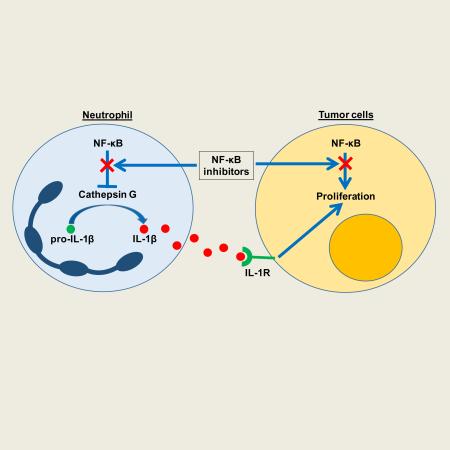
SUMMARY
While epithelial NF-κB signaling is important for lung carcinogenesis, NF-κB inhibitors are ineffective for cancer treatment. To explain this paradox, we studied mice with genetic deletion of IKKβ in myeloid cells and found enhanced tumorigenesis in KrasG12D and urethane models of lung cancer. Myeloid-specific inhibition of NF-κB augmented pro-IL-1β processing by cathepsin G in neutrophils, leading to increased IL-1β and enhanced epithelial cell proliferation. Combined treatment with bortezomib, a proteasome inhibitor that blocks NF-κB activation, and IL-1 receptor antagonist reduced tumor formation and growth in vivo. In lung cancer patients, plasma IL-1β levels correlated with poor prognosis and IL-1β increased following bortezomib treatment. Together, our studies elucidate an important role for neutrophils and IL-1β in lung carcinogenesis and resistance to NF-κB inhibitors.
Graphical Abstract
INTRODUCTION
The NF-κB pathway has become increasingly appreciated for its involvement in carcinogenesis as studies continue to uncover its roles in primary tumor growth, angiogenesis, and metastasis (Lin et al., 2010). In the lungs, NF-κB is activated in pre-malignant airway epithelial lesions, atypical adenomatous hyperplasia (AAH) lesions in the distal lungs, and invasive non-small cell lung cancer (NSCLC) (Tichelaar et al., 2005). Based on this information, inhibition of the NF-κB pathway has been tested as a therapy for lung cancer (Chen et al., 2011). The proteasome inhibitor bortezomib, which blocks degradation of the inhibitor of NF-κB (IκB) as well as other proteins that are regulated by the proteasome, is the best-studied agent for inhibiting NF-κB in humans; however, bortezomib has not been efficacious for NSCLC treatment (Besse et al., 2012; Fanucchi et al., 2006). The mechanism of resistance to bortezomib and other NF-κB inhibitor therapies is not known. Despite the disappointing results to date, numerous clinical trials have been attempted or are currently under way to test various combinations of bortezomib and other agents for cancer treatment. Our goals for these studies were to determine why NF-κB inhibitors are ineffective for NSCLC and to identify new approaches to overcome resistance to NF-κB inhibitors.
Our group and others have shown that NF-κB signaling in lung epithelial cells is crucial for lung tumor formation. In mice, expression of a constitutively active form of IKKβ (which activates canonical NF-κB) in airway epithelium results in a >3-fold increase in lung tumor formation after treatment with chemical carcinogens (Zaynagetdinov et al., 2012). In addition, studies using a variety of methods to block NF-κB signaling in lung epithelium have revealed a requirement for NF-κB signaling in lung cancer models driven by oncogenic forms of Kras and EGFR (Bassères et al., 2010; Meylan et al., 2009; Saxon et al., 2016; Stathopoulos et al., 2007; Xia et al., 2012). While some studies have shown short-term lung tumor regression following NF-κB inhibition (Bassères et al., 2014; Xue et al., 2011), pharmacologic NF-κB inhibition has not shown definitive long-term benefit in lung cancer models. Highlighting the challenges of NF-κB inhibition, Xue et al. showed that murine lung tumors developed resistance to therapy within a few weeks after treatment with bortezomib or an inhibitor of IκBα phosphorylation (BAY 11-7082) (Xue et al., 2011). Additionally, we showed that prolonged treatment with bortezomib enhanced, not hindered, lung tumor formation in urethane-treated mice (Karabela et al., 2012). While it is possible that tumor cells could develop intrinsic resistance to NF-κB inhibitors via the acquisition of additional mutations (Xue et al., 2011), this would likely translate into sporadic appearance of secondary resistance, as opposed to the uniform primary resistance to bortezomib observed in solid tumors (Besse et al., 2012; Fanucchi et al., 2006). Based on these observations, we postulated that systemic NF-κB inhibition evokes a pro-tumorigenic response from a nonepithelial cell population that overrides the anti-tumor effects resulting from NF-κB inhibition in epithelial cells.
Myeloid cells play important roles in both innate immunity and tumorigenesis (Giannou et al., 2015; Stathopoulos et al., 2010; Zaynagetdinov et al., 2011). It is now well-accepted that macrophages and neutrophils can act as pro- or anti-tumorigenic cells during tumorigenesis depending on signals that they receive from the tumor and the tumor stroma (Fridlender and Albelda, 2012; Rajnavolgyi et al., 2013). The role of NF-κB signaling in these cells during tumorigenesis is controversial and seems to be organ- and/or context-dependent. Some cancer models show that blocking NF-κB signaling in myeloid cells elicits a protective, anti-tumorigenic response (Greten et al., 2004; Takahashi et al., 2010). Others show that myeloid-specific NF-κB inhibition is detrimental and pro-tumorigenic (Enzler et al., 2011; Yang et al., 2014). In tumor-associated macrophages, blocking NF-κB can result in an anti-tumorigenic phenotype (Fong et al., 2008; Hagemann et al., 2008). On the other hand, a recent study showed that blocking NF-κB signaling in macrophages impedes their ability to mount anti-tumorigenic responses against melanoma cells (Yang et al., 2014).
For these studies, we postulated that inhibition of NF-κB signaling in myeloid cells could elicit pro-tumorigenic responses that limit the effectiveness of global (systemic) NF-κB inhibition. To test this hypothesis, we utilized a mouse model characterized by myeloid cell-specific deletion of IKKβ (IKKβΔmye mice; LysM-Cre/IKKβflox/flox) (Li et al., 2003). In carcinogen-induced and genetic lung cancer models, we found that blocking NF-κB signaling in myeloid cells enhances lung tumorigenesis through neutrophil-dependent production of IL-1β and that combined NF-κB and IL-1β targeted treatments reduces tumor formation and growth.
RESULTS
Neutrophils enhance lung tumorigenesis when NF-κB activity is inhibited in myeloid cells
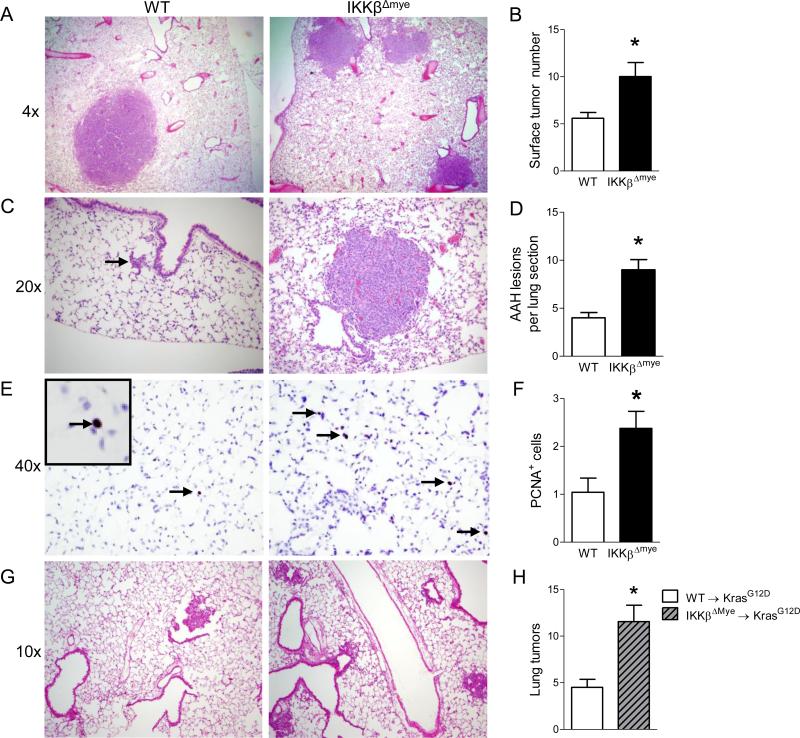
To determine the role of NF-κB signaling in myeloid cells during lung tumorigenesis, IKKβΔmye mice were fully back-crossed (>9 generations) to the tumor-susceptible FVB background. Deletion of IKKβ in myeloid cells in the bone marrow compartment was confirmed by western blot (Figure S1). Subsequently, IKKβΔmye mice and WT littermate controls were given a single intraperitoneal (IP) injection of the carcinogen urethane (1 g/kg). Urethane causes lung tumors primarily through induction of Kras mutations (You et al., 1989), but it can also induce a number of other driver mutations found in human cancers (Westcott et al., 2014). At week 16 after injection of urethane, we found that IKKβΔmye mice developed approximately twice as many lung tumors as WT mice (Figure 1A-B), indicating that inhibiting NF-κB signaling in myeloid cells promotes lung tumorigenesis. To determine if differences were detectable at an earlier stage of carcinogenesis, we harvested lungs at 6 weeks after urethane injection and identified a greater number of AAH lesions in lungs of IKKβΔmye mice compared to WT mice (Figure 1D). Unexpectedly, at 6 weeks post-urethane, we observed some fully formed tumors in the lungs of IKKβΔmye mice (Figure 1C). On lung sections, 58% (7/12) of IKKβΔmye lungs contained adenomas at 6 weeks post-urethane compared with 7.1% (1/14) of WT lungs (p<0.01 by Fisher's exact test). To investigate the mechanism of enhanced tumorigenesis in IKKβΔmye mice, we performed immunohistochemistry for markers of proliferation (PCNA) and apoptosis (cleaved caspase-3). Although we did not observe any differences in cleaved caspase-3 staining between IKKβΔmye and WT lungs, there were significantly more PCNA+ lung epithelial cells in IKKβΔmye mice compared to WT mice (Figure 1E-F and data not shown). To corroborate our findings from the urethane model, we utilized the LSL-KrasG12D (KrasG12D) lung tumor model (Tuveson et al., 2004). We performed bone marrow transplantation in KrasG12D mice using either WT (WT→ KrasG12D) or IKKβΔmye (IKKβΔmye→ KrasG12D) donors. Lung tumors were induced in these bone marrow chimeras by intratracheal (IT) instillation of adenoviral vectors expressing Cre recombinase (adeno-Cre). Similar to urethane-injected IKKβΔmye mice, IKKβΔmye→ KrasG12D mice developed twice as many lung tumors as WT→ KrasG12D mice at 8 weeks after adeno-Cre treatment (Figure 1G-H). Together, these studies show that blocking NF-κB signaling in myeloid cells promotes lung tumorigenesis is both chemical and genetic models of lung cancer.
Figure 1.
Inhibition of NF-κB signaling in myeloid cells increases lung tumorigenesis and epithelial cell proliferation. A) Representative photomicrographs and B) Number of lung tumors in WT and IKKβΔmye mice at 16 weeks after a single injection of urethane (n=16-22 mice per group). C) Representative photomicrographs showing an AAH lesion (red arrow) in the lung of WT mice or tumor in IKKβΔmye mice, and D) Number of AAH lesions counted per H&E-stained lung section (3 sections per mouse) from WT and IKKβΔmye mice harvested at week 6 after injection of urethane (n=9-10 mice per group). E) Immunostaining for PCNA+ cells and (F) Number of PCNA+ cells per lung section (averaged from 25 sequential fields taken at 40× magnification) from WT and IKKβΔmye mice harvested at week 6 after urethane injection (n=3-4 per group). G-H) Lethally-irradiated LSL-KrasG12D mice received bone marrow from WT (WT→KrasG12D) or IKKβΔmye (IKKβΔmye→KrasG12D) mice. Lung tumors were induced by instillation of IT adeno-Cre (1.5×107 PFU). G) Representative photomicrographs and H) Number of lung tumors in WT→KrasG12D and IKKβΔmye→KrasG12D mice at 8 weeks after adeno-Cre (n=4-9 mice per group) *p < 0.05. See also Figure S1.
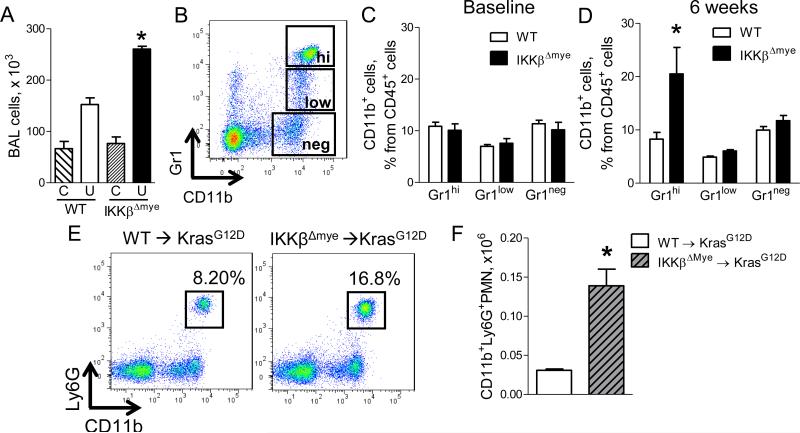
Since NF-κB is an important regulator of inflammation, we next investigated the role of myeloid NF-κB signaling on lung inflammation during tumorigenesis. No differences in inflammatory cells in bronchoalveolar lavage (BAL) fluid were observed between untreated WT and IKKβΔmye mice; however, at 6 weeks post-urethane injection, we observed increased inflammatory cells in BAL from IKKβΔmye mice, indicating that heightened lung inflammation in IKKβΔmye mice was an effect of carcinogen treatment (Figure 2A). To evaluate specific myeloid subpopulations, we performed flow cytometry on lung cells from IKKβΔmye and WT mice (Figure 2B). Consistent with findings in BAL, no differences in neutrophil, monocyte, or macrophage cell populations were observed between untreated WT and IKKβΔmye mice (Figure 2C). In contrast, we identified a selective increase in neutrophils in the lungs of IKKβΔmye mice at 6 weeks post-urethane injection compared to WT mice but no difference in total CD45+ cells (Figure 2D, S2). Additional studies in KrasG12D model bone marrow chimeras showed similar findings with increased lung neutrophils in IKKβΔmye→ KrasG12D mice at 8 weeks after IT adeno-Cre instillation compared to WT→ KrasG12D mice (Figure 2E-F).
Figure 2.
Neutrophils are increased in the lungs of mice lacking myeloid NF-κB signaling. A) Number of total BAL cells in WT and IKKβΔmye mice at baseline (C) and at 6 weeks after urethane injection (U) (n=7-9 mice per group; *p < 0.05 compared with urethane-treated WT mice). B) Representative FACS plots and (C-D) Percentages of viable CD45+/CD11b+/Gr1hi neutrophils (Gr1hi), CD45+/CD11b+/Gr1low monocytes (Gr1low), and CD45+/CD11b+/Gr1neg macrophages (Gr1neg) in the lungs of WT and IKKβΔmye mice at (C) baseline and (D) 6 weeks after urethane injection (n=4-11 mice per group; *p < 0.05 compared with WT). E) Representative FACS plots and (F) total viable CD45+/CD11b+/Ly6G+ neutrophils in the lungs of WT→KrasG12D and IKKβΔmye→KrasG12D mice 8 weeks after adeno-Cre (n=4 mice per group; *p < 0.05 compared with WT→KrasG12D). Ly6G identifies the granulocytic subgroup of the Gr1 marker. See also Figure S2.
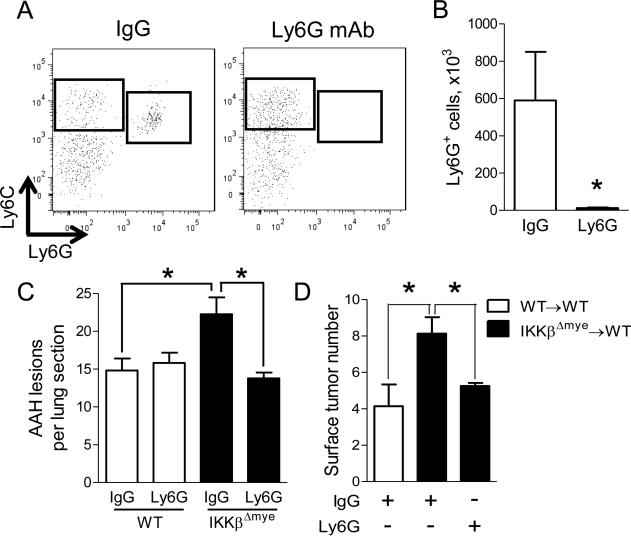
In order to determine if neutrophils were important for lung carcinogenesis, we performed neutrophil depletion using antibodies against Ly6G (Fleming et al., 1993). WT and IKKβΔmye mice were injected with urethane and administered anti-Ly6G antibodies or isotype control IgG antibodies (100 μg) twice weekly for 6 weeks. A marked reduction in lung neutrophils was confirmed by flow cytometry (Figure 3A-B). While neutrophil depletion significantly reduced AAH lesions in lungs of IKKβΔmye mice, we observed no effect of this treatment in WT mice (Figure 3C). Next, we tested the effect of neutrophil depletion on lung tumor formation. A bone marrow transplantation study was incorporated into this experiment to verify that enhanced tumorigenesis in IKKβΔmye mice following urethane treatment was due to bone marrow-derived leukocytes. Lethally-irradiated WT mice received bone marrow from IKKβΔmye (IKKβΔmye→WT) or WT (WT→WT) donors. Bone marrow chimeras were injected with urethane and administered anti-Ly6G antibodies or isotype control IgG antibodies (100 μg) twice weekly for 6 weeks. At week 16 after urethane injection, we observed increased tumor formation in the lungs of control (IgG-treated) IKKβΔmye→WT mice compared to control (IgG-treated) WT→WT mice (Figure 3D). In addition, neutrophil depletion using anti-Ly6G antibodies significantly reduced tumor formation in IKKβΔmye→WT mice compared to control (IgG-treated) IKKβΔmye→WT mice, identifying neutrophils as key mediators of increased tumor formation in the setting of myeloid NF-κB inhibition.
Figure 3.
Neutrophils promote lung tumorigenesis in the absence of myeloid NF-κB signaling. All mice were treated with isotype control IgG or anti-Ly6G depletion antibodies (100 g by IP injection) for the first 6 weeks following urethane injection. A) Representative FACS plots and (B) total viable CD45+/CD11b+/Ly6C+/Ly6G+ lung neutrophils demonstrating depletion efficiency in IKKβΔmye mice harvested 3 days after the last dose of antibody (n=4 mice per group). C) Number of AAH lesions per lung section from IgG- and anti-Ly6G-treated WT and IKKβΔmye mice at 6 weeks after urethane injection (n=6-9 mice per group). D) Lethally-irradiated WT mice received bone marrow from WT or IKKβΔmye mice. Lung tumors at 16 weeks after urethane injection in bone marrow chimera mice treated with IgG or anti-Ly6G antibodies for the first 6 weeks of tumorigenesis (n=6-8 mice per group). *p < 0.05.
Myeloid-specific NF-κB inhibition results in increased IL-1β production by neutrophils following carcinogen exposure
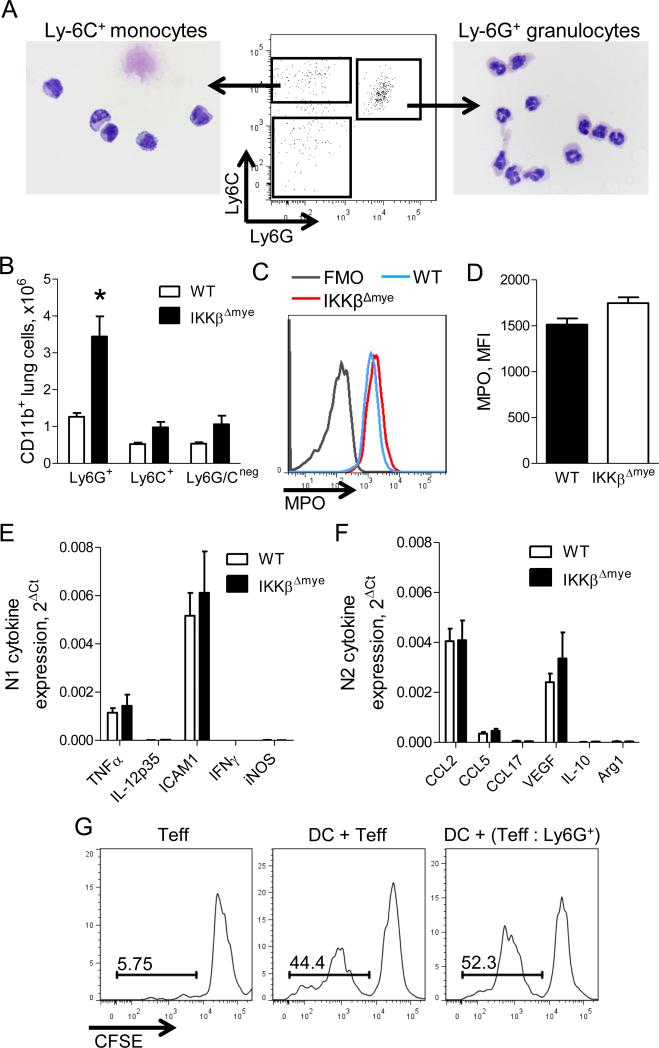
To determine how IKKβ-deficient neutrophils exert their pro-tumorigenic effects during lung carcinogenesis, we characterized neutrophils from IKKβΔmye and WT mice according to morphological appearance, maturity, and function. We sorted neutrophils (CD45+/CD11b+/Ly6C+/Ly6G+ cells) from lungs of urethane-treated IKKβΔmye and WT mice and confirmed that these cells had segmented nuclei, characteristic of mature neutrophils (Figure 4A). As early as 1 week post-urethane, IKKβΔmye mice had approximately 3-fold increase in neutrophils in the lungs compared to WT mice, while lung monocytes (CD45+/CD11b+/Ly6C+/Ly6G− cells), and macrophages (CD45+/CD11b+/Ly6C−/Ly6G− cells), as well as peripheral blood neutrophils, were comparable between groups (Figure 4B and data not shown). To determine if loss of NF-κB signaling affected maturation of neutrophils, we measured expression of myeloperoxidase (MPO), an enzyme produced by mature neutrophils, in Ly6G+ cells from IKKβΔmye and WT mice at 1 week after urethane injection (Figure 4C). Loss of NF-κB signaling in Ly6G+ cells from IKKβΔmye mice did not impair MPO production (Figure 4C-D). We also examined N1/N2 markers in lung neutrophils by real-time PCR but did not observe differences in anti-tumorigenic N1 markers (TNFα, IL-12p35, ICAM1, IFNγ, iNOS) or pro-tumorigenic N2 markers (CCL2, CCL5, CCL17, VEGF, IL-10, Arg1) between neutrophils from urethane-injected WT and IKKβΔmye mice (Figure 4E-F). Since a subset of Ly6G+ cells [granulocytic myeloid derived suppressor cells (MDSCs)] may support tumorigenesis through suppression of anti-tumor responses from T lymphocytes (Gabrilovich and Nagaraj, 2009), we assessed the ability of Ly6G+ cells isolated from lungs of urethane-treated IKKβΔmye mice to suppress effector T (Teff) cell proliferation in an allogeneic mixed lymphocyte reaction assay. As shown in Figure 4G, Ly6G+ cells from IKKβΔmye mice failed to suppress proliferation of Teff cells stimulated by allogeneic dendritic cells, indicating that Ly6G+ cells from IKKβΔmye mice do not act as MDSCs. These studies show that neutrophils from IKKβΔmye mice are mature cells that are not highly polarized towards N1 or N2 and do not exhibit immunosuppressive properties during early lung tumorigenesis.
Figure 4.
Mature neutrophils are increased in the lungs during early tumorigenesis in the absence of myeloid NF-κB signaling. A) FACS sorting strategy and photomicrographs demonstrating cell morphology of lung monocytes (CD45+/CD11b+/Ly6C+/Ly6G−) and neutrophils (CD45+/CD11b+/Ly6C+/Ly6G+) isolated from lungs of WT and IKKβΔmye mice at 1 week after urethane injection. B) Numbers of CD11b+/Ly6G+ neutrophils (Ly6G+), CD11b+/Ly6C+ monocytes (Ly6C+), and CD11b+/Ly6Gneg/Ly6Cneg macrophages (Ly6G/Cneg) in the lungs of WT and IKKβΔmye mice at 1 week after urethane injection (n=3 mice per group, representative of 2 independent experiments; *p<0.05 compared to WT). C) Flow cytometry plot (including fluorescence minus one [FMO] control) and (D) mean fluorescence intensity (MFI) showing expression of MPO in viable CD45+/CD11b+/Ly6G+ cells from lungs of WT and IKKβΔmye mice at 1 week after urethane injection (n=4 mice per group). Expression of (E) N1 and (F) N2 markers in CD45+/CD11b+/Ly6G+ cells isolated from lungs of IKKβΔmye mice at 1 week after urethane injection (n=4-5 mice per group). G) CD45+/CD11b+/Ly6G+ cells isolated from lungs of IKKβΔmye mice at 1 week after urethane injection do not impair the ability of allogeneic dendritic cells (DC) to induce proliferation of CFSE-labeled responder CD4+/CD25− T cells (Teff) (1:1, performed in duplicate).
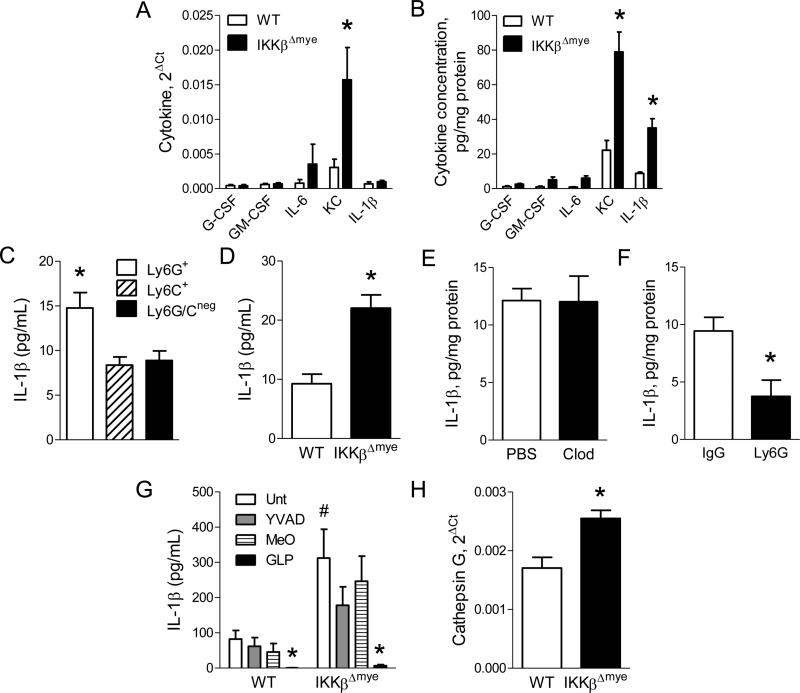
Since we did not identify differences in maturation or function of neutrophils from IKKβΔmye mice, we investigated whether differential production of inflammatory mediators could be responsible for increased tumorigenesis in the context of NF-κB inhibition. We measured mRNA and protein expression of a panel of cytokines (G-CSF, GM-CSF, IFNγ, IL-1β, IL-4, IL-6, IL-10, IL-12p40, KC, MCP-1, and MIP-1α) in the lungs of IKKβΔmye and WT mice at 1 week after urethane injection. Both KC mRNA and protein were increased in lungs of IKKβΔmye mice, while IL-1β protein, but not mRNA, was upregulated (Figure 5A-B). For IL-1β, increased protein without increased mRNA expression suggests increased pro-IL-1β processing. No differences in IL-1β protein levels in the lungs were detected between untreated WT and IKKβΔmye mice (data not shown). To determine the cellular source for increased IL-1β protein in IKKβΔmye mice, we sorted myeloid cells from lungs at 1 week after urethane injection and measured IL-1β in conditioned media. Neutrophils from IKKβΔmye mice secreted nearly twice as much IL-1β as monocytes or macrophages (Figure 5C) and produced more IL-1β per cell than lung neutrophils from urethane-injected WT mice (Figure 5D), identifying IKKβ-deficient neutrophils as the source of increased IL-1β protein levels in the lungs. To verify that neutrophils were the primary source of IL-1β, we performed macrophage and neutrophil depletion studies in urethane-treated IKKβΔmye mice. For macrophage depletion, urethane-treated IKKβΔmye mice were administered liposomal clodronate or vehicle (liposomal PBS) by IT injection (Zaynagetdinov et al., 2011) and harvested at 1 week after urethane. Macrophage depletion did not alter IL-1β protein in the lungs of IKKβΔmye mice (Figure 5E). For neutrophil depletion, urethane-treated IKKβΔmye mice received IP injections of 100 μg of anti-Ly6G or isotype control IgG antibodies (Chen et al., 2012; Fridlender et al., 2009) and lungs were harvested 1 week later. Neutrophil depletion in mice treated with anti-Ly6G antibodies was confirmed by flow cytometry (data not shown). Compared to IKKβΔmye mice treated with control IgG antibodies, anti-Ly6G antibody treatment significantly reduced IL-1β in the lungs (Figure 5F). Taken together, these studies point to IL-1β as a neutrophil-derived mediator that could support enhanced lung tumorigenesis.
Figure 5.
Neutrophils from IKKβΔmye mice produce increased IL-1β following urethane injection. Expression of cytokines by A) mRNA and B) protein in the lungs of WT and IKKβΔmye mice harvested 1 week after urethane (n=10-11 mice per group; *p < 0.05 compared with WT). C) Concentration of IL-1β in the conditioned media following 12-hour culture of lung Ly6G+ neutrophils, Ly6C+ monocytes, or Ly6G/Cneg macrophages isolated from IKKβΔmye mice at 1 week after urethane injection (n=3; *p < 0.05 compared with Ly6C+ and Ly6G/Cneg). D) Concentration of IL-1β in the conditioned media following 12-hour culture of equal numbers of lung Ly6G+ neutrophils from WT and IKKβΔmye mice isolated at 1 week after urethane injection (n=8 mice per group). E) IL-1β protein levels in lung homogenates at 1 week after urethane in the lungs of IKKβΔmye mice treated with liposomal clodronate or PBS on day 5 following urethane injection (n=6 mice per group). F) IL-1β protein levels at 1 week after urethane in the lungs of IKKβΔmye mice treated with anti-Ly6G antibodies (100 μg) or control IgG antibodies by IP injection on days −1, 2, and 5 relative to the day of urethane injection (n=3-5 mice per group; *p < 0.05 compared with IKKβΔmye mice treated with control IgG antibodies). Lung Ly6G+ neutrophils were isolated from WT and IKKβΔmye mice at 1 week after urethane injection. G) IL-1β concentration in the conditioned media after culture with inhibitors (all 100 M) of caspase-1 (Ac-YVAD-CMK), neutrophil elastase and proteinase-3 (MeOSuc-APPV-CMK), or cathepsin G (Z-GLP-CMK) (n=3-8 replicates per group; #p>0.05 compared to WT Unt; *p<0.05 compared to either WT or IKKβΔmye Unt). H) mRNA expression of cathepsin G in lung Ly6G+ neutrophils isolated from WT and IKKβΔmye mice at 1 week after urethane injection.
Serine proteases have been implicated in the regulation of IL-1β processing by neutrophils (Greten et al., 2007); therefore, we performed inhibitor studies to determine the mechanism of dysregulated IL-1β release by lung neutrophils from IKKβΔmye mice. Lung neutrophils were isolated from urethane-treated WT and IKKβΔmye mice and cultured in the presence of inhibitors of caspase-1 (Ac-YVAD-cmk; YVAD), neutrophil elastase and proteinase 3 (MeOSuc-APPV-CMK; MeO), or cathepsin G (Z-GLP-CMK; GLP). While caspase-1 inhibition partially reduced IL-1β release from IKKβΔmye neutrophils, inhibition of the serine protease cathepsin G blocked nearly all IL-1β secretion (Figure 5G). Additionally, gene expression of cathepsin G was upregulated in lung neutrophils from urethane-treated IKKβΔmye mice compared to WT mice, while no differences in expression were observed in caspase-1, neutrophil elastase, or proteinase 3 (Figure 5H and data not shown). These data implicate cathepsin G as the primary regulator of IL-1β processing by lung neutrophils in urethane-treated mice. Increased cathepsin G expression and/or activity in IKKβΔmye neutrophils likely accounts for the increased production of IL-1β by these cells.
Systemic NF-κB inhibition increases IL-1β production in mice and humans with lung cancer
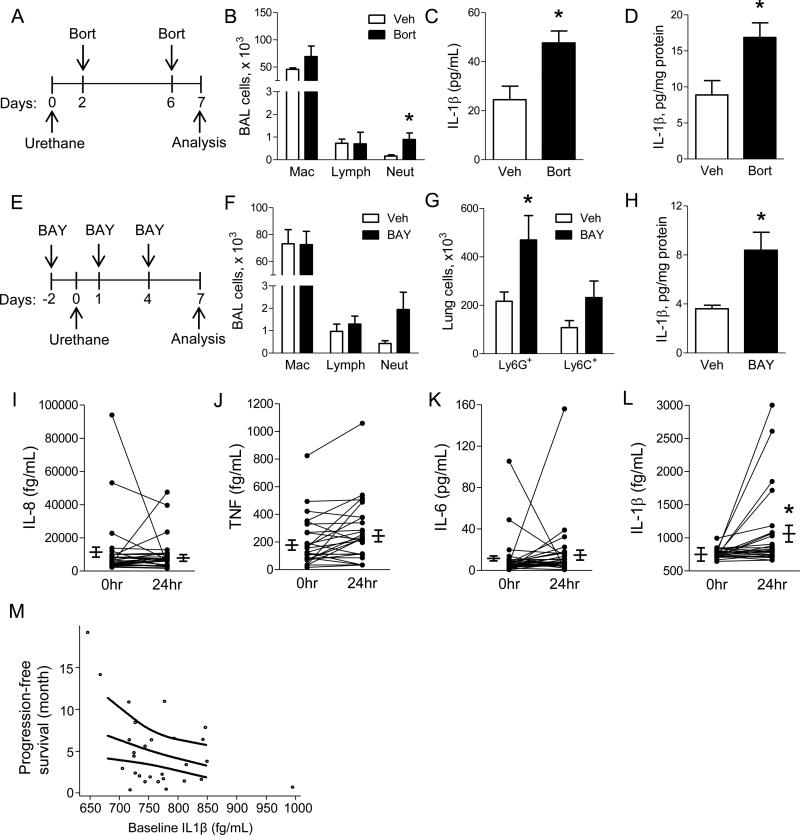
We next sought to determine whether IL-1β dysregulation could be detected following treatment with pharmacological NF-κB inhibitors in mice and human NSCLC patients. WT mice were treated with the proteasome inhibitor bortezomib (1 mg/kg) (Karabela et al., 2012; Xue et al., 2011) or vehicle by IP injection on days 2 and 6 following urethane injection and harvested at day 7 (Figure 6A). We observed elevated numbers of neutrophils in BAL from bortezomib-treated mice compared to vehicle-treated controls (Figure 6B). In addition, we found increased IL-1β protein in both serum and lungs of bortezomib-treated mice compared to mice treated with vehicle (Figure 6C-D). To test whether these effects were common to different classes of NF-κB inhibitors, we repeated our studies using BAY 11-7082 (BAY). NF-κB inhibition was verified by luciferase activity as a measure of NF-κB activity in vehicle- or BAY-treated NF-κB reporter mice (Everhart et al., 2006) after urethane injection (Figure S3). At 1 week after urethane injection, BAY treatment resulted in increased neutrophils in BAL and lung tissue (Figure 6E-G). BAY-treated mice also had elevated IL-1β protein in lung homogenates compared to vehicle-treated mice, similar to IKKβΔmye mice (Figure 6H). Unlike IKKβΔmye mice, however, KC expression was not increased in BAY-treated WT mice (Figure S4).
Figure 6.
Pharmacological inhibition of NF-κB increases IL-1β in mice and indicates worse survival in NSCLC patients. A) Schematic representation of NF-κB inhibition protocol using bortezomib (Bort). In addition to urethane, WT mice were treated with IP injections of Bort (1 mg/kg) or vehicle control (Veh). B) BAL cells in Bort- or Veh-treated WT mice at 1 week after urethane injection (n=4-5 mice per group; *p<0.05 compared to Veh). C) Serum and (D) lung IL-1β protein levels from Bort- or Veh-treated WT mice 1 week after urethane (n=6 mice per group). E) Schematic representation of the NF-κB inhibition protocol using BAY 11-7082 (BAY). In addition to urethane, WT mice were treated with IP injections of the specific NF-κB inhibitor BAY (10 mg/kg) or Veh. F) BAL cells in BAY- and Veh-treated WT mice at 1 week after urethane injection (n=8 mice per group). G) Number of Ly6G+ neutrophils and Ly6C+ monocytes in the lungs of BAY- or Veh-treated mice at 1 week after urethane injection (n=4-5 mice per group; *p<0.05 compared to Veh). H) IL-1β protein levels in the lungs of BAY- or Veh-treated mice at 1 week after urethane injection (n=8 mice per group). I) IL-8, (J) TNF, (K) IL-6, and (L) IL-1β protein levels in the plasma of NSCLC patients treated before (0hr) and 24hr after treatment with bortezomib (1 mg/m2) (n=28 patients; *p < 0.05 compared with 0 hr). M) Correlation analysis between progression-free survival and baseline plasma IL-1β protein levels in advanced NSCLC patients treated with bortezomib plus standard chemotherapy (p=0.026). See also Figures S3, S4 and Table S1.
To investigate the relevance of our mouse model findings to human NSCLC, we obtained blood samples from a completed study involving 28 chemotherapy-naïve individuals with advanced stage (III-IV) NSCLC (protocol NCT01633645) (Table S1). In this study, patients received one cycle of bortezomib followed by a standard chemotherapy/bortezomib combination regimen. In plasma obtained before and 24 hours after the first dose of bortezomib (1 mg/m2), we measured a panel of cytokines (IL-1β, IL-8, TNF, and IL-6) using cytometric bead array and found that treatment with bortezomib significantly increased IL-1β protein in the plasma of advanced NSCLC patients; however, no differences were detected in IL-8, TNF, or IL-6 (Figure 6I-L). In addition, we found that after controlling for age and performance status, IL-1β level at baseline significantly correlated with reduced progression-free survival in this cohort (p=0.026) (Figure 6M).
IL-1β promotes lung tumorigenesis, enhances epithelial cell proliferation, and mediates resistance to NF-κB inhibitor therapy
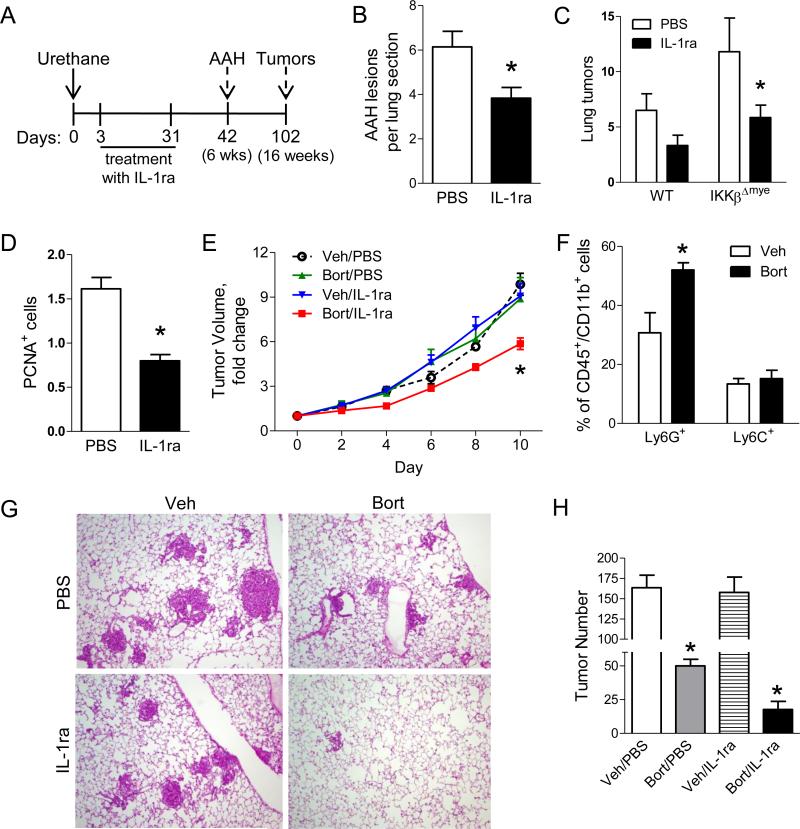
Since IL-1β production is increased in tumor models in the setting of myeloid and systemic NF-κB inhibition, we investigated the impact of IL-1β on lung tumorigenesis using the clinically available IL-1 receptor antagonist (IL-1ra, anakinra/Kineret®). IL-1ra (60 mg/kg/day) was delivered during the first 4 weeks after urethane injection to WT and IKKβΔmye mice usingsubcutaneously implanted osmotic pumps (Figure 7A). Osmotic pumps filled with vehicle (PBS) were used as controls. As shown in Figure 7B, IL-1ra treatment significantly decreased the number of AAH lesions in the lungs of IKKβΔmye mice at 6 weeks after urethane injection. To evaluate the impact of IL-1β signaling on tumor formation, we repeated these studies and harvested mice 16 weeks after urethane treatment. We found that IL-1ra treatment reduced lung tumors in IKKβΔmye mice by more than 50% compared to IKKβΔmye mice treated with vehicle (Figure 7C). Based on our finding that IKKβΔmye mice have increased lung epithelial cell proliferation during tumorigenesis, we tested whether IL-1β could exert its pro-tumorigenic effects by altering proliferation of epithelial cells. We performed PCNA immunostaining on lung sections from IL-1ra- and PBS-treated IKKβΔmye mice harvested 6 weeks after urethane and found reduced PCNA+ lung epithelial cells in IL-1ra-treated IKKβΔmye mice (Figure 7D), demonstrating that IL-1β signaling supports epithelial cell proliferation during tumorigenesis. Together, these results indicate a pro-tumorigenic role for IL-1β in the setting of NF-κB inhibition in myeloid cells.
Figure 7.
IL-1β facilitates lung tumorigenesis by stimulating epithelial cell proliferation and supports resistance to bortezomib therapy. A) Schematic representation of IL-1 receptor antagonist (IL-1ra) treatment protocol. WT and IKKβΔmye mice were injected with a single dose of urethane and treated by osmotic pump delivery of 60 mg/kg/day of IL-1ra or PBS for the first 4 weeks. B) Number of AAH lesions per H&E-stained lung section harvested from IKKβΔmye mice at week 6 after injection of urethane (n=9 mice per group, *p < 0.05 compared with PBS). C) Lung tumors on H&E-stained lung sections from WT and IKKβΔmye mice cut at predetermined depths (5 sections per mouse, n=7 mice per group; *p<0.05 compared with PBS-treated IKKβΔmye mice). D) Number of PCNA+ cells per lung section (averaged from 25 sequential fields taken at 40× magnification) from IKKβΔmye mice harvested at week 6 after urethane injection (n=9 mice per group; *p<0.05 compared with PBS). E) Fold change of subcutaneous LLC tumor volume over 10 days of treatment with vehicle control, bortezomib, IL-1ra, or bortezomib plus IL-1ra (n=6-9 mice per group; *p<0.05 compared with control). F-H) Inducible KrasG12D mice were treated with doxycycline (dox) for 4 weeks to develop lung tumors. (F) Percentage of Ly6G+ and Ly6C+ cells in the lungs of dox-inducible KrasG12D mice treated for 1 additional week with Bort or Veh plus dox (*p<0.05 compared to Veh). (G) Representative photomicrographs and (H) Numbers of surface lung tumors in mice treated with dox alone for 4 weeks followed by 4 weeks of treatment with dox plus vehicle control, bortezomib, IL-1ra, or bortezomib plus IL-1ra (n=6-7 mice per group; *p<0.05 compared with control.
Since IL-1β is dysregulated and supports tumor cell proliferation in the context of NF-κB inhibition, we next tested whether the addition of IL-1ra could improve the efficacy of NF-κB inhibitor therapy in two different lung cancer models. In the first model, we injected murine Lewis lung carcinoma (LLC) cells subcutaneously into the flanks of syngeneic WT mice. When tumors reached approx 100 mm2, mice were divided into four treatment groups: bortezomib, IL-1ra, bortezomib plus IL-1ra, or vehicle control. Bortezomib (or vehicle) was administered by IP injection twice weekly, and IL-1ra (or PBS control) was administered throughout the treatment course via osmotic pump. Whereas monotherapy with bortezomib or IL-1ra did not affect tumor growth, combination therapy with bortezomib and IL-1ra significantly reduced tumor growth compared with all other groups (Figure 7E). For the second model, we used doxycycline (dox)-inducible KrasG12D mice (Fisher et al., 2001). In a preliminary study, we treated mice with dox for 4 weeks followed by bortezomib twice weekly for 1 week and found increased neutrophils in the lungs compared to vehicle-treated mice (Figure 7F). Subsequently, we treated inducible KrasG12D mice with dox for 4 weeks and then randomized mice to treatment with bortezomib, IL-1ra, bortezomib plus IL-1ra, or vehicle control for 4 additional weeks. While treatment with bortezomib reduced tumor numbers compared to vehicle control and IL-1ra groups, lung tumors were reduced by 90% in mice administered combination therapy with bortezomib and IL-1ra (Figure 7G-H). In these studies, combination therapy with bortezomib and IL-1ra reduced tumor formation and growth and was more effective than bortezomib alone.
DISCUSSION
Our studies identify IL-1β as a targetable, pro-tumorigenic mediator that contributes to resistance of lung tumors to NF-κB inhibitors. We showed that inhibition of NF-κB in myeloid cells enhances lung tumorigenesis and paradoxically increases infiltration of neutrophils into the lungs. NF-κB-deficient neutrophils produced elevated levels of IL-1β, which was regulated by the serine protease cathepsin G. Consistent with studies in mice with myeloid-specific NF-κB inhibition, systemic delivery of pharmacological NF-κB inhibitors to WT mice significantly increased lung neutrophils and IL-1β production during lung tumorigenesis. In humans with advanced stage NSCLC, plasma IL-1β concentration inversely correlated with progression-free survival and IL-1β levels were increased following treatment with the proteasome inhibitor bortezomib. Neutrophil depletion studies and pharmacological IL-1ra treatment, both of which reduced lung tumors in the setting of myeloid NF-κB inhibition, support a causative role for neutrophil-derived IL-1β in lung tumorigenesis. Further, we demonstrated that combined treatment with bortezomib and IL-1ra reduces tumor formation and growth in vivo and that IL-1β exerts its pro-tumorigenic effects by stimulating lung epithelial cell proliferation. In addition to demonstrating an important role for IL-1β in promoting lung carcinogenesis and mediating resistance to NF-κB inhibitors, these data support broader utilization of rational combined biological therapies to treat lung cancer.
Together with existing literature, our findings suggest that the lung microenvironment could support both pro- and anti-tumorigenic outcomes resulting from inhibition of NF-κB signaling. Consistent with our previous studies showing pro-tumorigenic outcomes from long-term bortezomib treatment (Karabela et al., 2012), these data demonstrate that inhibition of NF-κB signaling specifically in myeloid cells enhances lung tumorigenesis. Our findings are also in agreement with a recent report in which myeloid NF-κB inhibition supported enhanced growth of melanomas (Yang et al., 2014). In opposition, previous studies using a colon cancer model and a model of lung cancer induced by oncogenic Kras plus cigarette smoke found that inhibition of NF-κB signaling in myeloid cells inhibited tumorigenesis (Greten et al., 2004; Takahashi et al., 2010). We suggest that differences in tumorigenic outcomes in response to myeloid-specific NF-κB inhibition may be due to differential effects on pre-existing inflammation in the tumor microenvironment. Both the azoxymethane plus dextran sulfate colon cancer model and the oncogenic Kras plus cigarette smoke model are highly inflammatory models in which myeloid NF-κB inhibition reduces carcinogenesis as well as cytokine expression and inflammatory cell infiltration (Greten et al., 2004; Takahashi et al., 2010). In contrast, the lung cancer models in our studies result in only mild inflammation, and myeloid NF-κB inhibition increases inflammation in these settings. Therefore, it may be that the overall impact of myeloid NF-κB inhibition on tumorigenesis is dependent upon the inflammatory environment. In environments with high levels of pre-existing inflammation, inhibition of NF-κB signaling may reduce protumorigenic inflammation by blocking transcription of NF-κB-dependent mediators, consequently suppressing tumor formation and growth. In contrast, up-regulation of IL-1β processing by neutrophils may play an important pro-tumorigenic role in less inflammatory environments, which may be more similar to human lung cancer, by providing important proliferation signals to mutated epithelial cells. In either case, it may be that combination biological approaches to block inflammatory signaling are superior to NF-κB inhibition alone.
In our studies, we discovered that both myeloid-specific and systemic inhibition of NF-κB induce an increase in lung neutrophils during lung carcinogenesis. This increase in lung neutrophils was not a result of increased circulating neutrophils, but could be related to increased recruitment or prolonged survival, which has been previously described for NF-κB-inhibited neutrophils (Hsu et al., 2011; Langereis et al., 2010). Although we found increased expression of the neutrophil chemoattractant KC in urethane-treated IKKβΔmye mice, WT mice treated with systemic NF-κB inhibitors also had increased neutrophils in the lungs but did not show increased KC expression, suggesting that KC is not the critical mediator of lung neutrophilia observed in our models. The N1/N2 neutrophil polarization paradigm has been used to explain anti- or protumorigenic functions of neutrophils (Fridlender et al., 2009). Several studies have shown that N2 tumor-associated neutrophils exert their pro-tumorigenic properties through production of angiogenic factors, matrix-degrading enzymes, and immunosuppression (Reviewed in Sionov et al., 2014). In contrast, our studies show that neutrophils with inhibited NF-κB signaling are not highly polarized towards N1 or N2 and are not immunosuppressive. Instead, NF-κB-deficient neutrophils have a unique pro-tumorigenic phenotype characterized by dysregulated processing of the inflammatory mediator IL-1β.
While we identified an important role for neutrophils in accelerating lung tumorigenesis in the context of NF-κB inhibition, other cell types may also contribute to this phenotype. Although not directly tested in our studies, interactions between neutrophils and macrophages may be important for creating a pro-tumorigenic environment in the lungs. This idea is supported by our previous finding that macrophages are important for urethane-induced tumorigenesis (Zaynagetdinov et al., 2011), as well as a recent study demonstrating that macrophages with inhibited NF-κB signaling are unable to mediate anti-tumor responses against metastatic melanoma cells (Yang et al., 2014). Future studies are necessary to fully elucidate interactions between inflammatory cell types and epithelial cells that regulate lung carcinogenesis.
A connection between elevated IL-1β and lung cancer in humans has been suggested by studies showing that a single nucleotide polymorphism (−31C-T) in IL1B increases IL-1β expression and lung cancer risk (Li and Wang, 2013; Lind et al., 2007). Our studies extend these findings by showing that IL-1β levels in plasma were inversely correlated with progression-free survival of NSCLC patients. Further, we found that plasma IL-1β levels of NSCLC patients increase following NF-κB inhibition with the proteasome inhibitor bortezomib, suggesting that our explanation for resistance to NF-κB inhibitor therapy is relevant to NSCLC patients. Although the mechanisms by which IL-1β impacts lung tumor biology are not fully understood, our findings suggest that IL-1β exerts its pro-tumorigenic effects by promoting proliferation of lung epithelial cells. Our observations are consistent with a prior report that IL-1β increases proliferation of human NSCLC cells (Wang et al., 2014).
We found that both myeloid-specific and systemic NF-κB inhibition increase IL-1β protein expression in the lungs. Although IL-1β mRNA expression is regulated by the NF-κB pathway (Cogswell et al., 1994), our findings are consistent with previous reports showing that NF-κB inhibition in myeloid cells increases IL-1β processing under conditions of septic shock and acute lung injury (Greten et al., 2007; Hsu et al., 2011; Huang et al., 2011). While IL-1β processing is thought to be primarily regulated by the inflammasome in most cells, serine proteases have been implicated in IL-1β processing by neutrophils (Greten et al., 2007; Guma et al., 2009). Our findings indicate that cathepsin G strongly regulates IL-1β production by neutrophils and that expression of cathepsin G is upregulated in neutrophils with inhibited NF-κB. Therefore, increased neutrophilia in the lungs during NF-κB inhibition and increased processing of pro-IL-1β by cathepsin G in individual neutrophils likely contribute to increased IL-1β production in this setting. Since cathepsin G has been correlated with tumor grade and clinical stage in NSCLC (Maksimowicz et al., 1997), future studies targeting this protease could be warranted.
Although IL-1 receptor blockade alone was ineffective in reducing tumor formation and growth in our models, these studies demonstrate that the addition of IL-1ra to NF-κB inhibition improves the effectiveness of NF-κB inhibitor therapy. In a heterotopic flank tumor model, combination therapy was the only regimen that slowed tumor growth compared to vehicle control. In the dox-inducible KrasG12D model, bortezomib monotherapy reduced tumor formation but combination therapy with bortezomib and IL-1ra was most effective. These findings indicate that the effects of bortezomib are variable and model-dependent. In contrast, we showed impressive responses to combined bortezomib/IL-1ra treatment in both tumor models tested. Of the 35 clinical trials included in the ClinicalTrials.gov database that investigate bortezomib in lung cancer, only three have used combined therapy with bortezomib and another targeted agent. Since combined targeted therapies may be the most direct way to manage disease and reduce nonspecific side effects from treatment (Gibbs, 2000), our studies support future human studies combining NF-κB inhibitors with IL-1ra or other targeted biological therapies aimed at overcoming resistance mechanisms.
EXPERIMENTAL PROCEDURES
Mouse studies
All animal care and experimental procedures were approved and conducted according to guidelines issued by the Vanderbilt University Institutional Animal Care and Use Committee. Lung tumors were induced in IKKβΔmye mice (IKKβfl/fl; LysM-Cre) (Li et al., 2003) and littermate wild-type (WT) controls by a single IP injection of urethane (ethyl carbamate, 1 g/kg) (Sigma-Aldrich). BAY 11-7082 (10 mg/kg body weight, Cayman Chemical) or bortezomib (1 mg/kg; Selleckchem) was delivered by IP injection as described (Xue et al., 2011). Lung tumors were induced in LSL-KrasG12D mice (Tuveson et al., 2004) using IT instillation of adeno-Cre (1.5×107 PFU). Lung tumors were established in mice expressing dox-inducible KrasG12D in CCSP+ lung epithelial cells [CCSP-rtTA (tet-O)- KrasG12D] and littermate WT controls (Fisher et al., 2001) via consumption of dox (0.5 g/L) in drinking water for 4 weeks. Subsequently, mice were treated with dox plus vehicle control, bortezomib, IL-1ra (60 mg/kg/d; Amgen), or combination bortezomib and IL-1ra for 4 weeks. Subcutaneous tumors in C57BL/6 mice were established by injection of 2.5×105 syngeneic Lewis Lung Carcinoma (LLC) cells in the right flank. When tumor size reached about 100 mm2, mice were randomized and treated with vehicle control, bortezomib, IL-1ra, or combination of bortezomib and IL-1ra. Tumor sizes were measured using Traceable digital calipers (Fisher Scientific). NF-κB reporter mice have been previously described (Everhart et al., 2006).
Human samples
Twenty-eight chemotherapy-naïve patients with inoperable locally advanced (Stage IIIB) or metastatic (Stage IV) NSCLC were treated with bortezomib (1 mg/m2) as part of a phase II clinical trial performed at the University Hospital of Crete (Protocol NCT01633645). Bortezomib was administered alone for the first cycle of treatment. All subsequent treatment cycles contained bortezomib plus gemcitabine and cisplatin. Plasma samples were collected before and 24 hours after the first dose of bortezomib. This trial (The “Velcade” project) was approved by the IRB of the University Hospital of Crete (No. 8433/21-09-2006) and the National Ethics Committee (No. 77659/22-11-2007).
Neutrophil depletion, macrophage depletion, and neutralization of IL-1 receptor
For neutrophil depletion, 100 μg of anti-Ly6G antibodies (Clone 1A8, BioLegend) or IgG2a isotype control antibodies (BioLegend) were delivered by IP injection twice a week for the first six weeks after urethane injection. Depletion of macrophages was conducted as previously described (Zaynagetdinov et al., 2011). To block IL-1β signaling, mice were treated with 60 mg/kg/day of IL-1 receptor antagonist (IL-1ra) (anakinra/Kineret®, Amgen) or PBS (vehicle control) delivered by subcutaneously implanted Alzet osmotic pumps (infusion rate of 0.5 μL/h, DURECT Corp.). After 2 weeks, osmotic pumps were replaced to complete a 4-week course of treatment.
Statistical analysis
Mouse data were analyzed using the GraphPad Prism 5.0 software (GraphPad Software Inc.), and values are presented as mean ± SEM. Pair-wise comparisons were made using Student's t-tests. For experiments conducted over several time points or with multiple comparisons, a twoway ANOVA with a Bonferroni post-test was used.
Data from the 28 chemotherapy-naïve subjects were analyzed using R software version 3.1.2 (www.r-project.org) and were expressed as median (interquartile range) for continuous variables and frequencies (proportions) for categorical variables. IL-1β, IL-8, TNF, and IL-6 before and 24 hours after initial treatment were compared using Student's t-test. Spearman correlation between baseline IL-1β and progression-free survival time in months was analyzed. We further applied a multivariable linear regression model to adjust for both subjects’ age at baseline and performance status. Normality of residuals of the linear model was diagnosed, and log transformation on progression-free survival time was performed to correct non-normal residuals if needed. p<0.05 was considered statistically significant for both mouse and human data.
Supplementary Material
HIGHLIGHTS.
Inhibition of NF-κB signaling in myeloid cells enhances lung tumorigenesis.
Carcinogen treatment induces IL-1β processing in neutrophils with NF-κB inhibition.
NF-κB targeting with bortezomib increases IL-1β production in NSCLC patients.
Combination therapy with bortezomib and IL-1R antagonist slows tumor growth in mice.
ACKNOWLEDGMENTS
This work was supported by a Grant from the Lung Cancer Initiative of North Carolina and Free to Breathe (R.Z.), by National Institutes of Health Grant T32HL094296 (R.Z.), by European Research Council Starting Independent Investigator Grant FP7-IDEAS-ERC-StG-2010-260524-KRASHIMPE (G.T.S), by the Department of Veterans Affairs (T.S.B), and by a Vanderbilt-Ingram Cancer Center Spore Grant 2010 (T.S.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS:
Conceptualization, A.G.M., R.Z., and T.S.B; Methodology, A.G.M., L.A.G., G.T.S., and R.Z; Investigation, A.G.M., T.P.S, D.S.C., W.H., J.A.S, L.A.G., P.W., V.V.P., G.T.S. and R.Z, Writing, A.G.M, R.Z., and T.S.B; Writing – Review & Editing, A.G.M., G.T.S., F.E.Y., R.Z. and T.S.B.; Funding Acquisition, R.Z., G.T.S., and T.S.B; Resources, M.K., F.E.Y and V.G., Supervision, A.G.M, R.Z, and T.S.B.
Further experimental procedures are described in the Supplemental Information.
The authors have no conflicting financial interests.
eTOC blurb
McLoed et al. show that inhibition of NF-κB signaling in myeloid cells augments lung tumorigenesis. Myeloid-specific or systemic NF-κB inhibition increases IL-1β production neutrophils, which enhances lung tumor formation. These studies highlight an important resistance pathway that limits efficacy of NF-κB inhibitors.
REFERENCES
- Bassères DS, Ebbs A, Cogswell PC, Baldwin AS. IKK is a therapeutic target in KRAS-Induced lung cancer with disrupted p53 activity. Genes Cancer. 2014;5:41–55. doi: 10.18632/genesandcancer.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassères DS, Ebbs A, Levantini E, Baldwin AS. Requirement of the NF-κB Subunit p65/RelA for K-Ras-Induced Lung Tumorigenesis. Cancer Res. 2010:3537–3546. doi: 10.1158/0008-5472.CAN-09-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse B, Planchard D, Veillard AS, Taillade L, Khayat D, Ducourtieux M, Pignon JP, Lumbroso J, Lafontaine C, Mathiot C, Soria JC. Phase 2 study of frontline bortezomib in patients with advanced non-small cell lung cancer. Lung Cancer. 2012;76:78–83. doi: 10.1016/j.lungcan.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, Chang YS. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol. Med. 2012;4:1276–1293. doi: 10.1002/emmm.201201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li Z, Bai L, Lin Y. NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front. Biosci. (Landmark ed) 2011;16:1172–1185. doi: 10.2741/3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, Gray JG. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 1994;153:712–723. [PubMed] [Google Scholar]
- Enzler T, Sano Y, Choo MK, Cottam HB, Karin M, Tsao H, Park JM. Cell-selective inhibition of NF-κB signaling improves therapeutic index in a melanoma chemotherapy model. Cancer Discov. 2011;1:496–507. doi: 10.1158/2159-8290.CD-11-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and Intensity of NF-κB Activity Determine the Severity of Endotoxin-Induced Acute Lung Injury. J. Immunol. 2006;176:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- Fanucchi MP, Fossella FV, Belt R, Natale R, Fidias P, Carbone DP, Govindan R, Raez LE, Robert F, Ribeiro M, Akerley W, Kelly K, Limentani SA, Crawford J, Reimers HJ, Axelrod R, Kashala O, Sheng S, Schiller JH. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- Fong CHY, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, Karin M, Lawrence T. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J. Exp. Med. 2008;205:1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannou AD, Marazioti A, Spella M, Kanellakis NI, Apostolopoulou H, Psallidas I, Prijovich ZM, Vreka M, Zazara DE, Lilis I, Papaleonidopoulos V, Kairi CA, Patmanidi AL, Giopanou I, Spiropoulou N, Harokopos V, Aidinis V, Spyratos D, Teliousi S, Papadaki H, Taraviras S, Snyder LA, Eickelberg O, Kardamakis D, Iwakura Y, Feyerabend TB, Rodewald HR, Kalomenidis I, Blackwell TS, Agalioti T, Stathopoulos GT. Mast cells mediate malignant pleural effusion formation. J. Clin. Invest. 2015;125:2317–2334. doi: 10.1172/JCI79840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000;287:1969–1973. doi: 10.1126/science.287.5460.1969. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O'Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, Yu GY, Lai LC, Temkin V, Sinzig U, Aung T, Nizet V, Weissman IL, Karin M. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat. Immunol. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Sugimoto S, Lai J, Okazaki M, Yamamoto S, Krupnick AS, Kreisel D, Gelman AE. Maintenance of IKKβ activity is necessary to protect lung grafts from acute injury. Transplantation. 2011;91:624–631. doi: 10.1097/TP.0b013e31820ba2a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabela SP, Psallidas I, Sherrill TP, Kairi CA, Zaynagetdinov R, Cheng DS, Vassiliou S, McMahon F, Gleaves LA, Han W, Stathopoulos I, Zakynthinos SG, Yull FE, Roussos C, Kalomenidis I, Blackwell TS, Stathopoulos GT. Opposing effects of bortezomib-induced nuclear factor-κB inhibition on chemical lung carcinogenesis. Carcinogenesis. 2012;33:859–867. doi: 10.1093/carcin/bgs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis JD, Raaijmakers HAJA, Ulfman LH, Koenderman L. Abrogation of NF-κB signaling in human neutrophils induces neutrophil survival through sustained p38-MAPK activation. J. Leukoc. Biol. 2010;88:655–664. doi: 10.1189/jlb.0809544. [DOI] [PubMed] [Google Scholar]
- Li C, Wang C. Current evidences on IL1B polymorphisms and lung cancer susceptibility: a meta-analysis. Tumour Biol. 2013;34:3477–3482. doi: 10.1007/s13277-013-0925-6. [DOI] [PubMed] [Google Scholar]
- Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J. Immunol. 2003;170:4630–4637. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets. 2010;14:45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind H, Haugen A, Zienolddiny S. Differential binding of proteins to the IL1B -31 T/C polymorphism in lung epithelial cells. Cytokine. 2007;38:43–48. doi: 10.1016/j.cyto.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Maksimowicz T, Chyczewska E, Chyczewski L, Nikliński J, Ostrowska H, Szyszko J, Furman M. Activity and tissue localization of cathepsin G in non small cell lung cancer. Rocz. Akad. Med. w Białymstoku. 1997;42(Suppl 1):199–216. [PubMed] [Google Scholar]
- Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajnavolgyi E, Nagy L, Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Saxon JA, Sherrill TP, Polosukhin VV, Sai J, Zaynagetdinov R, McLoed AG, Gulleman PM, Barham W, Cheng DS, Hunt RP, Gleaves LA, Richmond A, Young LR, Yull FE, Blackwell TS. Epithelial NF-κB signaling promotes EGFR-driven lung carcinogenesis via macrophage recruitment. Oncoimmunology. 2016 doi: 10.1080/2162402X.2016.1168549. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov RV, Fridlender ZG, Granot Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2014:1–34. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B, Blackwell TS. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18514–18519. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos GT, Sherrill TP, Karabela SP, Goleniewska K, Kalomenidis I, Roussos C, Fingleton B, Yull FE, Peebles RS, Blackwell TS. Host-derived interleukin-5 promotes adenocarcinoma-induced malignant pleural effusion. Am. J. Respir. Crit. Care Med. 2010;182:1273–1281. doi: 10.1164/rccm.201001-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar JW, Zhang Y, LeRiche JC, Biddinger PW, Lam S, Anderson MW. Increased staining for phospho-Akt, p65/RELA and cIAP-2 in pre-neoplastic human bronchial biopsies. BMC Cancer. 2005;5:155. doi: 10.1186/1471-2407-5-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, DePinho RA, Jacks T. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang LF, Wu J, Xu SJ, Xu YY, Li D, Lou JT, Liu MF. IL-1β-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res. 2014;74:4720–4730. doi: 10.1158/0008-5472.CAN-14-0960. [DOI] [PubMed] [Google Scholar]
- Westcott PMK, Halliwill KD, To MD, Rashid M, Rust AG, Keane TM, Delrosario R, Jen KY, Gurley KE, Kemp CJ, Fredlund E, Quigley DA, Adams DJ, Balmain A. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature. 2014;517:489–492. doi: 10.1038/nature13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Yeddula N, Leblanc M, Ke E, Zhang Y, Oldfield E, Shaw RJ, Verma IM. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat. Cell Biol. 2012;14:257–265. doi: 10.1038/ncb2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Meylan E, Oliver TG, Feldser DM, Winslow MM, Bronson R, Jacks T. Response and resistance to NF-κB inhibitors in mouse models of lung adenocarcinoma. Cancer Discov. 2011;1:236–247. doi: 10.1158/2159-8290.CD-11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hawkins O, Barham W, Gilchuk P, Boothby M, Ayers GD, Joyce S, Karin M, Yull F, Richmond A. Myeloid IKKβ Promotes Anti-tumor Immunity by Modulating CCL11 and the Innate Immune Response. Cancer Res. 2014;74:7274–7284. doi: 10.1158/0008-5472.CAN-14-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Candrian U, Maronpot RR, Stoner GD, Anderson MW. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc. Natl. Acad. Sci. U. S. A. 1989;86:3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R, Sherrill TP, Polosukhin VV, Han W, Ausborn JA, McLoed AG, McMahon FB, Gleaves LA, Degryse AL, Stathopoulos GT, Yull FE, Blackwell TS. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J. Immunol. 2011;187:5703–5711. doi: 10.4049/jimmunol.1100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R, Stathopoulos GT, Sherrill TP, Cheng DS, McLoed AG, Ausborn JA, Polosukhin VV, Connelly L, Zhou W, Fingleton B, Peebles RS, Prince LS, Yull FE, Blackwell TS. Epithelial nuclear factor-κB signaling promotes lung carcinogenesis via recruitment of regulatory T lymphocytes. Oncogene. 2012;31:3164–3176. doi: 10.1038/onc.2011.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.