Abstract
Background
Methamphetamine dependence is a significant public health concern without any approved medications for treatment. We evaluated ibudilast, a non-selective phosphodiesterase inhibitor, to assess the safety and tolerability during intravenous (IV) methamphetamine administration. We conducted a randomized, double-blind, placebo-controlled, within-subjects crossover clinical trial.
Methods
Participants received ibudilast (20 mg twice daily followed by 50 mg twice daily) and placebo, with order determined by randomization, and then underwent IV methamphetamine challenges (15 and 30 mg). We monitored cardiovascular effects, methamphetamine pharmacokinetics and reported adverse events.
Results
Ibudilast treatment had similar rates of adverse events compared to placebo and there was no significant augmentation of cardiovascular effects of methamphetamine. Pharmacokinetic analysis revealed no clinically significant change in maximum concentration or half-life of methamphetamine with ibudilast.
Conclusions
Methamphetamine administration during ibudilast treatment was well-tolerated without additive cardiovascular effects or serious adverse events, providing initial safety data to pursue ibudilast’s effectiveness for the treatment of methamphetamine dependence.
Keywords: ibudilast, methamphetamine, safety, pharmacokinetics
Introduction
Methamphetamine dependence causes devastating personal and public health consequences, and adult users of methamphetamine have remained stable over the last several years.1 The use of amphetamine-type stimulants, including methamphetamine, remains widespread globally, and appears to be increasing based on reported seizures and consumption levels.2 Current treatment options focus on behavioral therapies,3 and although these strategies are effective in reducing or eliminating use of the drug for some,3 combination pharmacotherapy with psychosocial interventions is likely to result in the greatest reduction in the negative consequences of methamphetamine dependence. However, there are no medications that have demonstrated proven effectiveness for treatment of methamphetamine dependence with the majority of trials focusing on medications targeting dopamine or other neurotransmitters.4
Previous research has shown the neurotoxic effects of methamphetamine, especially for dopaminergic systems in the ventral striatum,5 as well as clinical consequences of methamphetamine-induced neurotoxicity including neurocognitive decline6 and a poor response to behavioral therapies7. Given these results, medications that ameliorate methamphetamine-induced neurotoxicity may improve clinical outcomes in methamphetamine dependence. Multiple studies implicate glial cells in a variety of neurodegenerative diseases8,9 and glial cells may be important in modulating the rewarding properties of drugs of abuse, including methamphetamine.10 Furthermore, methamphetamine-induced glial activation may contribute to methamphetamine’s neurotoxicity and associated cognitive dysfunction via glial cell secretion of pro-inflammatory cytokines.11
Ibudilast is a non-selective phosphodiesterase (PDE) inhibitor with known molecular targets including macrophage inhibitory factor (MIF), and PDE-4 and -10 (with some PDE-3 and -11) that has activity as a modulator of CNS glial activation.12 Due to these glial modulating properties, ibudilast is an exciting medication candidate for the treatment of methamphetamine dependence. Ibudilast inhibited prime- and stress-induced resumption of methamphetamine drug-seeking behavior in rats13 and reduced morphine-induced nucleus accumbens dopamine levels in opioid dependent rats.14 Ibudilast is metabolized primarily by cytochrome P450 (CYP) 2B6 and CYP2E1 and weakly inhibits CYP1A2,15 while methamphetamine is primary metabolized by CYP2D6,16 making an ibudilast-methamphetamine pharmacokinetic interaction unlikely. As a result, ibudilast presents an opportunity for a novel approach to pharmacologic treatment of methamphetamine dependence through amelioration of methamphetamine-related cognitive dysfunction/degeneration via suppression of methamphetamine-induced glial activation and subsequent pro-inflammatory cytokine cascade.
Ibudilast has been used in Asia since 1989, mostly at doses of 30 mg per day or less for asthma, with a good safety record. Dosages higher than 30 mg per day (10–100 mg single dose and 20 mg twice daily to 50 mg twice daily) have been evaluated in three phase 1 and 2a clinical safety trials conducted in the US and Australia without any serious unexpected adverse events that required study discontinuation, death, or negative cardiac effects suggesting that ibudilast has an excellent safety profile.17,18 Preclinical and clinical pharmacokinetic and pharmacodynamic data suggest that CNS applications, such as addiction, will require higher doses to achieve clinically significant reductions in glial activation than those currently used for asthma and post-stroke dizziness. Dosages in our trial were chosen in order to sufficiently suppress methamphetamine-induced microglial activation.
We conducted a Phase I inpatient, double-blind, placebo-controlled clinical trial to assess the safety of ibudilast use in combination with methamphetamine for methamphetamine dependent participants. The aims of the study were to determine if ibudilast alters the cardiovascular response or pharmacokinetics of intravenous methamphetamine. Results of the methamphetamine subjective effects are reported in a separate manuscript.
Materials and Methods
Participants
One hundred and ten subjects provided informed consent and were screened and 18 were determined eligible for study participation and admitted to the hospital. 24 participants screened failed for medical conditions and 20 for psychiatric conditions, accounting for 48% of the total screen fail rate. Of these, 7 eligible patients withdrew voluntarily prior to receiving experimental medication as they chose not to stay in the hospital for 30 days. Eleven participants were admitted to the hospital and completed all experimental procedures, except one subject did not complete the 14-day follow-up (Supplemental Figure 1). No participants withdrew from the study because of medication adverse events.
Participants were recruited through advertisements and were paid for their participation. All participants met DSM-IV-TR criteria for methamphetamine dependence and were not seeking treatment for methamphetamine dependence at the time of study entry. Additional inclusion criteria included age between 18 and 55 years, English-speaking, and a self-reported history of using methamphetamine either via injection or smoking with at least one methamphetamine-positive urine prior to admission. Participants must have had normal vital signs, a baseline EKG in normal sinus rhythm without clinically significant arrhythmias, and normal laboratory findings (liver function tests ≤ 3 times the upper limit of normal; kidney function tests ≤ 2 times the upper limit of normal.)
Exclusion criteria included current dependence on cocaine, opioids, marijuana, or alcohol, current or past history of seizure disorder, history of head trauma, previous adverse reaction to methamphetamine, a current neurological disorder or major psychiatric disorder, or current, ongoing treatment with psychotropic medications or medications that interact with ibudilast. Heart disease, AIDS, asthma, and other unstable medical conditions were also exclusionary. This study was approved by the UCLA and UCLA-Harbor Institutional Review Boards and all subjects provided informed consent after being fully informed about potential risks of participation. The study was registered with clinicaltrials.gov (NCT01217970).
Study design
This phase I clinical trial was conducted at the Harbor-UCLA Inpatient General Clinical Research Center using a randomized, double-blind, placebo-controlled, within-subject crossover design (Table 1). Eligible participants were admitted to the clinical research unit for 27 days where they received experimental intravenous methamphetamine infusions during treatment with placebo as well as ibudilast at two doses, 20 mg twice daily (BID) and 50 mg BID. Participants were randomly assigned to receive either placebo followed by ibudilast 20 mg BID and ibudilast 50 mg BID or ibudilast 20 mg BID followed by ibudilast 50 mg BID and placebo to avoid starting any participants on ibudilast 50 mg BID. Participants were at each study condition for 3 days prior to any methamphetamine administration and were at each study dose for a total of 7 days. A 14 day post-discharge follow-up outpatient visit was completed to assess for any delayed adverse events.
Table 1.
Ibudilast Phase I clinical trial within-subject crossover design study schema
| Study Procedure | Day | Study Condition | Description |
|---|---|---|---|
| Admission | −2 | Admit to Harbor-UCLA General Clinical Research Center | |
| Randomization | Randomization to ibudilast or placebo as 1st condition | ||
| Condition 1 | 1 – 3 | ibudilast 20 mg BID or placebo | Take study condition to achieve steady state |
| MA Challenge 15 mg | 4 | MA 15 mg IV PM followed by cardiovascular, subjective effects, and PK draws | |
| PK | 5 | PK draws | |
| MA Challenge 30 mg | 6 | MA 30 mg IV PM followed by cardiovascular, subjective effects, and PK draws | |
| PK | 7 | PK draws | |
| Condition 2 | 8 – 10 | ibudilast 50 mg BID or 20 mg BID | Take study condition to achieve steady state |
| MA Challenge 15 mg | 11 | MA 15 mg IV PM followed by cardiovascular, subjective effects, and PK draws | |
| PK | 12 | PK draws | |
| MA Challenge 30 mg | 13 | MA 30 mg IV PM followed by cardiovascular, subjective effects, and PK draws | |
| PK | 14 | PK draws | |
| Condition 3 | 15 – 17 | placebo or ibudilast 50 mg BID | Take study condition to achieve steady state |
| MA Challenge 15 mg | 18 | MA 15 mg IV PM followed by cardiovascular, subjective effects, and PK draws | |
| PK | 19 | PK draws | |
| MA Challenge 30 mg | 20 | MA 30 mg IV PM followed by cardiovascular, subjective effects, and PK draws | |
| PK | 21 | PK draws | |
| MA Choice Session | Sampling of MA 15 mg IV followed by choice of MA 15 mg IV or money | ||
| Washout | 23 – 25 | Inpatient washout. Monitor safety. Termination physical, labs, EKG Discharge when stable |
|
| Health Check | 14 day post-discharge safety check | ||
abbreviations: twice-daily (BID); methamphetamine (MA); intravenous (IV); pharmacokinetics (PK); electrocardiogram (EKG)
Drugs
Ibudilast
Ibudilast is a non-selective PDE inhibitor approved for the treatment of asthma, post-stroke dizziness, and allergies in Asia. Ibudilast capsules (10 mg) were manufactured by Taisho Pharmaceuticals (Japanese-manufactured generic product), and imported by MediciNova, Inc. Taisho Pharmaceuticals also provided matching placebo capsules. The pharmacist prepared separate medication bottles for each treatment condition (ibudilast BID followed by placebo BID or placebo BID followed by ibudilast BID) with each medication bottle containing an entire course of study medication. Study medication was stored and dispensed by the Harbor-UCLA Research Pharmacy. An investigational new drug (IND #108996) application was obtained from the U.S. Food and Drug Administration for the use of ibudilast and methamphetamine in this study.
Participants self-administered study medication, either ibudilast or matching placebo capsules, under nurse supervision twice daily at 0700 and 1900. Each participant underwent experimental testing and methamphetamine infusions during treatment with placebo, ibudilast 20 mg BID, and ibudilast 50 mg BID. Participants always took ibudilast 20 mg BID prior to taking ibudilast 50 mg BID to achieve a dose titration and improve tolerability but whether placebo was received before or after the ibudilast conditions was determined randomly. At steady state of a multi-day 50 mg BID regimen, elimination half-life is about 21 – 28 hours, providing limited trough and peak variations.17,18 The half-life of ibudilast is about 19 hours in humans and therefore participants took ibudilast/placebo for three days prior to undergoing experimental methamphetamine infusion sessions to insure that ibudilast was at steady state at the time of testing.18
Methamphetamine
Methamphetamine challenge sessions occurred after treatment conditions had reached steady state (on the 4th and 6th day) with sessions separated by 2 days to allow for pharmacokinetic analysis. During methamphetamine challenge sessions, participants were given an infusion of saline at 1000 followed by either a 15 mg (on the 4th day) or 30 mg (on the 6th day) infusion of methamphetamine administered via IV push over 2 minutes using an automatic pump. Participants never received more than 30 mg IV methamphetamine on a single day, which represents an adequate dose to test safety interactions, similar to doses used for narcolepsy and attention deficit hyperactivity disorder generally ranging from 10 to 40 mg/day.
Physiological measures
Systolic and diastolic blood pressure, heart rate, and an EKG “rhythm strip” were collected 15 minutes prior to each infusion and following each saline/methamphetamine infusion at regular intervals (2, 5, 10, 15, 30, 45, 60, 75, 90, 120, 180, 240 minutes for saline infusions and additional 300 and 360 minutes for safety following methamphetamine infusions.) In addition, heart rate and blood pressure were assessed three times daily throughout the hospitalization.
Plasma samples/Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (LC-ESI-MS/MS)
Samples were collected for methamphetamine pharmacokinetic analysis following each infusion at regular intervals (5, 15, 30, 60, 90, 120, 240, and 360 minutes, and at 12, 24, 36, and 41 hours after each infusion.) Plasma concentrations of amphetamine and methamphetamine were assessed via liquid chromatographic-tandem mass spectrometry to determine if ibudilast alters the pharmacokinetics of intravenous methamphetamine. The method below was modified from a previously published method for seligiline, norseligiline, methamphetamine and amphetamine.19 Plasma samples were also drawn for pharmacokinetic analysis of ibudilast and its primary metabolite (6,7-dihydrodiol-ibudilast); however, there were errors in the handling and processing of some specimens, and we did not have sufficient samples to re-run the analyses.
Sample Preparation
Calibrators were prepared from serial dilutions of a stock solution of reference materials (Cerriliant, Round Rock, TX) in methanol. Blank human plasma (University of Utah blood bank) was fortified to achieve concentrations of 2/1, 4/2, 8/4, 12/6, 16/8, 20/10, 40/20, and 100/50 ng/mL methamphetamine/amphetamine. Calibration curves were run in duplicate with one set at the beginning and one set at the end of the run. Solutions for preparation of quality control samples (QCs) were prepared similarly to those for calibrators, but from separate aliquots of the reference materials. Pools of QCs were prepared at 80/40, 14/7 and 6/3 ng/mL methamphetamine/amphetamine, and 0.5 mL aliquots stored at −20°C until the day of use at N ≥ 2 per concentration. Aliquots (0.5 mL) of study samples, calibrators and QCs were fortified with 0.025 mL of internal standard (0.1 µg/mL methamphetamine-d8/amphetamine-d5), made basic by addition of 0.1 mL ammonium hydroxide, and extracted into 4 mL of 4:1 n-butyl chloride:acetonitrile. The organic phase was transferred to a clean tube acidified with 0.1 mL of 0.1% HCl in methanol and dried at 30°C under 10 psi. air in a turbovap. The extracts were reconstituted with 50 µL of 0.1% formic acid in water, and transferred to autosampler vials.
LC-ESI-MS/MS
Chromatography utilized a Thermo Finnigan (San Jose, CA) Surveyor LC pump equipped with an inline solvent degasser, a thermo-statted autosampler, and a 100×3.0 mm, 3 µm MetaSil Basic column (MetaChem Technologies Inc., CA) column. The mass spectrometer was a Thermo Finnigan (San Jose, CA) TSQ Quantum equipped with an Xcalibur (v 2.0) operating software with ThermoFinnigan LCquan software (v 2.0) for the quantitative calculations. The LC was interfaced to the MS by means of an ESI source. The injection volume was 10 µL. Isocratic separation was performed with 85% 0.1% formic acid in water and 15% acetonitrile, at a flow rate of 0.2 mL/min. The instrument was operated under selective reaction monitoring mode. The capillary temperature was 270°C, and ESI spray voltage 3.0 kV. High purity nitrogen was used for both sheath and auxiliary gas. High purity argon was used for collision gas. The m/z 150.2 (MH+) to 91.2, 158.2 to 93.2, 136.2 to 91.2 and 141.2 to 93.2 transitions were used to analyze methamphetamine, methamphetamine-d8, amphetamine and amphetamine-d5, respectively. The concentrations of methamphetamine and amphetamine were determined by the peak area ratio of the analyte and its internal standard with comparison to the calibration curve. All curves were quadratic with 1/X2 weighting.
Accuracy and Precision
Over the course of the analyses of study samples QCs were run with an N = 34 for methamphetamine and N=31 for amphetamine. For methamphetamine, the respective accuracies (% target) for the low, medium and high QCs were 100.2, 97.1 and 100.9%, and the respective precisions (%CV) were 3.8, 4.4 and 5.5%. For amphetamine, the respective accuracies for the low, medium and high QCs were 100.3, 98.6 and 102.8%, and the respective precisions (%CV) were 5.3, 3.8 and 8.6%.
Data analysis
The primary outcome measures were cardiovascular responses following methamphetamine infusion and safety indicators, including adverse events. Descriptive statistics for demographic and adverse events were calculated for all completed participants. For each cardiovascular effect, 132 functional data curves (-15 to 360 min) out of 11 samples (12 curves each) were observed in this experimental study. The 12 curves vary in terms of combinations of variables: treatment (categorical: placebo, ibudilast 20 mg BID, ibudilast 50 mg BID), methamphetamine dosage (categorical: saline, 15 mg, 30 mg), and study day (continuous). Functional features were extracted for each curve. Each feature represents the first peak in the curve within 30 minutes minus its own baseline value at −15 minute. A linear regression model with Gaussian variance was performed with responses as 132 features extracted, predictors as treatment, methamphetamine dosage, interaction between treatment and methamphetamine dosage, and adjusted with covariates (study day, sequence of treatment, gender, age, and methamphetamine use reported in previous 30 days.)
For pharmacokinetic analysis, the method was similar to cardiovascular effect. We analyzed both plasma methamphetamine and amphetamine. Peak concentration (Cmax) was the observed maximum value during the collection period of 0 (pre-dose) to 18 hours. The time to peak concentration (Tmax) was the time at which Cmax was observed. The area under the curve represents the total drug exposure over time, either to the last sample time (AUC) or the estimated total drug exposure (AUC∞). Pharmacokinetic parameters (AUC, Tmax, Cmax, and elimination rates) were calculated using the times of sample collection reported by the Investigator. AUC, AUC∞, T½, and λz, were all calculated from R package “PK” version 1.3–2. Each subgroup was analyzed using 11 samples. The terminal exponential rate constant (λz) was determined by linear regression analysis of data points occurring during the terminal (log) linear phase of the plasma concentration-time profile. The PK package does not provide this value, but it could be calculated from the noncompartmental terminal exponential half-life (T½). AUC was calculated using the linear trapezoidal rule on the arithmetic means at the different time points. Extrapolation was used to determine AUC∞ by assuming an exponential decay on the last tail time points. The features were used as responses for Gaussian linear model with the same predictors and covariates as in the cardiovascular effect models.
Results
Demographics
There were a total of 11 participants, 7 who identified as white (63.6%) and 1 each identifying themselves as Hispanic, African-American, Native Hawaiian/Other Pacific Islander, and American Indian. The mean age of the subjects was 42.4 years (standard deviation of 6.7 years) and the mean days of self-reported methamphetamine use in the 30 days before the start of the study was 18.8 days (standard deviation of 7.7 days). The majority (81.8%) of participants were male.
Adverse events
The adverse events were mild to moderate in severity, were not more common during ibudilast treatment than placebo, and are typical adverse events observed during methamphetamine studies (Table 2). There were no serious adverse events or deaths during the trial. Insomnia (reported by 45% of the placebo group, 73% of the ibudilast 20 mg BID group, and 82% of the ibudilast 50 mg BID group), nicotine craving (36%, 45%, and 45% respectively), gastrointestinal upset (35%, 27%, and 35% respectively), and headache (45%, 55%, and 27% respectively) were the most common reported side effects during the study. The proportions of participants reporting adverse events were generally similar between the two doses of ibudilast, with no or only a slight increase in events at the higher dose. However, more subjects reported headache at the lower dose compared to the higher dose (55% vs. 27%).
Table 2.
Proportion of participants (N=11) reporting adverse events during treatment with placebo, ibudilast 20 mg twice daily (BID), and ibudilast 50 mg BID
| Adverse event | Placebo | Ibudilast 20 mg BID |
Ibudilast 50 mg BID |
p value* |
|---|---|---|---|---|
| Insomnia | 45% (5) | 73% (8) | 82% (9) | 0.236 |
| Nicotine craving | 36% (4) | 45% (5) | 45% (5) | 0.368 |
| Gastrointestinal upset | 35% (4) | 27% (3) | 36% (4) | 0.819 |
| Headache | 45% (5) | 55% (6) | 27% (3) | 0.247 |
| Musculoskeletal pain | 0% (0) | 18% (2) | 18% (2) | 0.264 |
| Pain at IV site | 27% (3) | 18% (2) | 18% (2) | 0.779 |
| Rash | 9% (1) | 9% (1) | 18% (2) | 0.717 |
| Vivid dreams | 18% (2) | 18% (2) | 18% (2) | 1.000 |
| Constipation | 0% (0) | 0% (0) | 9% (1) | 0.368 |
| Dizziness | 0% (0) | 0% (0) | 9% (1) | 0.368 |
| Dysuria | 9% (1) | 0% (0) | 9% (1) | 0.607 |
| Ectopic heart beats | 0% (0) | 0% (0) | 9% (1) | 0.368 |
| Fever/chills/hot flashes | 0% (0) | 18% (2) | 9% (1) | 0.223 |
| Pruritis | 0% (0) | 18% (2) | 9% (1) | 0.223 |
| Sore throat | 0% (0) | 9% (1) | 9% (1) | 0.368 |
| Tinnitus | 0% (0) | 0% (0) | 9% (1) | 0.368 |
| Backache | 0% (0) | 9% (1) | 0% (0) | 0.368 |
| Chest congestion | 9% (1) | 0% (0) | 0% (0) | 0.368 |
| Sedation | 0% (0) | 9% (1) | 0% (0) | 0.368 |
Cochrane’s Q test
One participant had mild anemia, and another had a mild elevation of alanine transaminase on study termination labs. No other participants had lab abnormalities, and the abnormal values were determined (based on previous history of participants) not to be due to the medication. Another participant had an episode of ectopic heart beats detected on routine cardiac monitoring without subjective symptoms or change in vital signs. The ectopic beats were attributed to the methamphetamine infusion (although a medication effect could not be ruled out), but this was not serious and the participant completed the remainder of study activities without problems.
Cardiovascular results
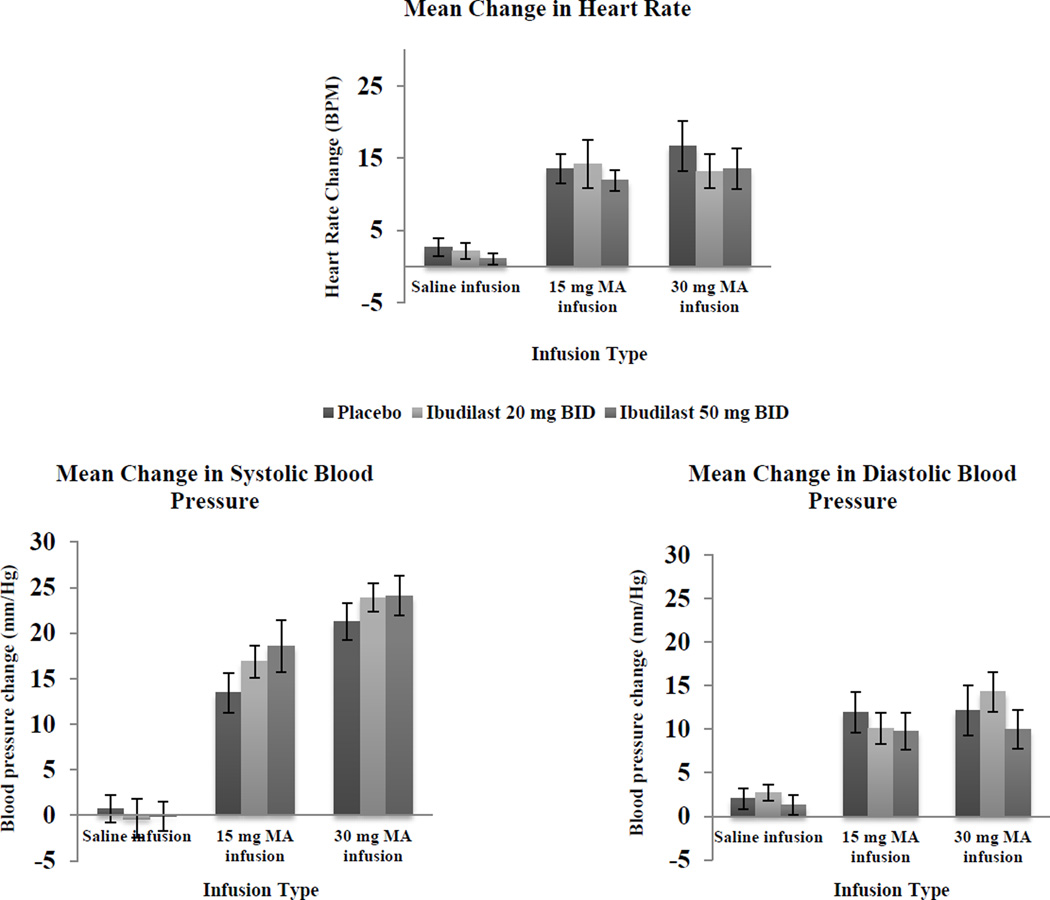
Mean changes in heart rate and blood pressure following saline or methamphetamine infusion with both doses of ibudilast and placebo are shown in Figure 1. Using a linear regression model controlling for age, gender, study day, and baseline methamphetamine use, methamphetamine infusion was associated with increased heart rate, systolic blood pressure, and diastolic blood pressure, with a higher methamphetamine dose (30 mg vs. 15 mg) associated with greater increases in all 3 cardiovascular measures (p < 0.001). There was no statistically significant main effect of ibudilast at either dose on mean change in heart rate (p = 0.76 for ibudilast 20 mg BID, p = 0.42 for ibudilast 50 mg BID), systolic blood pressure (p = 0.68 for ibudilast 20 mg BID, p = 0.76 for ibudilast 50 mg BID), or diastolic blood pressure (p = 0.81 for ibudilast 20 mg BID, p = 0.80 for ibudilast 50 mg BID) compared to placebo. Nor were there any significant interactions between ibudilast dose and methamphetamine dose on any of the cardiovascular measures (all p > 0.05).
Figure 1.
Mean peak change in cardiovascular parameters for intravenous saline or methamphetamine (MA; 15 mg and 30 mg) during treatment with ibudilast 20 mg twice daily (BID), 50 mg BID, or placebo BID in MA dependent volunteers (N=11). Error bars represent the standard error of the mean.
Pharmacokinetic analysis
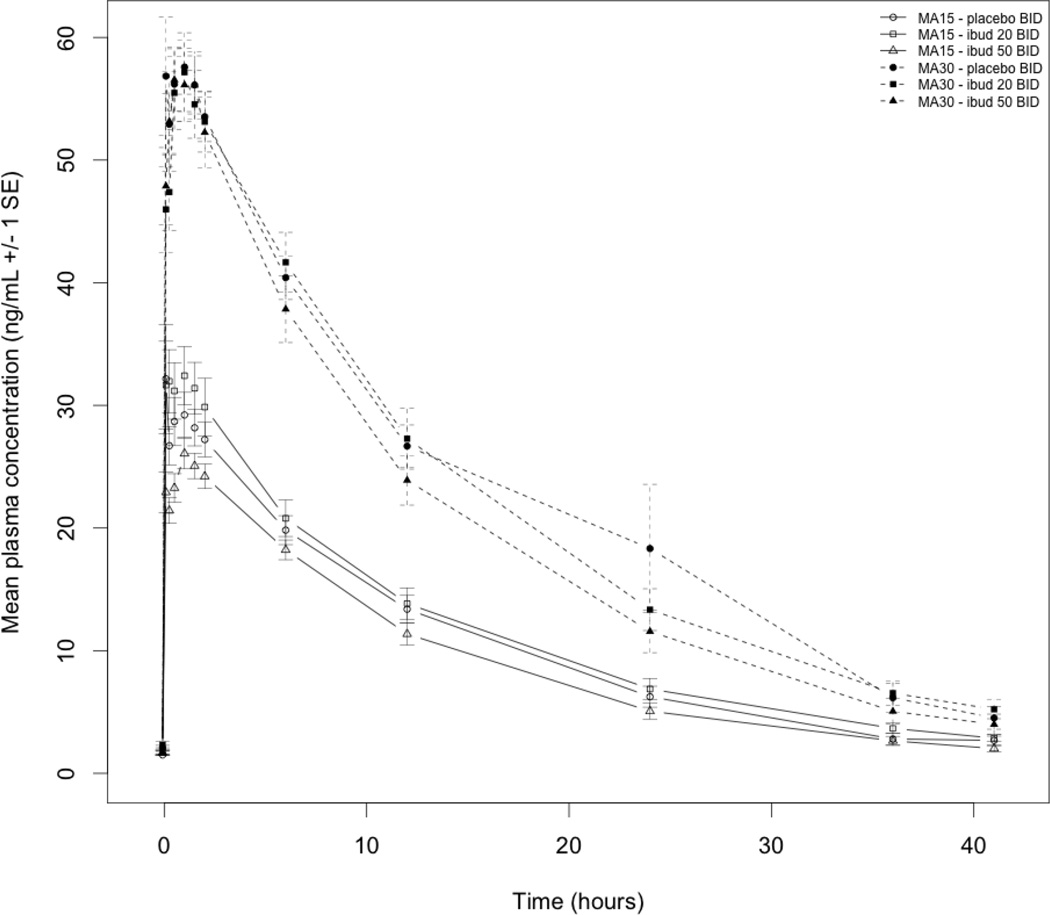
The AUCto last time point for methamphetamine was significantly lower for ibudilast 50 mg BID, but not ibudilast 20 mg BID, compared to placebo following the 15 mg methamphetamine infusion (Table 3). There were no significant differences in Cmax, Tmax, AUC∞ or T1/2 of methamphetamine for either ibudilast dose compared to placebo following the 15 and 30 mg methamphetamine infusions. Figure 2 shows methamphetamine concentration versus time without significant differences between study conditions for each infusion dose.
Table 3.
Methamphetamine (MA) pharmacokinetic parameters following intravenous MA (15 mg and 30 mg) during treatment with ibudilast 20 mg twice daily (BID), 50 mg BID, or placebo BID in MA dependent volunteers (N=11).
| Methamphetamine Pharmacokinetic Parameter |
Methamphetamine dose (mg) |
Group Mean (Standard Error) | ||
|---|---|---|---|---|
| Placebo | Ibudilast 20 mg BID |
Ibudilast 50 mg BID |
||
| Cmax (ng/ml) | 15 | 34.6 (4.1) | 36.6 (2.8) | 27.8 (1.4) |
| 30 | 64.9 (3.3) | 61.7 (2.7) | 61.9 (3.3) | |
| Tmax (hr) | 15 | 0.69 (0.21) | 0.93 (0.20) | 1.33 (0.18) |
| 30 | 0.64 (0.17) | 0.92 (0.16) | 0.95 (0.18) | |
| AUCto last time point | 15 | 435 (33) | 472 (39) | 377 (23)* |
| 30 | 943 (69) | 895 (65) | 808 (64) | |
| AUC∞ (ng hr/ml) | 15 | 797 (286) | 539 (43) | 417 (26) |
| 30 | 1018 (82) | 994 (76) | 879 (79) | |
| T½ (hr) | 15 | 13.3 (2.7) | 11.4 (0.5) | 11.2 (0.6) |
| 30 | 10.6 (0.9) | 11.5 (0.7) | 10.1 (1.0) | |
p < 0.01 for ibudilast 50 mg BID versus placebo
Figure 2.
Methamphetamine (MA) concentration versus time following intravenous MA (15 mg and 30 mg) during treatment with ibudilast (ibud) 20 mg twice daily (BID), 50 mg BID, or placebo BID in MA dependent volunteers (N=11)
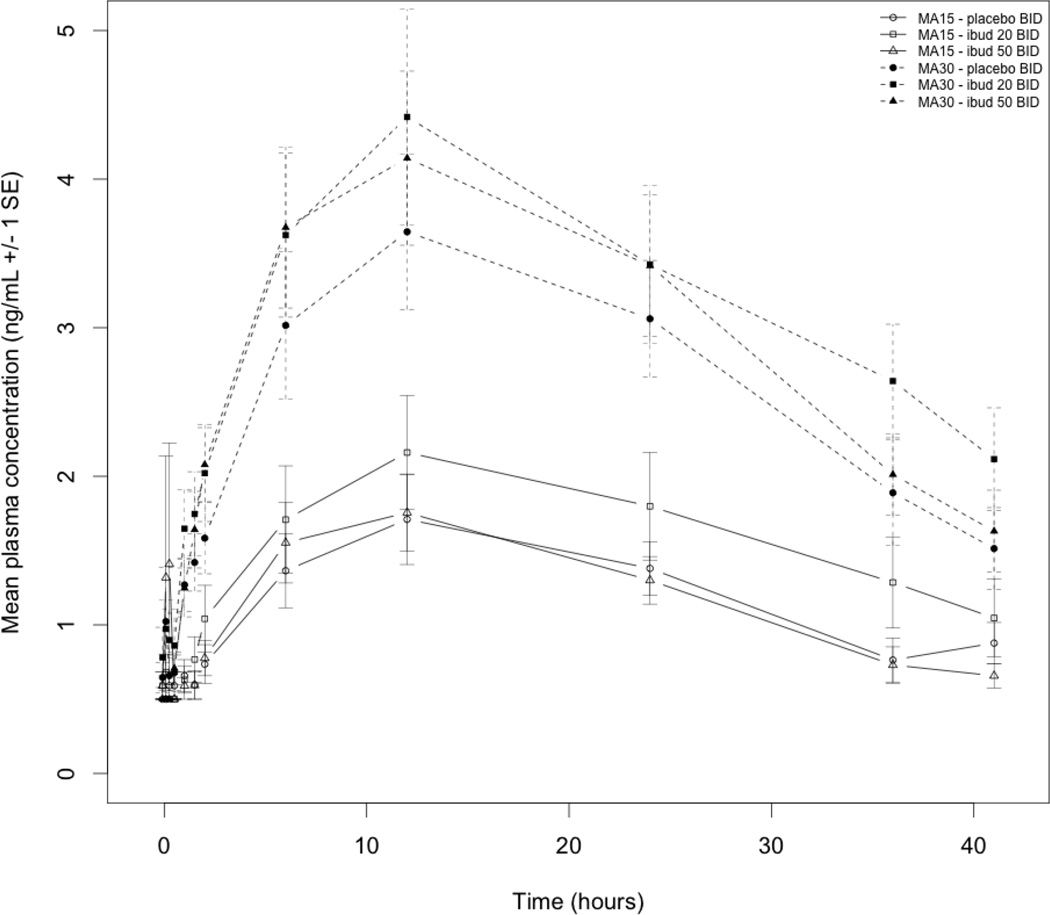
As a metabolite of methamphetamine, amphetamine pharmacokinetic analysis was also performed and there were no significant differences for Cmax , Tmax, or AUC∞ (Table 4). Figure 3 shows amphetamine concentration versus time without significant differences between study conditions for each infusion dose.
Table 4.
Amphetamine pharmacokinetic parameters following intravenous methamphetamine (MA; 15 mg and 30 mg) during treatment with ibudilast 20 mg twice daily (BID), 50 mg BID, or placebo BID in MA dependent volunteers (N=11).
| Amphetamine Pharmacokinetic Parameter |
Methamphetamine dose (mg) |
Group Mean (Standard Error) | ||
|---|---|---|---|---|
| Placebo | Ibudilast 20 mg BID |
Ibudilast 50 mg BID |
||
| Cmax (ng/ml) | 15 | 1.80 (0.28) | 2.26 (0.39) | 2.59 (0.73) |
| 30 | 3.93 (0.51) | 4.42 (0.72) | 4.14 (0.59) | |
| Tmax (hr) | 15 | 9.83 (1.45) | 7.80 (1.57) | 8.20 (1.66) |
| 30 | 10.4 (1.2) | 12.0 (0.0) | 12.0 (0.0) | |
| AUC∞ (ng hr/ml) | 15 | 89.8 (25.6) | 116 (31) | 65.8 (10.7) |
| 30 | 167 (27) | 221 (32) | 203 (42) | |
Figure 3.
Amphetamine concentration versus time following intravenous methamphetamine (MA; 15 mg and 30 mg) during treatment with ibudilast (ibud) 20 mg twice daily (BID), 50 mg BID, or placebo BID in MA dependent volunteers (N=11)
Discussion
Results of this phase 1 safety-interaction study of ibudilast and methamphetamine suggest that ibudilast was safe and well tolerated during administration of intravenous methamphetamine. Adverse events during co-administration of ibudilast at steady state and methamphetamine were mild to moderate and typical of those observed with methamphetamine administration. There was no significant effect of ibudilast on heart rate or blood pressure and ibudilast did not augment methamphetamine-induced increases in heart rate or blood pressure. There were minimal clinically significant changes in methamphetamine pharmacokinetic parameters with ibudilast and none for amphetamine. Demonstrating a lack of clinically significant interactions between a medication and methamphetamine is critical prior to advancing a candidate medication to additional testing in an outpatient phase 2 trial. Results of this study suggest that ibudilast at doses up to 50 mg BID exhibits sufficient safety and tolerability with methamphetamine to support advancement to a phase 2 outpatient efficacy trial (NCT01860807).
Ibudilast is a vasodilator in preclinical studies most likely due to ibudilast’s activity as a non-selective PDE inhibitor.20 PDE3 inhibitors are cardiac inotropes and have been associated with cardiac arrhythmias.21 Ibudilast inhibits PDE3A, PDE4, PDE10, and PDE11 in in vitro studies20 but at doses used in humans primarily inhibits PDE4 and PDE10. There were no significant changes in heart rate or blood pressure or any serious cardiac arrhythmias with ibudilast overall or following methamphetamine challenge suggesting that despite cardiovascular effects of other PDE inhibitors, ibudilast does not augment the cardiovascular risks associated with methamphetamine use possibly due to relative selectivity for PDE4 and PDE10 in vivo.
Methamphetamine is metabolized by CYP2D6, but selectivity for this CYP gene product has not yet been demonstrated.16 Ibudilast is metabolized primarily by CYP2B6 and CYP2E1, inhibits CYP1A2, and has no known effect on CYP2D6.15 There have been no important drug-drug interactions reported with extensive clinical use of ibudilast in Asia. Minimal clinically significant pharmacokinetic interactions were observed between ibudilast and methamphetamine in this trial likely due to the lack of demonstrated effects of ibudilast on CYP2D6. The decrease in AUCto last time point with high dose ibudilast for the 15 mg methamphetamine infusion did not appear to have clinical significance, but raises some questions about factors controlling methamphetamine clearance.
Limitations of the study include a small sample size with participants free of known cardiovascular disease or arrhythmias or those dependent on other substances (alcohol, opioids) and thus may not adequately account for cardiovascular risks in the general methamphetamine using population. The significant psychiatric22 and medical23 comborbidities associated with methamphetamine dependence explain the higher exclusion rate. The exclusion criteria were similar to other safety studies with methamphetamine dependence24 as the study was designed to obtain preliminary data on the safety of ibudilast with methamphetamine (which has not been done previously.) Given its lack of adverse events or significant pharmacokinetic changes in this population, the data would support further assessment of ibudilast in a larger outpatient population. A second limitation is that ibudilast was administered over a relatively short period and thus the design could only detect common adverse events once initial steady state is achieved. The present results may not be representative of findings in clinical populations taking this medication for longer periods of time (which would be the expected regimen). Lastly, doses of methamphetamine administered in this study, 15 mg and 30 mg, are low compared to doses typically used illicitly (50 mg and more)25 but were selected to provide a model of potential cardiovascular effects with illicit use while balancing the risks to study participants.
In summary, in this phase 1 clinical trial ibudilast appeared safe from a cardiovascular perspective and was well tolerated in methamphetamine dependent volunteers following intravenous methamphetamine administration. There were minimal clinically significant effects of ibudilast on methamphetamine pharmacokinetics. Further studies testing the efficacy of ibudilast for methamphetamine treatment are warranted based on the preliminary results of this study.
Supplementary Material
Acknowledgments
MediciNova, Incorporated provided study medication and matching placebo. We would like to thank the excellent nursing staff at the Harbor-UCLA General Clinical Research Center for their care of our participants. Thanks to Marisa Briones, PhD, for her assistance in data analysis strategy and manuscript preparation.
Source of Funding:
This work was supported by a grant from the National Institute on Drug Abuse (R01 DA029804 to S.S.) Dr. DeYoung has received funding from Alkermes for travel and meeting expenses. Dr. Heinzerling has received clinical research supplies from Pfizer, Inc., Medicinova, Inc., and Alkermes and is an advisor to Gilead. Dr. Shoptaw has received clinical research supplies from Pfizer, Inc., and Medicinova, Inc.
Footnotes
Conflicts of Interest:
All other authors declare no conflicts of interest.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. 14-4863. [Google Scholar]
- 2.United Nations Office on Drugs and Crime. World Drug Report 2015. Vienna, Austria: Division for Policy Analysis and Public Affairs; 2015. (United Nations publication, Sales No. E.15.XI.6) [Google Scholar]
- 3.Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: an update. Drug Alcohol Rev. 2013;32:449–460. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang GJ, Smith L, Volkow ND, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? The Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 9.Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol pathol. 2011;39:115–123. doi: 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayaraghavan S. Glial-neuronal interactions--implications for plasticity and drug addiction. AAPS J. 2009;11:123–132. doi: 10.1208/s12248-009-9085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narita M, Suzuki M, Kuzumaki N, et al. Implication of activated astrocytes in the development of drug dependence: differences between methamphetamine and morphine. Ann N Y Acad Sci. 2008;1141:96–104. doi: 10.1196/annals.1441.032. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno T, Kurotani T, Komatsu Y, et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Beardsley PM, Shelton KL, Hendrick E, et al. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson MR, Lewis SS, Coats BD, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanftner LM, Gibbons JA, Gross MI, et al. Cross-species comparisons of the pharmacokinetics of ibudilast. Xenobiotica. 2009;39:964–977. doi: 10.3109/00498250903254340. [DOI] [PubMed] [Google Scholar]
- 16.Lin LY, Di Stefano EW, Schmitz DA, et al. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos. 1997;25:1059–1064. [PubMed] [Google Scholar]
- 17.Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–2904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- 18.Rolan P, Gibbons JA, He L, et al. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol. 2008;66:792–801. doi: 10.1111/j.1365-2125.2008.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slawson MH, Taccogno JL, Foltz RL, et al. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) in human plasma by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Anal Toxicol. 2002;26:430–437. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- 20.Gibson LC, Hastings SF, McPhee I, et al. The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur J Pharmacol. 2006;538:39–42. doi: 10.1016/j.ejphar.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Kanlop N, Chattipakorn S, Chattipakorn N. Effects of cilostazol in the heart. J Cardiovasc Med (Hagerstown) 2011;12:88–95. doi: 10.2459/JCM.0b013e3283439746. [DOI] [PubMed] [Google Scholar]
- 22.Zweben JE, Cohen JB, Christian D, et al. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]
- 23.Albertson TE, Derlet RW, Van Hoozen BE. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214–219. [PMC free article] [PubMed] [Google Scholar]
- 24.Newton TF, Roache JD, De La Garza R, 2nd, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- 25.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.