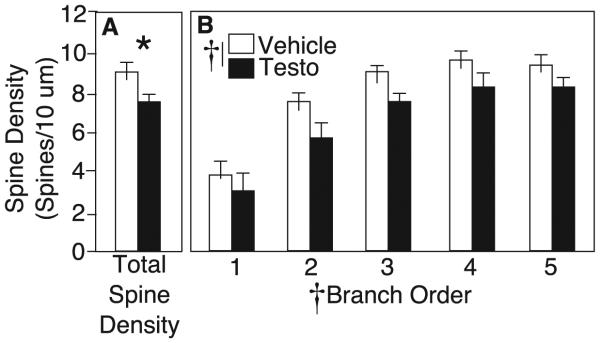
MSNs have elaborate dendritic arbors with numerous dendritic spines (Figure 1). Spine density throughout the entire dendritic tree averaged 9.1±0.3 spines/10 µm in vehicle-treated rats, comparable to previously reported densities in the rat Acb (Robinson et al. 2001; Li et al. 2003). Testosterone-treated rats had an average density of 7.7±0.5 spines/10 µm (Figure 2A). By RM- ANOVA, there was a significant main effect of drug across all branch orders (F1,14 = 5.455, p<0.05; Figure 2B). There was also a significant main effect of branch order on spine density, with density increasing as branch order increased for both treatment groups (F4,11 = 11.105, p<0.05; Figure 2B). In vehicle controls, spine density increased from an average of 3.8±0.7 spines/10 µm on primary branches to 9.3±0.7 spines/10 µm on 5th-order branches. There was no interaction of drug x branch order, suggesting that testosterone decreased spines uniformly throughout the dendritic tree.
Figure 2.
A) Total dendritic spine density (mean±SEM) for vehicle (white bars) and testosterone-treated rats (black bars) on medium spiny neurons in the nucleus accumbens shell. B) Dendritic spine density on 1st−through 5th-order branches of medium spiny neurons. Asterisk indicates p<0.05 by Student’s t-test. Cross indicates p<0.05 by RM-ANOVA.
3.2 Branch Order
The average highest branch order observed for vehicle controls was 5.8±0.4, comparable to previously published findings on branching in Acb MSNs of rats (Wang et al. 2012). Testosterone did not alter average highest branch order (6.1± 0.7, p>0.05; data not shown).
3.3 Spine Number
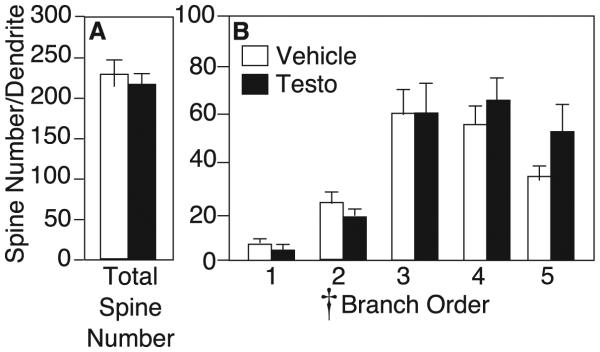
Although testosterone decreased dendritic spine density, it did not affect total spine number (vehicle: 228.6±17.4 spines, testosterone: 218.8±20.0 spines, p>0.05; Figure 3A). By RM- ANOVA, there was a significant main effect of branch order on spine number, with more spines on distal than proximal branches (F4,11 = 13.14, p<0.05). However, there was no interaction of drug x branch order, and no effect of testosterone on spine number at any portion of the dendritic tree (F1,14 = 0.01, p>0.05; Figure 3B).
Figure 3.
A) Average spine number per dendrite (mean±SEM) for vehicle (white bars) and testosterone-treated rats (black bars) on medium spiny neurons in the nucleus accumbens shell. B) Average spine number on 1st− through 5th-order branches of medium spiny neurons. Cross indicates p<0.05 by RM-ANOVA.
3.4 Dendritic Length
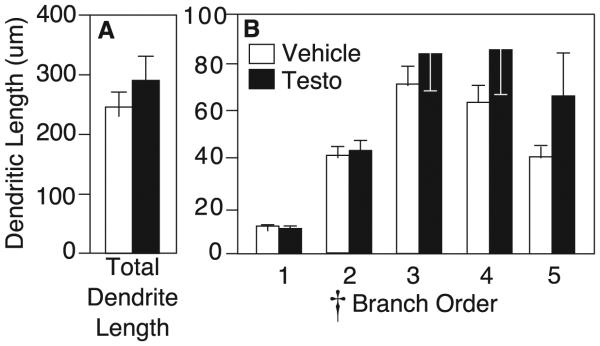
Branch order analysis by RM-ANOVA revealed a significant main effect of branch order on branch length, with middle branches longer than the most proximal and most distal branches (F4,11 = 10.783, p<0.05; Figure 4B). However, testosterone treatment did not affect dendritic length. Average total dendritic length for testosterone-treated rats was 296.0±39.4 µm/dendrite, compared to 256.6±24.5 µm/dendrite for vehicle controls (p>0.05; Figure 4A). By RM-ANOVA, there was no interaction of drug x branch order, and no effect of testosterone on branch length at any order throughout the dendritic tree (F1,14 = 0.760, p>0.05).
Figure 4.
A) Average dendrite length (mean±SEM) for vehicle (white bars) and testosterone-treated rats (black bars) on medium spiny neurons in the nucleus accumbens shell. B) Average length of 1st− through 5th-order branches of medium spiny neurons. Cross indicates p<0.05 by RM-ANOVA.
3.5 Dendritic Arborization
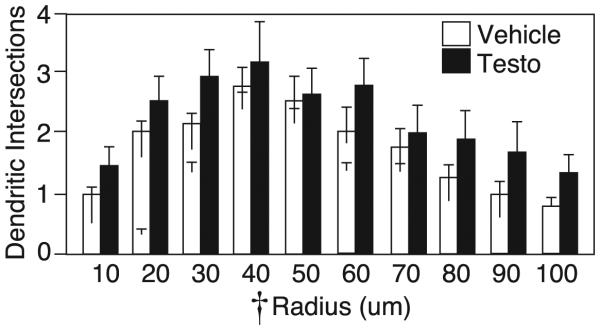
Sholl analysis revealed a significant main effect of ring radius on dendritic intersections (F9,6 = 7.79 p<0.05, by RM-ANOVA) with intersections decreasing as distance from the soma increased. Although testosterone-treated rats appeared to exhibit a trend toward more dendritic intersections per radius, no significant effect of drug on dendritic arborization was detected (F1,14 = 1.14, p>0.05, Figure 5). Furthermore, there was no significant interaction between drug and radius on dendritic intersection (F9,6 = 0.53, p>0.05).
Figure 5.
Sholl analysis of dendritic intersections (mean±SEM) with rings at increasing radii of medium spiny neurons in the nucleus accumbens shell for vehicle (white bars) and testosterone-treated rats (black bars). Cross indicates p<0.05 by RM-ANOVA.
4. DISCUSSION
The current study used chronic, high-dose testosterone treatment in male rats as a model for AAS use to investigate effects on neuronal morphology in AcbSh. As hypothesized, testosterone significantly decreased dendritic spine density of MSNs. Thus, AAS mirror the effects of opioids on dendritic spine density in Acb (Robinson and Kolb, 1999). These results also correspond with previous studies showing similar physiological effects of AAS and opioids (Wood, 2008). Decreased spine density means that AAS may modulate information processing in MSNs in Acb, a region crucial to reward, motivation, decision making and cognition. These alterations may underlie the cognitive and behavioral changes observed in human AAS users and in AAS-treated laboratory animals.
It appears that AAS enhance the effects of physiologic testosterone levels on dendritic spine density throughout the brain. The medial amygdala (Me), a region crucial to male social and sexual behavior, and the CA1 region of the hippocampus are sensitive to androgens. In males, dendritic spine density in Me and CA1 is decreased by castration, but rescued by testosterone replacement at physiologic levels (Gomez and Newman 1991, Leranth et al. 2003). AAS treatment exaggerates this effect by increasing dendritic spine density in Me and CA1 relative to controls with physiologic testosterone levels (Cunningham et al. 2007). The current findings suggest that AAS also enhance the effects of physiologic testosterone on Acb. In Acb, females have greater spine density than males (Forlano and Woolley, 2010; Wissman et al. 2011), and vehicle-treated males have greater spine density than AAS-treated males (current study). Thus, higher testosterone corresponds to lower spine density in Acb. Although previous studies report higher Acb spine densities in male rats compared with vehicle-treated males in the present study, this likely reflects the different methods (DiI vs Golgi) used to visualize neuronal morphology. In particular, the DiI labeling used by Forlano and Woolley (2010) and Wissman et al. (2011) yields higher spine densities than Golgi impregnation in pyramidal cells of CA1. (Woolley et al. 1997). The opposing effects on spine density in Acb compared to Me and CA1 may seem contradictory. However, it is common for drugs of abuse to exert opposite effects on spine density in different brain regions. For instance, morphine decreases spine density in Acb and medial PFC, but increases density in the orbital PFC (Robinson and Kolb, 2004). If AAS indeed work through opioidergic mechanisms, they may also decrease dendritic morphology in the medial PFC while increasing density in the orbital region of the PFC.
Interestingly, the decrease in spine density in testosterone rats cannot be attributed solely to either an increase in dendritic length or a decrease in total spine number. Rather, the significant decrease in spine density results from non-significant changes in both dendritic length and spine number in Acb. This finding corresponds with previous studies. Forlano and Woolley (2010) have shown that despite a sex difference in spine density in Acb, there is no sex difference in any measure of arborization. Likewise, the current study found a decrease in Acb spine density of AAS-treated rats with no effect on dendritic ring intersections by Sholl analysis. In this way, AAS-treated rats resemble rats with depleted Acb DA. Medial forebrain bundle lesions deplete Acb DA input, and result in decreased spine density in both the core and shell of Acb with no effect on dendritic length (Meredith et al. 1995).
The mechanism of action for AAS effects on Acb MSNs is unclear. Acb is not traditionally considered a hormone-responsive brain region and does not contain many classical androgen receptors (Simmerly et al. 1990). However, gonadal hormones can modulate behavior without binding classical receptors. For instance, the reinforcing effects of AAS do not require classical androgen receptors (Sato et al. 2010). Furthermore, some behavioral effects of androgens occur too quickly to be mediated by gene transcription, suggesting AAS may act on non-genomic receptors in Acb (Foradori et al. 2008). Additionally, changes in Acb may occur secondary to AAS effects on Acb afferents. For instance, the VTA contains classic androgen receptors and projects to Acb (Swanson, 1982; Simmerly et al. 1990). Because Acb spine density correlates with DA release (Meredith et al. 1995), AAS may decrease spine density by decreasing the activity of VTA DA projection neurons. One or both of these mechanisms may underlie AAS influence on Acb.
Like other drugs of abuse, AAS are rewarding and can cause dependence (Wood, 2008). Male hamsters will self-administer AAS, even to the point of death by overdose (Johnson and Wood, 2001; Wood et al. 2004). Human studies show that AAS users develop symptoms of dependence, evidenced by continued use despite negative consequences and withdrawal when AAS use was discontinued (Brower et al. 1990). Indeed, 57% of AAS users meet diagnostic criteria for dependence (Brower et al. 1991). While the neural mechanisms underlying AAS dependence are not well understood, the current study shows that AAS mirror opioid effects on Acb spine density. Indeed, the magnitude of spine density decrease induced by AAS was 15.4%, similar to the magnitude of decrease induced by opioids (Robinson et al. 2002). This finding is consistent with previous data suggesting that AAS my influence the brain via opioidergic mechanisms. For instance, pretreatment with the opioid receptor antagonist naltrexone blocks both the physiologic and reinforcing effects of AAS (Peters and Wood, 2005). Among human users, AAS abuse is a common comorbidity of opioid use (Kanayama et al. 2003), and AAS seem to interact with opioids in accidental overdose (Thiblin et al. 2000). Studies by Nyberg and colleagues support a link between AAS and opioids, showing that AAS increase B-endorphin levels in the VTA (Johansson et al. 1997) and disrupt dynorphin and enkephalin levels in multiple brain regions including the Acb (Johansson et al. 2000). Thus, AAS may exert their reinforcing effects on humans and animals and their effect on Acb spine density by influencing the opioid system.
In addition to causing dependence, AAS are implicated in behavioral disturbances such as increased risk taking and altered decision making—behaviors dependent on Acb (Floresco, 2015; Wallin et al. 2015). When rats in the present study were tested for decision-making in an effort discounting task, we found that testosterone increased the rats' willingness to work for food reward (Wallin et al, 2015). We cannot determine if the lower MSN spine density reported here was responsible for the behavioral effects reported previously. However, because 90% of all excitatory synapses occur on dendritic spines (Harris and Kater, 1994) and synapse distribution affects excitability of neurons (Rall, 1967) AAS may alter decision making behavior by modulating MSN function in AcbSh. AAS use in human adolescent males is associated with risky behavior, including unsafe sex, carrying a weapon, not wearing a helmet or seatbelt, and concomitant use of alcohol and other drugs (Middleman et al. 1995). We have previously shown increased risk taking by AAS-treated male rats in a task involving physical risk (punishment discounting). AAS-treated rats were more willing to choose a risky reward paired with a chance of footshock compared to vehicle controls (Cooper et al. 2014). However, AAS effects on risk taking are context dependent: in a similar paradigm involving reward uncertainty (probability discounting), AAS-treated rats exhibited decreased risk taking (Wallin et al. 2015). This behavior corresponds with decreased excitatory activity in AcbSh, as pharmacological inactivation of AcbSh also decreases risk taking in probability discounting (Stopper and Floresco, 2011).
AAS effects on decision making in punishment and probability discounting are opposite to effects of the stimulant amphetamine (AMPH). AMPH increases risk taking on probability discounting and decreases it in punishment discounting (Simon et al. 2009; St Onge and Floresco, 2009). Additionally, AMPH increases spine density in Acb (Robinson et al. 2001). Thus, AAS and AMPH exhibit opposing effects on both Acb-dependent behavior and Acb spine density (current study). Furthermore, pretreatment with AAS blunts both the motor response and Acb DA release induced by stimulant treatment (Birgner et al. 2007; Kurling-Kailanto et al. 2010). Therefore AAS seem to oppose the effects of stimulants, and may alter decision making by suppressing DA release in Acb. This is consistent with findings that DA depletion decreases dendritic spine density in AcbSh (Meredith et al. 1995). Alteration of the structure and function of the mesolimbic DA system provides a potentially powerful mechanism for AAS to influence cognition and behavior, and may explain alterations in decision making and AAS dependence. Understanding AAS effects on the mesolimbic DA system is a step toward understanding how AAS relate to other drugs of abuse in their addictive potential, cognitive disturbances and behavioral consequences.
HIGHLIGHTS.
We use high-dose testosterone treatment to model anabolic-androgenic steroid abuse in rats.
Testosterone treatment decreases dendritic spine density in the nucleus accumbens.
Testosterone treatment appears to mirror opioid effects and oppose stimulant effects on the mesolimbic dopamine system.
ACKNOWLEDGMENTS
Funding for this study was provided by NIDA Grant R01-DA029613 to RIW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. MPTP neurotoxicity and testosterone induce dendritic remodeling of striatal medium spiny neurons in the C57Bl/6 mouse. Parkinson’s disease. 2011 doi: 10.4061/2011/138471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of MDMA (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neurosci. 2009;160(2):264. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Miller BR, Rebec GV. Electrophysiological and structural alterations in striatum associated with behavioral sensitization to (±) 3, 4- methylenedioxymethamphetamine (ecstasy) in rats: role of drug context. Neurosci. 2010;171(3):794–811. doi: 10.1016/j.neuroscience.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgner C, Kindlundh-Högberg AM, Nyberg F, Bergström L. Altered extracellular levels of DOPAC and HVA in the rat nucleus accumbens shell in response to sub-chronic nandrolone administration and a subsequent amphetamine challenge. Neurosci Lett. 2007;412(2):168–72. doi: 10.1016/j.neulet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Eliopulos GA, Blow FC, Catlin DH, Beresford TP. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am J Psychiat. 1990;147(4):510. doi: 10.1176/ajp.147.4.510. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic-androgenic steroid dependence. Br J Addict. 1991;86(6):759–68. doi: 10.1111/j.1360-0443.1991.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone effects on risk tolerance and motor impulsivity in male rats. Psychoneuroendocrino. 2014;40:201–212. doi: 10.1016/j.psyneuen.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham RL, Claiborne BJ, McGinnis MY. Pubertal exposure to anabolic androgenic steroids increases spine densities on neurons in the limbic system of male rats. Neuroscience. 2007;150(3):609–615. doi: 10.1016/j.neuroscience.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front neuroendocrin. 2008;29(2):169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS. Quantitative analysis of pre-and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol. 2010;518(8):1330–1348. doi: 10.1002/cne.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. Anat Rec. 1991;231(4):498–509. doi: 10.1002/ar.1092310412. [DOI] [PubMed] [Google Scholar]
- Hall RC, Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46(4):285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexilibility to synaptic function. Annu Rev Neurosci. 1994;17:341–71. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Johansson P, Hallberg M, Kindlundh A, Nyberg F. The effect on opioid peptides in the rat brain, after chronic treatment with the anabolic androgenic steroid, nandrolone decanoate. Brain Res Bull. 2000;51(5):413–8. doi: 10.1016/s0361-9230(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Johansson P, Ray A, Zhou Q, Huang W, Karlsson K, Nyberg F. Anabolic androgenic steroids increase β-endorphin levels in the ventral tegmental area in the male rat brain. Neurosci Res. 1997;27(2):185–189. doi: 10.1016/s0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73(4):285–292. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Ann Arbor: Institute for Social Research. The University of Michigan; 2013. Monitoring the Future national survey results on drug use: 2012 Overview, Key findings on adolescent drug use. [Google Scholar]
- Kanayama G, Cohane GH, Weiss RD, Pope HG. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiat. 2003;64(2):156–160. doi: 10.4088/jcp.v64n0208. [DOI] [PubMed] [Google Scholar]
- Kurling-Kailanto S, Kankaanpää A, Seppälä T. Subchronic nandrolone administration reduces cocaine-induced dopamine and 5-hydroxytryptamine outflow in the rat nucleus accumbens. Psychopharmacol. 2010;209(3):271–81. doi: 10.1007/s00213-010-1796-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacol. 2003;28(6):1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23(5):1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Ypma P, Zahm DS. Effects of dopamine depletion on the morphology of medium spiny neurons in the shell and core of the rat nucleus accumbens. J Neurosci. 1995;15(5):3808–3820. doi: 10.1523/JNEUROSCI.15-05-03808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleman AB, Faulkner AH, Woods ER, Emans SJ, Durant RH. High-Risk Behaviors Among High School Students in Massachusetts Who Use Anabolic Steroids. Pediatrics. 1995;96(2):268–272. [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press (US); Washington (DC): 2011. [PubMed] [Google Scholar]
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci. 1997;111(1):219. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130(4):971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Pope HG, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am J Addict. 2013;23(4):371–377. doi: 10.1111/j.1521-0391.2013.12118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr., Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: An Endocrine Society scientific statement. Endocr Rev. 2014;35(3):341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter-versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46(4):271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Malenka RC, Nestler EJ. The Addicted Synapse: Mechanisms of Synaptic and Structural Plasticity in Nucleus Accumbens. Trends Neurosci. 2010;33(6):267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM, Johansen JA, Jordan CL, Wood RI. Membrane androgen receptors may mediate androgen reinforcement. Psychoneuroendocrinology. 2010;35(7):1063–73. doi: 10.1016/j.psyneuen.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387. Pt 4. [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Chang C, Muramatsu M. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacol. 2009;34(10):2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco S. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacol. 2009;34(3):681–97. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn Affect Behav Ne. 2011;11(1):97–112. doi: 10.3758/s13415-010-0015-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Lindquist O, Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J Forensic Sci. 2000;45(1):16–23. [PubMed] [Google Scholar]
- WADA Anti-Doping Testing Figures Report. 2012 www.wada-ama.org. [Google Scholar]
- Wallin KG, Alves JM, Wood RI. Anabolic–androgenic steroids and decision making: Probability and effort discounting in male rats. Psychoneuroendocrinology. 2015;57:84–92. doi: 10.1016/j.psyneuen.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin KG, Wood RI. Anabolic-androgenic steroids impair set-shifting and reversal learning in male rats. Eur Neuropsychopharmacol. 2015;25(4):583–590. doi: 10.1016/j.euroneuro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012;217(2):337–51. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang G-Z, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61(1-2):217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Armstrong A, Fridkin V, Shah V, Najafi A, Jakowec M. 'Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase. Physiol Behav. 2013;110:6–12. doi: 10.1016/j.physbeh.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology. 2004;171(3):298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Wood RI. Anabolic–androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrin. 2008;29(4):490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17(5):1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]