Abstract
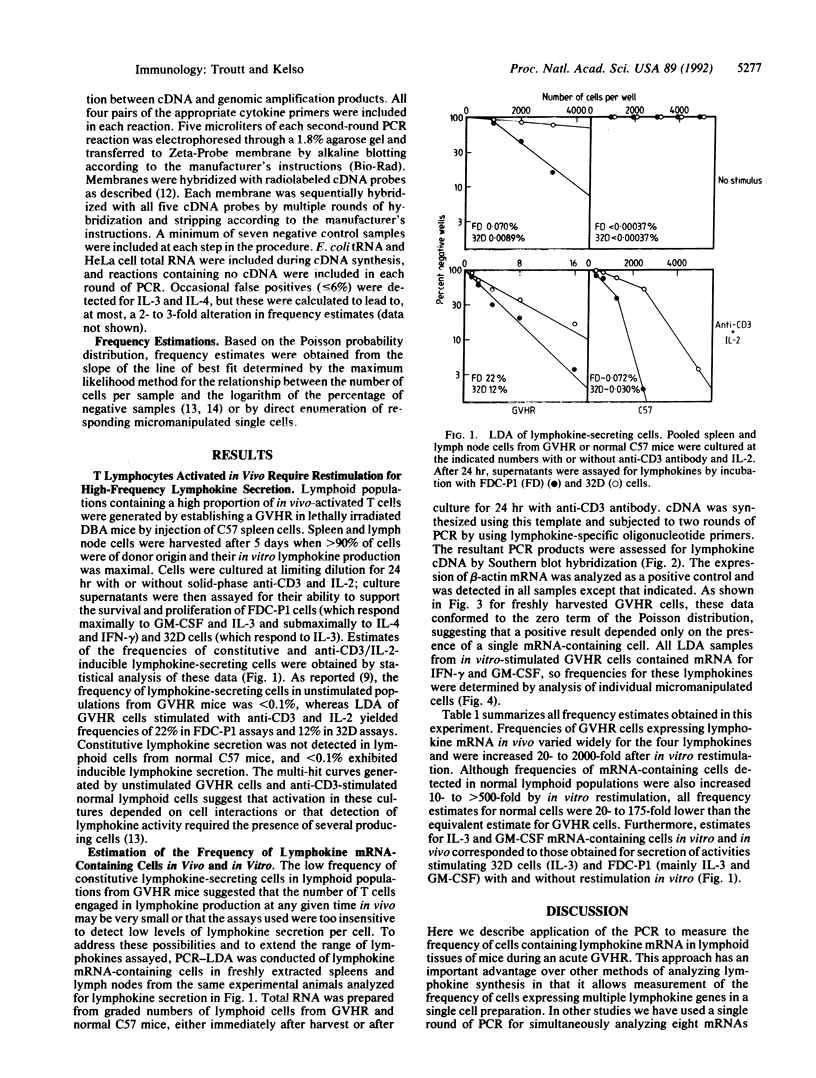
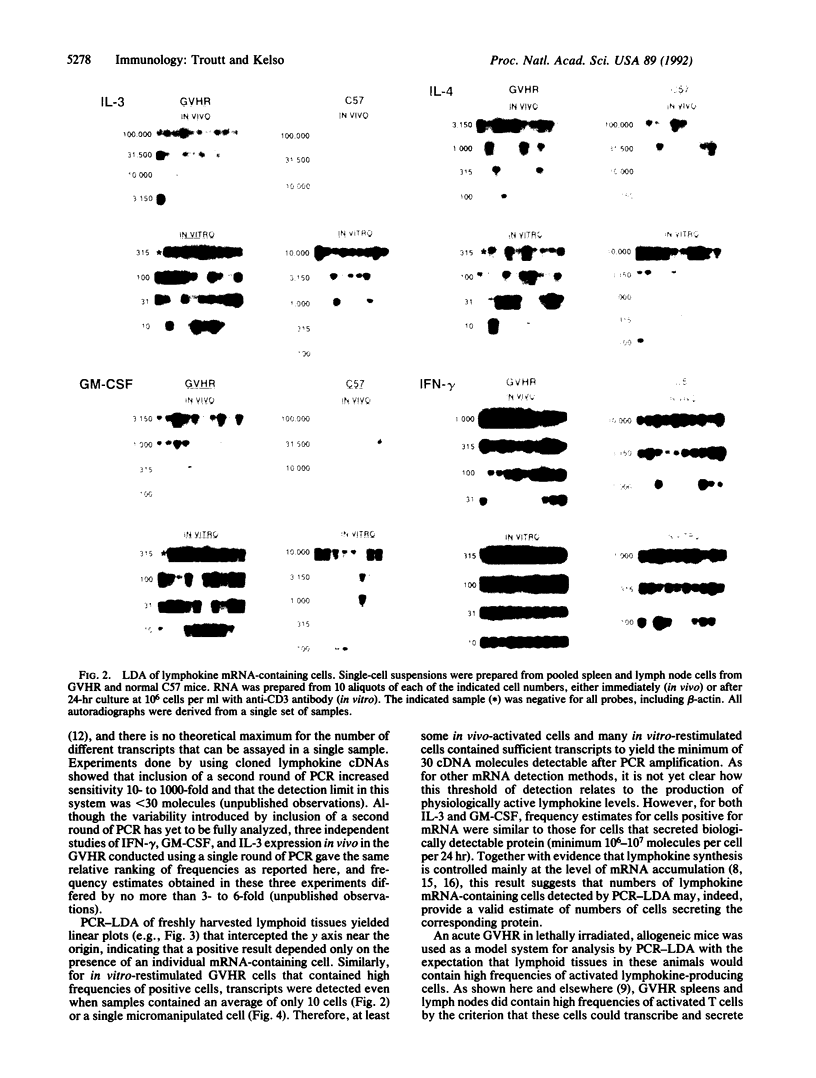
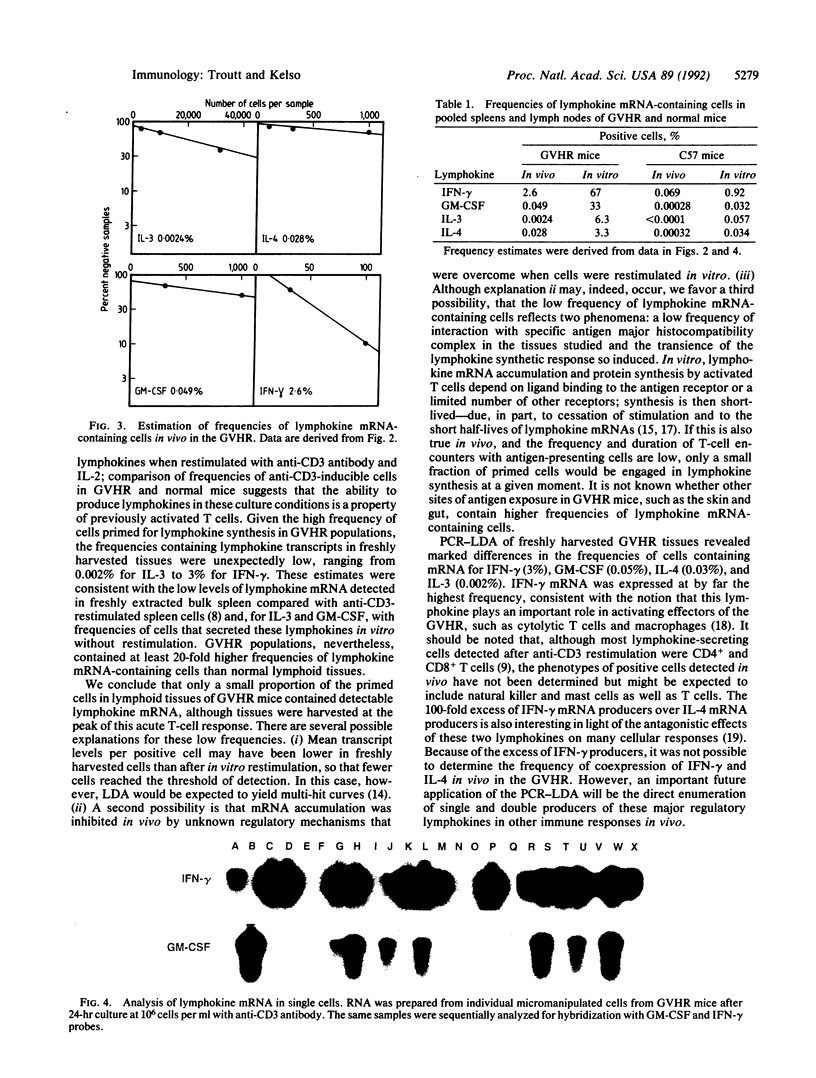
A method of enumerating lymphokine mRNA-containing cells in vivo was developed by combining limiting dilution analysis with PCR amplification of cDNA. Single-hit kinetics revealed that the PCR-limiting dilution analysis could detect a single positive cell among greater than 40,000 negative cells. With this method, spleens and lymph nodes of mice undergoing an acute allogeneic graft-versus-host reaction were found to contain lymphokine mRNA-expressing cells at frequencies of 3% for interferon gamma, 0.05% for granulocyte/macrophage colony-stimulating factor, 0.002% for interleukin 3, and 0.03% for interleukin 4; these frequencies were 20- to 175-fold higher than in lymphoid tissues of normal mice. In contrast to their low frequencies of lymphokine mRNA-containing cells in vivo, graft-versus-host reaction populations restimulated in vitro for 24 hr with anti-CD3 antibody yielded frequencies ranging from 3% for interleukin 4 to nearly 70% for interferon gamma. Furthermore, lymphokine transcripts were also detected in single micromanipulated cells from these populations. Because frequencies of anti-CD3-inducible lymphokine mRNA-containing cells in normal mice were only 0.03-1%, it was concluded that lymphoid tissues of graft-versus-host reaction mice contained high frequencies of cells that had been primed for lymphokine synthesis. Only a small fraction of these cells, however, expressed lymphokine mRNAs at a given time point in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Gough N. M., Kelso A. GM-CSF expression is preferential to multi-CSF (IL-3) expression in murine T lymphocyte clones. Growth Factors. 1989;1(4):287–298. doi: 10.3109/08977198909000253. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold K. C., Lancki D. W., Dunn D. E., Arai K., Fitch F. W. Activation of lymphokine genes during stimulation of cloned T cells. Eur J Immunol. 1986 Dec;16(12):1533–1538. doi: 10.1002/eji.1830161211. [DOI] [PubMed] [Google Scholar]
- Kelso A. Cytokines: structure, function and synthesis. Curr Opin Immunol. 1989 Dec;2(2):215–225. doi: 10.1016/0952-7915(89)90191-x. [DOI] [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Kelso A., Gough N. M. Coexpression of granulocyte-macrophage colony-stimulating factor, gamma interferon, and interleukins 3 and 4 is random in murine alloreactive T-lymphocyte clones. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9189–9193. doi: 10.1073/pnas.85.23.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Troutt A. B., Maraskovsky E., Gough N. M., Morris L., Pech M. H., Thomson J. A. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol Rev. 1991 Oct;123:85–114. doi: 10.1111/j.1600-065x.1991.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Troutt A. B., Kelso A. Co-engagement of CD3 with LFA-1 or ICAM-1 adhesion molecules enhances the frequency of activation of single murine CD4+ and CD8+ T cells and induces synthesis of IL-3 and IFN-gamma but not IL-4 or IL-6. Int Immunol. 1992 Apr;4(4):475–485. doi: 10.1093/intimm/4.4.475. [DOI] [PubMed] [Google Scholar]
- Mohler K. M., Butler L. D. Differential production of IL-2 and IL-4 mRNA in vivo after primary sensitization. J Immunol. 1990 Sep 15;145(6):1734–1739. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Reeves R., Magnuson N. S. Mechanisms regulating transient expression of mammalian cytokine genes and cellular oncogenes. Prog Nucleic Acid Res Mol Biol. 1990;38:241–282. doi: 10.1016/s0079-6603(08)60713-8. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]





