Abstract
Psoralen and UVA (PUVA) has immunosuppressive and proapoptotic effects, which are thought to be responsible alone or in combination for its therapeutic efficacy. However, the molecular mechanism by which PUVA mediates its effects are not well understood. Activation of the serotonin (5-hydroxytryptamine, 5-HT) pathway has been suggested to be involved in the modulation of T cell responses and found to mediate UVB-induced immune suppression. In particular, the activation of the 5-HT2A receptor has been proposed as one mechanism responsible for UV-induced immune suppression. We therefore hypothesized that 5-HT may play a role in PUVA-induced effects. The model of systemic suppression of delayed-type hypersensitivity (DTH) to Candida albicans was used to study immune function after exposure of C3H and KITW-Sh/W-Sh mice to a minimal inflammatory dose of topical PUVA. The intraperitoneal injection of the 5-HT2 receptor antagonist ketanserin or cyproheptadine or an anti-5-HT antibody immediately before PUVA exposure entirely abrogated suppression of DTH but had no significant effect on inflammation, as measured by swelling and cellular infiltration of the skin, and apoptosis as determined by the number of sunburn cells in C3H mice. Importantly, the systemic injection of 5-HT recapitulated PUVA immune suppression of DTH but did not induce inflammation or apoptosis in the skin. KITW-Sh/W-Sh mice (exhibiting myelopoietic abnormalities, including lack of 5-HT-containing mast cells) were resistant to PUVA-induced suppression of DTH but not local skin swelling. Thus, this points towards a crucial role of 5-HT signaling in PUVA-induced immune suppression but not inflammation or apoptosis in situ in the skin.
Keywords: mast cells, immune suppression, ultraviolet radiation, PUVA, serotonin
INTRODUCTION
Similar to UVB radiation (1, 2) PUVA (reviewed in (3)) has long been known to have profound proapoptic and immunosuppressive effects. This property is likely to explain (at least partly) its therapeutic efficacy. PUVA has been shown to suppress contact hypersensitivity and/or delayed type hypersensitivity (DTH) in mice (4-7), guinea pigs (8, 9) as well as humans (10-12). PUVA can exert profound, suppressive effects on the immune cells found infiltrating the skin of psoriatic patients (12, 13). Indeed, epidermal and dermal CD3+T lymphocytes, as well as CD4+, CD8+, and IL-2 receptor+ subsets, were strongly suppressed by PUVA, with virtual elimination of IL-2 receptor+ (activated) T cells in some patients. Vallat and colleagues also found that consistent with diminished lymphocyte activation, HLA-DR expression by epidermal keratinocytes was markedly reduced in PUVA-treated skin (13). These findings are supported by Coven et al (14) who showed that the depletion of CD3+ lymphocytes in the epidermis of psoriatic plaques correlated with the clinical response to PUVA. In comparison to immunosuppressive cyclosporine, which is another conventional treatment option for psoriasis, PUVA therapy led to more complete reversal of pathological epidermal and lymphocytic activation (13). These changes are proposed to be the cellular basis for the sustained remission of psoriatic disease after PUVA treatment.
PUVA's proapoptotic effects may be due to direct DNA damage caused by interstrand cross-links (15). However, the molecular events and mediators downstream of this DNA damage that lead to PUVA-induced effects are not well understood (16). Using a combination of platelet activating factor (PAF) receptor knockout mice as well as PAF receptor antagonists we previously showed that PAF, a biophospholipid, was involved in PUVA-induced apoptosis and immune suppression (3). PAF receptor antagonists can have dual efficacy in blocking both PAF and serotonin (5-hydroxytryptamine, 5-HT) signaling (3, 17-19) and activation of the 5-HT pathway has been suggested to be involved in the modulation of T cell responses (20, 21) and found to mediate UVB-induced immune suppression (18). 5-HT is a component of both rodent and human mast cell secretory granules (22). Although the importance of human mast cell-derived 5-HT is controversial, 5-HT was shown to be synthesized and released by human mast cells derived from peripheral blood progenitors (23). PUVA treatment itself leads to the in vitro release of numerous inflammatory mediators (including 5-HT) from mast cells of serosa (24) and skin (25). We therefore asked whether 5-HT may play a role in PUVA-induced immune suppression. We addressed this question using the murine model of suppression of induction of DTH to C. albicans, and compared the ability of 5-HT to modulate immunity to its role in inflammation. As 5-HT is a well known product of activated mast cells (20) and mast cells are involved in UV-induced immune suppression (2, 26) we used mast cell-deficient mice (27) to study the functional role of 5-HT in our system. We found that 5-HT signaling was crucial in the induction of PUVA-induced systemic suppression of delayed type hypersensitivity but not local inflammation.
MATERIALS AND METHODS
Animals
Specific pathogen-free female C3H mice/HeNCr (MTV−) mice were purchased from the National Cancer Institute Frederick Cancer Research Facility Animal Production Area (Frederick, MD). KITW-Sh/W-Sh mast cell deficient mice were purchased from Jackson laboratories and bred in our own facility at the University of Texas M.D. Anderson Cancer Center. Male and female C57BL/6 wild type litter mates served as controls. All animals were maintained with alternating 12-h light and dark cycles and controlled temperature and humidity in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with current regulations of the United States Department of Health and Human Services. Water and food were provided ad libitum. All animal procedures were approved by the University of Texas M.D. Anderson Cancer Center Institutional Animal Care and Use Committee. Mice were 8-20 weeks old at the start of and were age- and sex-matched within each experiment.
Reagents to study the 5-HT pathway
The selevtive 5-HT2 receptor antagonist ketanserin and the antihistamine cyproheptadine with anti-5-HT properties were purchased from Biomol Research Labs, Inc. Stock solutions were prepared by dissolving each in a 50% DMSO/PBS buffer and diluted further in PBS before injection into mice. The doses of the antagonists (500 nmol per mouse) injected intraperitoneally (i.p.) immediately before PUVA exposure were based on previous studies (18, 28), in which such concentrations totally blocked UVB-induced immune suppression. 5-HT was purchased from Sigma-Aldrich (product no. H9523, Saint Louis, MO) for i.p. injection (500 nmol per mouse). Anti-5-HT antiserum (produced in rabbit) (Sigma product number S 5545) (100 μg i.p. per mouse) was used for in vivo i.p. injection to neutralize 5-HT activity. As a control, an equal amount of normal rabbit serum (Sigma-Aldrich) was used. Anti-cis urocanic acid antibody was a gift from Mary Norval and a dose of 5 μg i.p. per mouse or an equivalent amount of isotype antibody was injected, as previously described (18, 28).
PUVA and ultraviolet radiation treatment
The backs of the mice were shaved with electric clippers 1 day before PUVA treatment. Groups of mice were painted on their backs either with 100 μl of vehicle (100% ethanol) or 8-MOP (Sigma-Aldrich, Saint Louis, Missouri) in ethanol (at a concentration of 1 mg/ml), or were left untreated. The mice were then kept for 30 min in individual compartments of standard cages, separated with Plexiglass dividers to allow penetration of 8-MOP. UVA irradiation was provided by bank of six Sylvania F15T8/BLB lamps (emission range, 320 to 400 nm; peak at 365 nm; Danvers, MA). During UVA irradiation, the mice were housed five per cage, individually separated, on a shelf 20 cm below the fluorescent light bulbs under a wire cage top. The mice were UVA-irradiated at a mean UVA irradiance of 4.5 mW/cm2, as measured by an IL 700 spectroradiometer with a SEE 033 UVA detector (International Light Inc., Newburyport, MA) (3). UVB irradiation was produced by an Oriel solar simulator, as previously described (28).
Jet fuel treatment
Jet fuel JP-8 (lot # 3509) was provided for the experiments by Operational Toxicology Branch, Air Force Research Laboratory, Wright Patterson Air Force Base, Dayton, OH. Storage, handling, and application of the jet fuel to mice was done, as previously described (29, 30). Briefly, undiluted jet fuel (300 μl) was applied with a pipette to the dorsal shaved skin of the animals. Housing of the mice during and after jet fuel application was also done, as previously described (29, 30).
Quantification of macroscopic skin inflammation
Inflammation was assessed by measuring the double-skin-fold-thickness (31, 32) of dorsal skin of the mice with a spring-loaded engineer's micrometer (Swiss Precision Instruments) before, 24 and 48 hours after PUVA or UV exposure. Skin swelling was determined for individual mice by subtracting the double-skin-fold-thickness before treatment from that after PUVA or UV exposure. The skin swelling values of individual mice were averaged for the different treatment groups.
Delayed type hypersensitivity assay
A DTH assay was performed as previously described (3). Mice were treated with PUVA on Day 0. Five days later they were immunized by the subcutaneous injection of 2 × 107 formalin-fixed Candida albicans into each flank of the unirradiated abdomen. Nine days later, each hind footpad was measured with the engineer's micrometer, the thickness recorded, and the animals were challenged by injecting 50μl of Candida antigen (Antigen Supply House) into each hind footpad. The thickness of each hind footpad was measured again 18-24 h later and the mean footpad swelling for each mouse (left food + right foot / 2) was recorded. The mean footpad swelling and standard deviation was calculated for each experimental group. The specific footpad swelling was calculated by subtracting the mean footpad swelling found in control mice that were not immunized, but were challenged, from the swelling observed in groups of mice that were both immunized and challenged. The percent suppression of DTH was determined by the following formula: [1-(A/B)] ×100, where A represents the specific footpad swelling in sensitized and PUVA (or solar simulated UV) treated mice (i.e. experimental group), and B represents that in sensitized, unirradiated mice (i.e. positive control group).
Histology
Three mice per treatment group were euthanized 48 hrs after PUVA exposure. Approximately 1 cm2 of dorsal skin was excised per mouse, fixed immediately in 4% buffered formaldehyde, processed routinely, and sectioned at 5 μm for hematoxylin and eosin staining. At least two nonsequential sections of each specimen were examined for histological alterations. Sunburn cells were counted in the interfollicular epidermis in a total of at least 10 random high power fields per section (at 400x microscopic magnification). Counts were expressed as the number of sunburn cells per mm length of epidermis, as determined with a calibrated eyepiece micrometer. Epidermal hyperplasia was assessed by counting epidermal cell layers and measuring the thickness of the epidermis from the basal layer to the stratum corneum. To quantitate inflammatory cellular infiltration in the dermis of the specimens, an eyepiece counting grid with area of 0.04 mm2 (Olympus, Vienna, Austria) was used at 400x microscopic magnification. All infiltrating cells (including leucocytes, macrophages, and fibroblasts) or neutrophils (identified by their cellular morphology with segmented nucleus) within the grid were counted in at least 10 grid fields at randomly selected locations in the dermis per specimen to calculate cell density per mm2.
Mast cell and 5-HT staining
Deparaffinized 5μm thick serial tissue sections were used for immunohistochemical staining using polyclonal anti-5-HT antibody (product no. S 5545, Sigma, St. Louis, MO) at a concentration of 1:1000 or anti-tryptase antibody (clone AA1, code no. M7052, DAKO, Glostrup, Denmark) at a concentration of 1:1000. Biocare Mach 2 Double stain 2, developed with Biocare Vulcan Fast red, or DAB from Dako (K5001) was performed according to the manufacturer's instructions. Giemsa staining was done according to standard protocol (33, 34). Sections from a carcinoid tumor and normal intestinal mucosa (with enterochromaffin cells) served as positive controls for 5-HT staining.
Statistical analysis
The readings of the DTH experiments as well as histological and immunohistochemical stainings were conducted in a blinded fashion. All data presented are expressed as means + S.E.M. Statistical differences among control and experimental groups were determined by use of one-way ANOVA, followed by Tukey's multiple comparison test (Prism Statistical Software; GraphPad). Statistical significance was set at a P-level < 0.05.
RESULTS
5-HT2 blockade abrogates PUVA-induced systemic suppression of delayed type hypersensitivity but not local inflammation
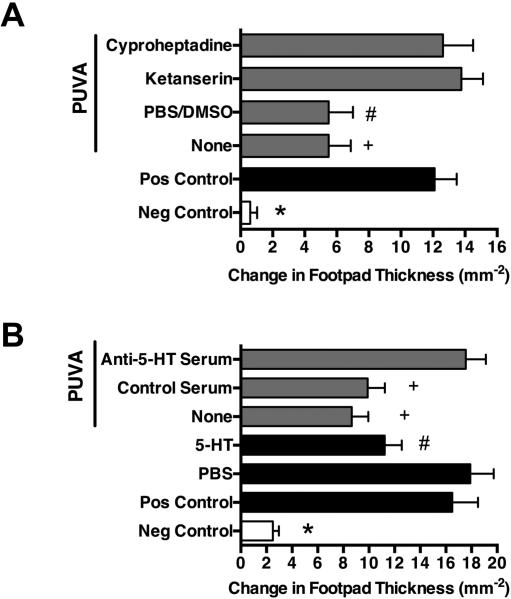
We used the mouse model of induction of DTH to investigate the effect of topical PUVA on immune function with a minimal inflammatory PUVA dose of 50 kJ/m2 UVA, as determined in previous dose response studies (3). This UVA dose alone (without psoralen photosensitzation) was unable to induce IL-10 production and functional immune suppression as well as inflammation or p53 upregulation, as previously shown (3). As depicted in Figure 1, PUVA significantly suppressed immune function (by 56-57%, as calculated by the formula given in materials and methods) and this was totally abrogated by pharmacological 5-HT receptor blockade with ketanserin or cyproheptadine (Figure 1 A) or anti-5-HT antibody treatment (Figure 1B) or anti-cis-UCA antibody (Figure S 1A) given immediately before PUVA exposure. PBS/DMSO vehicle or control serum treatment did not significantly alter PUVA-induced immune suppression. In contrast, treatment with ketanserin or cyproheptadine (Figure 2 A, Figure S2, and Table S1) or anti-5-HT antibody (Figure 2B and Table S1) as well as anti-cis-UCA antibody (Figure S1 B) did not significantly affect PUVA-induced skin inflammation, as measured macroscopically by skin swelling (Figure 2) or microscopically by cellular skin infiltration and hyperplasia (Table S1 and Figure S1). Importantly, there was also no statistical significant difference in the number of sunburn cells among the different PUVA-treated groups compared to the untreated control group (Table S1), suggesting that 5-HT does not play a role in PUVA-induced apoptosis.
Figure 1. 5-HT2 receptor blockade abrogates PUVA-induced systemic immune suppression and 5-HT administration mimics the effect of PUVA on immune function.
PUVA-induced systemic immune suppression was studied in the model of DTH to C. albicans. Groups of C3H mice were treated with topical 8-MOP and 50 kJ/m2 of UVA or i.p. injection with 5-HT or PBS as control 5 days before immunization with C. albicans. Nine days later the mice were footpad challenged with Candida antigen. DTH was measured 24 h after challenge by change of footpad thickness. The background response (Neg Control) was measured in mice that were not immunized and challenged. Mice that were immunized (but not PUVA-treated) and challenged served as the positive control group (Pos Control). A) The i.p. injection of mice immediately before PUVA exposure with the 5-HT2 antagonist ketanserin or cyproheptadine entirely abrogated PUVA-induced suppression of induction of DTH whereas the control injection with PBS/DSMO had no effect compared to the non-injected group (None). B) Similar to PUVA, 5-HT injection did lead to suppression of DTH whereas PBS control injection did not. N=5, negative control; n=10-13, other experimental groups. * P < 0.001; + P = 0.01; # P < 0.05 vs. respective control groups of antagonists (A) and PBS (B). Mean + S.E.M. shown.
Figure 2. 5-HT2 antagonists do not affect PUVA-induced inflamamtion nor does 5-HT lead to inflammation of the skin.
A) Inflammation of PUVA-exposed dorsal skin of C3H mice was quantified by measuring double skin fold thickness (DSFT) before and 48 hours after exposure. Injection with 5-HT2 antagonist ketanserin or cyproheptadine did not alter PUVA-induced inflammation, compared to PBS/DMSO control, given immediately before PUVA exposure. B) I.p. 5-HT injection did not lead to measurable skin inflammation nor did anti-5-HT serum given immediately before PUVA exposure abrogate PUVA-induced inflammation. N=10-13 per group; * P < 0.001 vs. untreated control group (Control). Mean + S.E.M. shown.
Systemic 5-HT administration mimics the immune suppressive effect of PUVA
As shown in Figure 1B, the i.p. injection of 5-HT did mimic PUVA-induced immune suppression (Figure 1A). However, i.p. 5-HT injection did not lead to inflammation of the skin (Figure 2B) and 5-HT receptor blockade with ketanserin or cyproheptadine or anti-5-HT antibody treatment was ineffective in reducing PUVA-induced skin inflammation as determined macroscopically by skin swelling (Figure 2) or microscopically by cellular skin infiltration and hyperplasia (Table S1 and Figure S1). In a previous study (35), intradermal injection of 5-HT did also not significantly alter the skin thickness of K.5hTGF-beta1 transgenic mice (prone to psoriatic skin alterations) or their WT littermates at the site of injection compared to control injection. Together, this indicated that 5-HT was crucial for the induction of PUVA-induced immune suppression (as measured by systemic delayed type hypersensitivity) but not local inflammation.
Mast cells are possibly required for PUVA-induced systemic immune suppression but not local inflammation
Staining serial skin sections with Giemsa to identify mast cells and anti-5HT antibodies by immunohistochemistry suggested that mast cells were the cellular source of UV-induced, immune suppressive 5-HT in skin (Figure 3). 5-HT-positive mast cells were dispersed and clustered in the dermis of mice, irrespective of treatment (Table S2). 5HT-positive cells contained multiple small metachromatic granules in a large cytoplasm, suggesting that these cells are mast cells and source of 5-HT. Double staining with anti-5-HT and anti-tryptase antibody indicated that a large portion of mast cells contained 5-HT (Figure S3). KITW-Sh/W-Sh mice could not be immmune suppressed by PUVA (Figure 4A), suggesting that mast cells may have been required for PUVA-induced immune suppression. As a positive “suppressor” control for this experiment mice were exposed to UVB or JP-8 jet fuel, because previous work by us and others demonstrated that these agents induce immune suppression in a mast cell dependent manner (36-38). Whereas PUVA was strongly immunosuppressive in wild type (WT) mice by 78% (as calculated by the formula given in materials and methods), similar to jet fuel and UV(B) (78% and 89% suppression in that experiment) (Figure 4A), all agents completely failed to induce immune suppression in mice that lacked mast cells. In contrast, mast cells were dispensable for PUVA-induced skin inflammation since the amount of skin swelling was similar in mast-cell-deficient and WT mice at 24 hours (Figure 4B) and at 48 hours (Figure 4C) at its maximum. This was in contrast to the skin swelling response in solar simulated UV-irradiated WT mice, which exhibited at the dose used no skin significant skin swelling at 24 hours and less swelling at 48 hours compared to the mast cell-deficient mice. Finally we asked whether exposure to PUVA might alter mast cells density and 5-HT expression in the skin. We found that at least in the short-term (48 hours after PUVA exposure) this was not the case since there were no significant differences in 5-HT and mast cell levels in the skin between the PUVA-treated mouse groups compared to the untreated group (Figure 3 and Table S2). Slightly higher mast cell numbers were obtained for Giemsa staining as well as tryptase staining compared to 5-HT staining.
Figure 3. PUVA exposure does not alter the number of mast cells and 5-HT expression in the skin.
Representative skin sections are shown from PUVA-treated C3H mice 48 hours after exposure (PUVA) and control mice (Control). Normal gut and carcinoid tumor tissue is shown as postive control for 5-HT staining. 5-HT-positive cells contained multiple small metachromatic granules in a large cytoplasma (see right panel at higher magnification), suggesting that these cells are mast cells and the primary source of 5-HT. Bar, 100 μm and 50 μm (green-framed magnified areas). Quantification of data is provided in Supplementary Table 1.
Figure 4. Mast cells may be crucial for PUVA-induced systemic immune suppression but not local inflammation.
PUVA-induced systemic immune suppression was studied in the model of DTH to C. albicans. Groups of mice mast cell deficient KitW-Sh/W-Sh mice or wild type mice (WT) were treated with topical 8-MOP and 50 kJ/m2 of UVA, 15 kJ/m2 of solar simulated UV irradiation or jet fuel (JP-8) 5 days before immunization with C. albicans. For further experimental details on DTH and control groups see Figure legend 1. A) Treatment with PUVA, solar simulated UV or jet fuel led to significant suppression of DTH in WT mice but not in mast cell deficient mice. N=5, negative control; n=10, other experimental groups; + P < 0.01 vs. positive control group (Pos Control). B) and C) PUVA did lead to significant inflammation as measured by double skin fold thickness of dorsal skin (DSFT) at B) 24 hours and C) 48 hours in both wild type (WT) and mast cell deficient mice with no significant differences between them. Skin swelling induced by solar simulated UV was greater in mast cell-deficient mice compared to WT. * P < 0.001 vs. untreated control group (None). $ P < 0.001 vs. UV group of WT mice. § P < 0.05 vs. UV group of WT mice. Mean + S.E.M. shown.
DISCUSSION
Using a pharmacological and targeted genetic approach, our results show that 5-HT signaling through 5HT(2) receptors is crucial for PUVA-induced immune suppression but not cutaneous inflammation (Figure 1 and 2) or apoptosis. Blocking either 5-HT(2) receptors with the antagonists ketanserin or cyproheptadine or blocking 5HT itself with anti-5-HT or anti-cis-UCA antibodies were all able to prevent PUVA-induced immune suppression. However, none of these treatments could block PUVA-induced inflammation or apoptosis suggesting that another mechanism, most likely involving PAF (3) and/or products of arachidonic acid metabolism (39) is involved in these biological events. That antagonizing the 5-HT2 receptor is most important to block PUVA-induced immune suppression is supported by the fact that ketanserin preferentially blocks this receptor and that treatment with an anti-cis-UCA antibody that has been shown to block binding to the 5-HT2 receptor (18) also blocked PUVA-induced suppression of DTH (Figure S2). The ability of anti-cis-UCA antibodies to block PUVA-induced immune suppression is most likely due to the fact that the ring-like structure of cis-UCA is immunologically similar to the epitope recognized by the anti-5-HT antibody (18). Both 5-HT and cis-UCA have been shown to bind with high affinity to the 5-HT2A receptor (18).
One way how 5-HT may mediate immune suppression by an immunotoxin such as PUVA is that the release of 5-HT may interfere with the induction of the immune response at the systemic level, possibly in lymph nodes to which mast cells have been shown to migrate upon UV exposure (36). Supporting this idea are data demonstrating that altering mast cell migration via the C-X-C motif chemokine receptor 4-C-X-C motif chemokine ligand 12 (CXCR4-CXCL12) chemokine pathway has been shown to protect against UVB and jet-fuel-induced immune suppression (36, 38) as well as UV-induced skin cancer formation (40) in mice. A previous study from our laboratory has revealed that PUVA-induced suppression of the induction of DTH can be blocked by PAF inhibition (3). Together with the present observation on the role of 5-HT, this points towards a redundancy of pathways involved in PUVA-induced immune suppression, similar to that for UVB.
Work by Sreevidya et al (28) has demonstrated that UV carcinogenesis in mice can be inhibited by the administration of the 5-HT2A antagonists ketanserin and 1-(1-Naphthyl) piperazine. These 5-HT2 antagonists also reduced UV-induced hypertrophy, sunburn cell formation, and apoptosis induced by a single dose of UVB radiation. Subsequent work of the investigators indicated that 5-HT2 (as well as PAF) receptor antagonists worked by accelerating DNA repair (41). This contrasts with the results presented here using 5-HT receptor antagonists including ketanserin in the context of PUVA-induced inflammation and sunburn cell formation. However, our results are consistent with previous work by Ikai et al (42), who used mast cell-deficient W/Wv mice to show that mast cells do not make a major contribution to sunburn cell formation and ear swelling response following topical PUVA treatment. One explanation for these differences may be overlapping but not identical mechanisms of UVB's and PUVA's effects. That there are differences in the effects of PUVA and UVB is also evident in the differential skin swelling response after PUVA vs. UVB exposure between mast cell-deficient mice and their WT littermates (Figure 4B and C). Modulating the mechanistic profile of PUVA may have great potential in improving the safety of the treatment in patients with various skin diseases because long-term PUVA treatment increases the risk of non-melanoma skin cancer (43, 44) and possibly malignant melanoma (45). Indeed, similar to UVB radiation PUVA is a complete carcinogen, leading to carcinogenesis by enhancing tumor initiation, promotion, and/or immune suppression (reviewed in (46)).
In the mouse model PUVA-associated suppression of contact hypersensitivity has been linked to the activation of antigen specific transferable T suppressor cells (6) (now called regulatory T cells; Tregs) (50-53), which are thought to favor skin cancer formation. Indeed, suppression of immunity to the contact allergen dinitrochlorobenzene and DTH has also been reported in patients with psoriasis treated with PUVA (10, 11). On the other hand, we found that induction of Tregs and downregulation of the Th17 axis was linked to improvement of psoriasiform skin changes in TGF-beta1 transgenic mice treated by PUVA (35, 54, 55).
We speculate that mast cells are important in PUVA-induced immune suppression. This is supported by a bulk of evidence indicating that mast cells are a major cellular mediator of jet fuel- (38), UVA- (56) and UVB-modulated immunity (36-38, 56-60). Importantly, the 5-HT2a receptor is expressed by mast cells (61) and previous studies investigating cis-UCA have shown that mast cells could be indirectly activated through cutaneous nerve fibers to release mast cell activating neuropeptides (57, 62). It's perhaps not surprising that mast cells were found to be dispensable for PUVA-induced inflammation. Grimbaldeston et al (60) used mast cell-deficient mice to demonstrate that mast cell-derived IL-10 was required to prevent inflammatory leucocyte infiltration and limit skin edema and inflammation induced by chronic UVB radiation. More recently, Schweintzger et al (34) have shown that mast cells were required for inducing phototolerance and abating UVB-induced scratching of the skin. Mast cell-deficient mice (KitW-Sh/W-Sh) were prone to develop photo itch and more skin edema and epidermal hyperplasia upon UVB exposure and the implications of these findings were discussed in a commentary by De Gruijl (63) in the context of increased scratching observed in carcinogenesis studies in mice after chronic (UVA-1) irradiation (64, 65). Consistent with a protective role of mast cells, we now observed that mast cell-deficient mice developed significant more skin swelling than their WT littermates after exposure to a single dose of solar simulated UV but not PUVA (Figure 4B and C). Besides lacking mast cells the increased skin swelling response to UVB exposure in the mast cell-deficient KitW-Sh/W-Sh mice could be at least partially contributed to their depigmented skin caused by the mutation of the c-kit locus, resulting in impaired melanogenesis (27). However, differences in pigmentation cannot solely be responsible for differences in the immune responses between mast cell-deficient and wild type mice because, if pigment has a protective role, then there should have been more but not less immune suppression in mast cell-deficient mice compared to wild type controls. Indeed, exactly the opposite was observed (Figure 4A), again reinforcing the importance of mast cells.
Mast cell-deficient KitW-Sh/W-Sh mice also display abnormalities in myelopoiesis making them an imperfect model to investigate the role of mast cells (66). Despite these limitations, previous studies have shown that a lack of mast cells in mast cell-deficient mice was the primary reason for the immunological alterations related to UVB exposure (2, 26, 27). However, before making any definite conclusions on the role of mast cells in PUVA-induced immune suppression experiments involving mast cell reconstitution (34) or using newer models of mast cell deficiency (67-69) are definitely needed.
PUVA, through its ability to induce apoptosis and/or suppress cell-mediated immune response, continues to be one of the most effective treatment modalities for various skin disorders, including psoriasis (70) and cutaneous T cell lymphoma (71). Combined with previous reports, the results of our study provide unique opportunities to target the specific molecular (5-HT) players responsible for PUVA's mechanistic profile. That being said, administration of the serotonin receptor antagonist ketanserin did neither affect inflammation nor psoriatic phenotype of K5.hTGF-beta1 transgenic mice (35), whose skin lesions respond to PUVA treatment (35, 54). If it turns out that PUVA-induced apoptosis out-weights immune suppression in its significance for clinical therapeutic efficacy, then blockade of the 5-HT pathway may improve the safety profile of PUVA by reducing its tumor promoting effects (through diminishing immune suppression). Indeed, a computational model of psoriatic epidermis revealed that keratinocyte apoptosis was sufficient to explain (311nm) UVB phototherapy-induced psoriatic plaque resolution (72). Moreover, PUVA-induced cell-cycle arrest and subsequent apoptosis in human T lymphocytes (73, 74) is a process that might be particularly important for therapeutic efficacy in cutaneous T-cell lymphoma (75). This is consistent with current clinical knowledge that phototherapy clears psoriatic and cutaneous T cell lymphoma skin lesions only at directly exposed but not shielded skin sites (76). Said so, the pharmacological inhibition of the release of mast cell mediators did not improve psoriatic plaques in patients of a clinical study (77). Moreover, evidence is accumulating that cells of the innate immune system, including neutrophils, γδT cells, innate lymphoid cells and mast cells are the main source of IL-17, as one of the key cytokines in psoriasis (78). Interfering with 5-HT receptor signaling (23) may offer the opportunity to reduce side effects without affecting PUVA's efficacy in the treatment of diseases such as psoriasis and CTCL.
Supplementary Material
Acknowledgements
The authors thank Nasser Kazimi for assistance with the experiments reported here; Mrs. Ulrike Schmidbauer, Mrs. Margit Gogg-Kamerer, and Elisabeth Steinbauer for performing histological and/or immunohistochemical stainings; and Jeffrey P. Walterscheid, Dat X. Nghiem and Gerardo Ramos for help and advice. S.N.B. was supported by a National Health and Medical Research Council C. J. Martin Fellowship (307726). S.E.U. was supported by a grant for the US National Institutes of Health (CA131207).
Abbreviations
- 5-HT
5-hydroxtryptamine (serotonin)
- DTH
delayed type hypersensitivity
- PUVA
psoralen plus UVA
- Treg
regulatory T cells
Footnotes
Author contribution
P.W., S.N.B and A.Y.L.-F. performed the experimental work. G.H. provided expertise and support in performing immunohistochemical stainings. P.W. and S.N.B. analysed the data. P.W. and S.E.U. designed the research study. P.W. drafted the paper. All authors approved of the submitted and final version of the manuscript.
Conflict Of Interest
The authors state no conflict of interest
Supporting Information
Additional supporting data are found in the supplementary information of this article.
Table S1. PUVA-induced histologic alterations in the skin of mice.
Table S2. Number of mast cells and 5-HT-positive cells in the skin of mice.
Figure S1. Blockade of cis-UCA abrogates PUVA-induced systemic immune suppression but not inflammation.
Figure S2. 5-HT2 receptor and 5-HT blockade does not affect PUVA-induced inflammation and hyperplasia of the skin.
Figure S3. Mast cell stain for the presence of 5-HT.
REFERENCES
- 1.Duthie MS, Kimber I, Norval M. Br J Dermatol. 1999;140:995–1009. doi: 10.1046/j.1365-2133.1999.02898.x. [DOI] [PubMed] [Google Scholar]
- 2.Ullrich SE, Byrne SN. J Invest Dermatol. 2012;132:896–905. doi: 10.1038/jid.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf P, Nghiem DX, Walterscheid JP, et al. Am J Pathol. 2006;169:795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcalay J, Ullrich SE, Kripke ML. Photochem Photobiol. 1989;50:217–220. doi: 10.1111/j.1751-1097.1989.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 5.Aubin F, Dall'Acqua F, Kripke ML. J Invest Dermatol. 1991;97:50–54. doi: 10.1111/1523-1747.ep12478010. [DOI] [PubMed] [Google Scholar]
- 6.Kripke ML, Morison WL, Parrish JA. J Invest Dermatol. 1983;81:87–92. doi: 10.1111/1523-1747.ep12542071. [DOI] [PubMed] [Google Scholar]
- 7.Ullrich SE. Photodermatol Photoimmunol Photomed. 1991;8:116–122. [PubMed] [Google Scholar]
- 8.Morison WL, Parrish JA, Woehler ME, Krugler JI, Bloch KJ. J Invest Dermatol. 1981;76:484–488. doi: 10.1111/1523-1747.ep12521182. [DOI] [PubMed] [Google Scholar]
- 9.Morison WL, Kripke ML. Cell Immunol. 1984;85:270–277. doi: 10.1016/0008-8749(84)90298-3. [DOI] [PubMed] [Google Scholar]
- 10.Strauss GH, Bridges BA, Greaves M, Hall-Smith P, Price M, Vella-Briffa D. Lancet. 1980;2:556–559. doi: 10.1016/s0140-6736(80)91992-3. [DOI] [PubMed] [Google Scholar]
- 11.Strauss GH, Bridges BA, Greaves M, Vella-Briffa D, Hall-Smith P, Price M. Lancet. 1980;2:1134–1135. doi: 10.1016/s0140-6736(80)92562-3. [DOI] [PubMed] [Google Scholar]
- 12.Morison WL. Natl Cancer Inst Monogr. 1984;66:243–246. [PubMed] [Google Scholar]
- 13.Vallat VP, Gilleaudeau P, Battat L, et al. J Exp Med. 1994;180:283–296. doi: 10.1084/jem.180.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coven TR, Walters IB, Cardinale I, Krueger JG. Photodermatol Photoimmunol Photomed. 1999;15:22–27. doi: 10.1111/j.1600-0781.1999.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 15.Caffieri S. Photochem Photobiol Sci. 2002;1:149–157. doi: 10.1039/b107329j. [DOI] [PubMed] [Google Scholar]
- 16.Shirsath N, Mayer G, Singh TP, Wolf P. Exp Dermatol. 2015 Jun 29; doi: 10.1111/exd.12779. 10.1111/exd.12779. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Martins MA, Lima MC, Bozza PT, et al. Eur J Pharmacol. 1993;237:17–22. doi: 10.1016/0014-2999(93)90087-x. [DOI] [PubMed] [Google Scholar]
- 18.Walterscheid JP, Nghiem DX, Kazimi N, et al. Proc Natl Acad Sci U S A. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walterscheid JP, Ullrich SE, Nghiem DX. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ameisen JC, Meade R, Askenase PW. J Immunol. 1989;142:3171–3179. [PubMed] [Google Scholar]
- 21.Askenase PW, Geba GP, Levin J, et al. Int Arch Allergy Immunol. 1995;107:145–147. doi: 10.1159/000236958. [DOI] [PubMed] [Google Scholar]
- 22.Ringvall M, Ronnberg E, Wernersson S, et al. J Allergy Clin Immunol. 2008;121:1020–1026. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Mandel MJ, Lim HW. Photodermatol. 1988;5:211–217. [PubMed] [Google Scholar]
- 25.Guhl S, Stefaniak R, Strathmann M, et al. J Invest Dermatol. 2005;124:453–456. doi: 10.1111/j.0022-202X.2004.23523.x. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs NK, Tye J, Norval M. Photochem Photobiol Sci. 2008;7:655–667. doi: 10.1039/b717398a. [DOI] [PubMed] [Google Scholar]
- 27.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE. Cancer Res. 2008;68:3978–3984. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos G, Limon-Flores AY, Ullrich SE. Toxicol Sci. 2007;100:415–422. doi: 10.1093/toxsci/kfm247. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich SE, Lyons HJ. Toxicol Sci. 2000;58:290–298. doi: 10.1093/toxsci/58.2.290. [DOI] [PubMed] [Google Scholar]
- 31.Wolf P, Cox P, Yarosh DB, Kripke ML. J Invest Dermatol. 1995;104:287–292. doi: 10.1111/1523-1747.ep12612828. [DOI] [PubMed] [Google Scholar]
- 32.Wolf P, Donawho CK, Kripke ML. J Invest Dermatol. 1993;100:254–259. doi: 10.1111/1523-1747.ep12469038. [DOI] [PubMed] [Google Scholar]
- 33.Wolf P, Gruber-Wackernagel A, Bambach I, et al. Exp Dermatol. 2014;23:428–430. doi: 10.1111/exd.12427. [DOI] [PubMed] [Google Scholar]
- 34.Schweintzger NA, Bambach I, Reginato E, et al. Exp Dermatol. 2015;24:491–496. doi: 10.1111/exd.12687. [DOI] [PubMed] [Google Scholar]
- 35.Singh TP, Huettner B, Koefeler H, et al. Am J Pathol. 2011;178:699–708. doi: 10.1016/j.ajpath.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne SN, Limon-Flores AY, Ullrich SE. J Immunol. 2008;180:4648–4655. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limon-Flores AY, Chacon-Salinas R, Ramos G, Ullrich SE. Toxicol Sci. 2009;112:144–152. doi: 10.1093/toxsci/kfp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imokawa G, Tejima T. J Invest Dermatol. 1989;92:296–300. doi: 10.1111/1523-1747.ep12276882. [DOI] [PubMed] [Google Scholar]
- 40.Sarchio SN, Scolyer RA, Beaugie C, et al. J Invest Dermatol. 2014;134:1091–1100. doi: 10.1038/jid.2013.424. [DOI] [PubMed] [Google Scholar]
- 41.Sreevidya CS, Fukunaga A, Khaskhely NM, et al. J Invest Dermatol. 2010;130:1428–1437. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikai K, Danno K, Horio T, Narumiya S. J Invest Dermatol. 1985;85:82–84. doi: 10.1111/1523-1747.ep12275365. [DOI] [PubMed] [Google Scholar]
- 43.Stern RS, Puva Follow-Up Study J Am Acad Dermatol. 2012;66:553–562. doi: 10.1016/j.jaad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Nijsten TE, Stern RS. J Invest Dermatol. 2003;121:252–258. doi: 10.1046/j.1523-1747.2003.12350.x. [DOI] [PubMed] [Google Scholar]
- 45.Stern RS, Nichols KT, Vakeva LH. N Engl J Med. 1997;336:1041–1045. doi: 10.1056/NEJM199704103361501. [DOI] [PubMed] [Google Scholar]
- 46.Wolf P, Kreimer-Erlacher H, Seidl H, Back B, Soyer HP, Kerl H. J Invest Dermatol. 2004;122:190–200. doi: 10.1046/j.0022-202X.2004.22118.x. [DOI] [PubMed] [Google Scholar]
- 47.Kreimer-Erlacher H, Seidl H, Back B, Cerroni L, Kerl H, Wolf P. J Invest Dermatol. 2003;120:676–682. doi: 10.1046/j.1523-1747.2003.12085.x. [DOI] [PubMed] [Google Scholar]
- 48.Nataraj AJ, Wolf P, Cerroni L, Ananthaswamy HN. J Invest Dermatol. 1997;109:238–243. doi: 10.1111/1523-1747.ep12319764. [DOI] [PubMed] [Google Scholar]
- 49.Stern RS, Bolshakov S, Nataraj AJ, Ananthaswamy HN. J Invest Dermatol. 2002;119:522–526. doi: 10.1046/j.1523-1747.2002.01814.x. [DOI] [PubMed] [Google Scholar]
- 50.Maeda A, Beissert S, Schwarz T, Schwarz A. J Immunol. 2008;180:3065–3071. doi: 10.4049/jimmunol.180.5.3065. [DOI] [PubMed] [Google Scholar]
- 51.Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. J Allergy Clin Immunol. 2011;128:826–833. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz A, Schwarz T. J Invest Dermatol. 2010;130:1914–1921. doi: 10.1038/jid.2010.59. [DOI] [PubMed] [Google Scholar]
- 53.Schweintzger N, Gruber-Wackernagel A, Reginato E, et al. Br J Dermatol. 2015;173:519–526. doi: 10.1111/bjd.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh TP, Schon MP, Wallbrecht K, et al. J Immunol. 2010;184:7257–7267. doi: 10.4049/jimmunol.0903719. [DOI] [PubMed] [Google Scholar]
- 55.Singh TP, Schon MP, Wallbrecht K, Wolf P. Exp Dermatol. 2012;21:228–230. doi: 10.1111/j.1600-0625.2011.01437.x. [DOI] [PubMed] [Google Scholar]
- 56.Ullrich SE, Nghiem DX, Khaskina P. Photochem Photobiol. 2007;83:1095–1100. doi: 10.1111/j.1751-1097.2007.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hart PH, Grimbaldeston MA, Swift GJ, Hosszu EK, Finlay-Jones JJ. Photochem Photobiol. 1999;70:807–812. [PubMed] [Google Scholar]
- 58.Hart PH, Grimbaldeston MA, Finlay-Jones JJ. J Photochem Photobiol B. 2000;55:81–87. doi: 10.1016/s1011-1344(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 59.Lu LF, Lind EF, Gondek DC, et al. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 60.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 61.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, et al. J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 62.Khalil Z, Townley SL, Grimbaldeston MA, Finlay-Jones JJ, Hart PH. J Invest Dermatol. 2001;117:886–891. doi: 10.1046/j.0022-202x.2001.01466.x. [DOI] [PubMed] [Google Scholar]
- 63.de Gruijl FR. Exp Dermatol. 2015;24:489–490. doi: 10.1111/exd.12742. [DOI] [PubMed] [Google Scholar]
- 64.de Laat JM, Seite S, Groenendijk M, van Vloten WA, de Gruijl FR. Exp Dermatol. 1997;6:292–297. doi: 10.1111/j.1600-0625.1997.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 65.Rebel H, der Spek CD, Salvatori D, van Leeuwen JP, Robanus-Maandag EC, de Gruijl FR. Int J Cancer. 2015;136:271–277. doi: 10.1002/ijc.29002. [DOI] [PubMed] [Google Scholar]
- 66.Michel A, Schuler A, Friedrich P, et al. J Immunol. 2013;190:5534–5544. doi: 10.4049/jimmunol.1203355. [DOI] [PubMed] [Google Scholar]
- 67.Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Adv Immunol. 2015;126:45–127. doi: 10.1016/bs.ai.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowak EC, de Vries VC, Wasiuk A, et al. J Exp Med. 2012;209:2127–2135. doi: 10.1084/jem.20120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peschke K, Dudeck A, Rabenhorst A, Hartmann K, Roers A. Methods Mol Biol. 2015;1220:403–421. doi: 10.1007/978-1-4939-1568-2_25. [DOI] [PubMed] [Google Scholar]
- 70.Inzinger M, Heschl B, Weger W, et al. Br J Dermatol. 2011;165:640–645. doi: 10.1111/j.1365-2133.2011.10396.x. [DOI] [PubMed] [Google Scholar]
- 71.Wackernagel A, Hofer A, Legat F, Kerl H, Wolf P. Br J Dermatol. 2006;154:519–523. doi: 10.1111/j.1365-2133.2005.07008.x. [DOI] [PubMed] [Google Scholar]
- 72.Weatherhead SC, Farr PM, Jamieson D, et al. J Invest Dermatol. 2011;131:1916–1926. doi: 10.1038/jid.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santamaria AB, Davis DW, Nghiem DX, et al. Cell Death Differ. 2002;9:549–560. doi: 10.1038/sj.cdd.4401007. [DOI] [PubMed] [Google Scholar]
- 74.Johnson R, Staiano-Coico L, Austin L, Cardinale I, Nabeya-Tsukifuji R, Krueger JG. Photochem Photobiol. 1996;63:566–571. doi: 10.1111/j.1751-1097.1996.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 75.Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. J Invest Dermatol. 1996;107:235–242. doi: 10.1111/1523-1747.ep12329711. [DOI] [PubMed] [Google Scholar]
- 76.Gilchrest BA, Parrish JA, Tanenbaum L, Haynes HA, Fitzpatrick TB. Cancer. 1976;38:683–689. doi: 10.1002/1097-0142(197608)38:2<683::aid-cncr2820380210>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 77.van de Kerkhof PC, Goos M, Christophers E, Baudin M, Dupuy P. Skin Pharmacol. 1995;8:25–29. doi: 10.1159/000211327. [DOI] [PubMed] [Google Scholar]
- 78.Keijsers RR, Joosten I, van Erp PE, Koenen HJ, van de Kerkhof PC. Exp Dermatol. 2014;23:799–803. doi: 10.1111/exd.12487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.