Introduction
In the United States in 2013, nearly 1.7 million adults were diagnosed with cancer, and, when adjusted for life expectancy, the 5-year relative survival rate was 68%. In contrast, only 11,630 children between the ages of 0-14 years will develop cancer, and the 5-year relative survival rate from 2002-2008 was 83%. 1 Neurologic complications are common in patients with systemic malignancies, and neurologists are often consulted for diagnosis and management. While there are numerous reports in the literature reviewing neurologic complications and their prognoses in adults with cancer,2 there remains a relative paucity of literature concerning the neurologic sequelae in the pediatric cancer population. Pediatric cancer patients receive many of the same therapies as their adult counterparts including surgery, chemotherapy, and radiation; however, because children are still growing and developing, they may experience different adverse effects. Furthermore, prostate, lung, colon, and breast cancer comprise a large plurality of malignancies in adults while CNS tumors and leukemia/lymphoma represent the majority of malignancies in children.1 Therefore, it is important to recognize complications in the pediatric population as disparate from those occurring in adults. Diagnosing such complications correctly and initiating treatment quickly may reduce pain and prevent further progression and possibly, permanent deficits.
We review the most recent literature on the neurological complications of cancer organized by frequency in the pediatric population. The complications below occur primarily in the acute and subacute settings. Please refer to the paper by Roddy et al. in this issue for a discussion of some of the neurologic sequelae in survivors of childhood cancer3.
Headache
In general, childhood headaches are rarely caused by a serious underlying disorder and as a result, can safely be attributed to primary headache syndromes.4 Primary headache syndromes will not be discussed in this paper. Instead, emphasis will be placed on recognizing important clues in the history and on physical examination in pediatric oncology patients with headache to guide the evaluation and treatment.
Intracranial parenchymal or leptomeningeal metastatic disease
Headaches are the most common presenting symptom of children with space occupying intracranial lesions, and, over the course of their disease, up to two thirds of children will complain of headache.5,6 Pathology in the brain parenchyma alone typically does not cause headache, as brain tissue is devoid of pain receptors. Primary intracranial tumors, metastatic disease, or CSF involvement can result in pain secondary to direct local invasion and/or compression of pain sensitive intracranial structures or increased intracranial pressure resulting from mass effect or hydrocephalus5 (Table 1).
Table 1.
Structures that cause pain44
| Vasculature | Dura | Cranial nerves (CN) that carry pain fibers | Extracranial structures | Additional structures |
|---|---|---|---|---|
| Large arteries at the base of the brain Proximal portions of their immediate branches Meningeal arteries Venous channels of the brain and dura |
Dura itself Tentorium Diaphragma sellae |
CN V CN VIICN IX CN X |
Orbit Sinus Middle ear Teeth |
Periosteum of the skull Subcutaneous tissue Muscle Skin |
Certain characteristics of headache should prompt further evaluation in the pediatric population. In children who carry a diagnosis of a primary headache syndrome, any change in the established headache pattern, an increase in severity or frequency of headaches, or changes in the pain quality should warrant additional workup.7 A history of early morning headache or a headache that awakens a child from sleep is worrisome. Acute headaches associated with episodes of confusion or any focal neurological deficits are also of concern. Headaches that worsen with positional change, cough, or Valsalva suggest raised intracranial pressure, as do nausea/vomiting, peripheral visual loss, diplopia, gait unsteadiness, urinary incontinence, or seizures. Finally, a migrainous headache in a patient without a family history of migraine should also prompt neuroimaging.
New onset headache in children less than 3 years of age should prompt a workup, as primary causes of headache are not common at this age. Table 2 lists the signs and symptoms in young children, nonverbal children, or children with cognitive delay.
Table 2.
Signs/ symptoms associated with headaches in younger or nonverbal children
| Changes in mood | Changes in behavior | Physical examination findings |
|---|---|---|
| Irritability Restlessness |
Failure to thrive Stagnation or loss in developmental milestones Poor educational performance due to impaired concentration/-memory Behavioral problems Excessive sleepiness |
Unexpected increase in head circumference Abnormally patent or bulging fontanel Papilledema Restricted upgaze Cranial nerve palsies Motor abnormalities |
Hydrocephalus
Obstructive or noncommunicating hydrocephalus is the result of obstruction in the normal CSF flow through the ventricular system as a result of either mass effect or direct tumor growth. Metastatic disease to the leptomeninges and/or the CSF may cause non-obstructive or communicating hydrocephalus as cellular debris and increased CSF protein impair CSF absorption.
Therapy related
Chemotherapeutic agents, both intravenous and intrathecal, may cause headaches. Intrathecal chemotherapy may cause chemical meningitis presenting with headaches, fever, and nuchal rigidity 2-4 hours post-lumbar puncture; however, CNS infection must be excluded in this immunosuppressed patient population. Retinoids, steroids and therapy related metabolic or endocrinologic disruption-may cause pseudotumor cerebri which may result in headaches. Low pressure headaches which worsen upon sitting or standing may develop after lumbar puncture due to CSF leak. Hydration, intravenous caffeine or a blood patch may be used to treat post-lumbar puncture headache. In a patient who has a history of post-lumbar puncture headaches and requires more lumbar punctures, using a smaller bore needle with minimal trauma may prevent headache. Patients receiving external beam irradiation to the brain may develop headaches due to pseudoprogression, transient increase in lesion size, or surrounding edema presumably due to cytotoxic damage. Steroids can help mitigate symptoms.
Infectious causes
The development of headaches plus associated fever and nuchal rigidity manifesting 24 hours after intracranial or intrathecal chemotherapy is most commonly seen in bacterial meningitis. Of note, immunosuppressed patients may not mount a fever or develop nuchal rigidity. Thus, regardless of the time course or clinical presentation, a lumbar puncture should still be strongly considered unless contraindicated (see below for contraindications to lumbar procedures). In children immunosuppressed to prevent graft rejection following transplant, mortality associated with meningitis approaches 50%, with a delay in diagnosis significantly increasing morbidity and mortality8. Atypical pathogens including, but not limited to atypical bacteria, human herpesvirus 6, cytomegalovirus, Epstein–Barr virus, varicella zoster virus, herpes simplex virus, enterovirus D3, and parasitic infections, must be considered and appropriate coverage initiated.
Brain abscesses can be seen in severely immunocompromised patients, particularly those who have received bone marrow transplant. These lesions are typically ring enhancing on the T1 post-contrast MRI of the brain. Other infectious processes that can result in headache include upper respiratory illness resulting in sinusitis or otitis media. Infectious disease consultation may be helpful.
Intracranial hemorrhage
Spontaneous intracranial hemorrhage or hemorrhage into CNS lesions may cause headaches, especially in the setting of therapy induced thrombocytopenia (most likely with platelet count <10 K/mcL) or anticoagulation. When intracranial hemorrhage causes headache, the mechanism may be related to mass effect of the bleed and or meningeal or ependymal irritation. Specifically, L-asparaginase is associated with cerebral thrombosis with secondary intracranial hemorrhage or primary hemorrhage because of coagulation protein deficiencies.9 Bevacizumab and other anti-VEGF agents may also increase the risk of bleeding.
Cerebral venous sinus thrombosis
Cancer can cause hypercoagulability, and, as a result, patients may develop thrombi. In cerebral venous sinus thrombosis, headaches are typically of gradual onset with pain located along the vertex and can be associated with increased intracranial pressure. Focal deficits can occur in the setting of venous congesting strokes, seizures, or encephalopathy. Dehydration is a risk factor for developing cerebral venous sinus thrombosis which is common in children with chronic nausea and poor oral intake. Cerebral venous sinus thrombosis may be seen following the use of L-asparaginase use and/or steroids.9
Evaluation of headaches
History and examination
The differential diagnosis for headaches is usually elicited from the history. Excellent clinical examination, including funduscopy, is essential but may be difficult in encephalopathic or young patients who are not able to fully participate.
Imaging
While MRI of the brain with and without contrast is the gold standard in evaluating intracranial pathology, non-contrast head CT is the preferred modality for detection of intracranial hemorrhage. For patients with suspected hydrocephalus, a shorter non-contrast MRI technique (called MRI hydrocephalus at our institution), may be considered especially in young or unwell patients unable to undergo full MRI. MR venogram can evaluate for cerebral venous sinus thrombosis. Anesthesia may be necessary in younger patients to obtain the highest quality imaging by minimizing motion generated artifact.
Lumbar puncture
CSF should be sampled to rule out a CNS infection or metastatic leptomeningeal disease. Neuroimaging with MR brain imaging or head CT should be performed prior to performing lumbar puncture in patients who are encephalopathic, not otherwise able to tolerate a comprehensive examination, have focal neurological findings, have known leptomeningeal disease, known brain metastasis, or a history of hydrocephalus. If neuroimaging reveals obstructive hydrocephalus, low lying cerebellar tonsils, or other signs of herniation, a lumbar puncture should not be performed. Additionally, prothrombin time/partial thromboplastin time and more importantly platelet count should be checked to rule out any contraindicating coagulopathy.
Treatment of headaches
Treatment depends upon the underlying etiology. Steroids may be helpful in cases of mass related increased intracranial pressure. Lumbar puncture related low pressure headaches may be treated with IV caffeine or a blood patch. Migraine or tension headaches can be treated with a variety of therapies for non-cancer related head pain, as long as the therapies do not compromise ongoing tumor directed therapy (for example avoid the use of enzyme inducers which may alter the metabolism of certain chemotherapies).
Seizures
Seizures are a common complication in children with primary brain tumors.10 Approximately half of all children with a structural lesion develop epilepsy.5 However, even in the absence of a structural lesion, 8-10% of children with hematologic cancers and 10% of children post bone marrow transplant can develop seizures with one-third progressing to epilepsy.11 An increase in seizure frequency, regardless of known intracranial pathology, can be due to a single or any multitude of provoking etiologies (see Table 3).12 Busulfan, used as a myeloablative conditioning agent in children undergoing hematopoietic stem cell transplantation may lower seizure threshold13. Chimeric antigen receptor T-cell therapy, a promising new treatment for certain leukemias and other malignancies, may also be neurotoxic and result in intractable seizures.14 Levetiracetam is used as prophylaxis in patients at high risk for seizures.
Table 3.
Conditions that can provoke seizures.
| Change in intracranial pathology | Infections in the immunocompromised | Chemotherapy/other treatments | Antibiotics | Medication side effects | Antiepileptic drug (AED) treatment failure |
|---|---|---|---|---|---|
| Progression of disease/recurrence of disease | Meningitis | Busulfan | Imipenem Cefepime | Metabolic/electrolyte derangements, particularly in sodium, magnesium, and calcium | Drug-drug interactions with antiepileptics |
| Intracranial process affecting the hypothalamic-pituitary axis or systemic tumor excretion of antidiuretic hormone | Encephalitis, particularly with HSV | Cyclosporine Ifosfamide | Penicillin Cephalosporins | Hepatic and/ or renal impairment accelerating toxic-metabolic disorders or decreasing drug clearance | Competition for protein binding changes serum antiepileptic drug levels |
| Surgical resection(s) | Intracranial abscess formation | Intrathecal cytarabine | Critical drug-drug interactions with AEDs | ||
| PRES | CAR T-cell therapy | Neurotoxicity | |||
| Radiation therapy induced edema or pseudoprogression | Combination of intrathecal liposomal cytarabine and high dose methotrexate | Elevation in blood pressure | |||
| Intracranial hemorrhage | L-asparaginase | ||||
| Stroke, venous congestion stroke | Intrathecal methotrexate |
While convulsive or tonic-clonic seizures are more overtly identifiable, patients in nonconvulsive or subclinical status epilepticus have frequent or continuous electrographic seizures without loss of consciousness or convulsions.12 Clinical findings can include any of the following: agitation or other mood disturbance, disruptive behavior, mutism, staring, oral automatisms, hallucinations, or lethargy.
Posterior reversible encephalopathy syndrome (PRES)
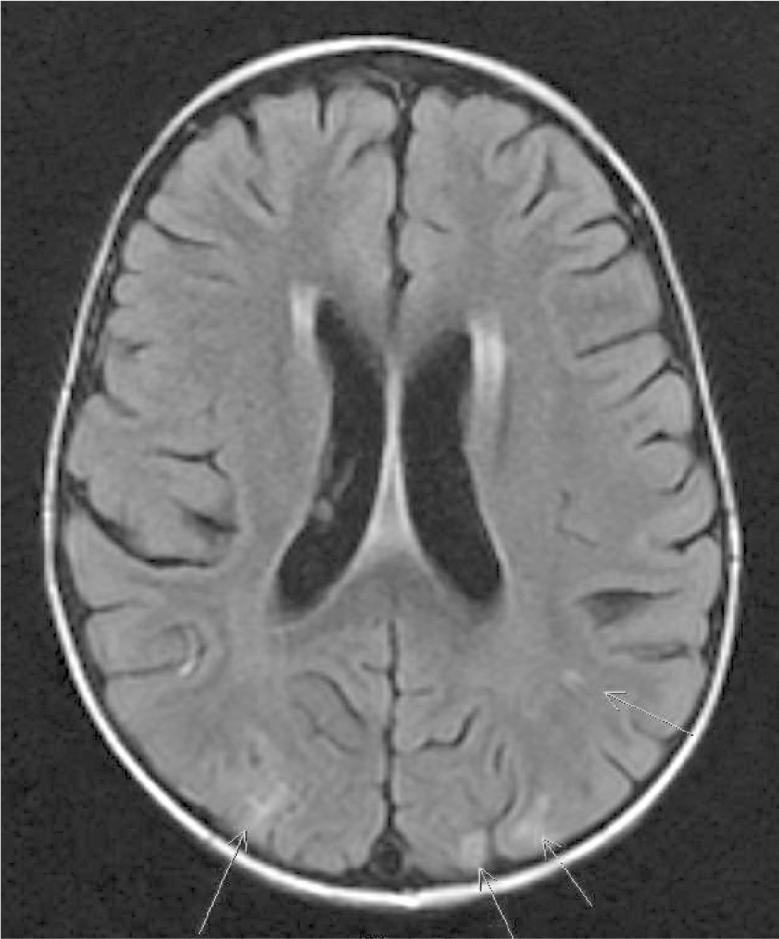
PRES is seen primarily in cases of hypertension. Clinically patients develop headaches, seizures, changes in vision, and altered mentation.15 Radiographically, imaging reveals edematous changes visible on fluid-attenuated inversion recovery and diffusion weighted imaging sequences primarily in the posterior regions of the brain (Figure 1). While a majority of children will be hypertensive for their age at the time of diagnosis, an acute increase in systolic blood pressure is not necessary for the development of PRES in children who are also receiving cancer-directed treatment.16 Chemotherapeutic agents may cause endothelial dysfunction with subsequent disruption of the blood-brain barrier and, as such, result in a loss of the protective autonomic vasoconstrictive ability seen primarily in the posterior circulation of the brain.17
Figure 1.
MRI of the brain fluid-attenuated inversion recovery sequences from a child with posterior reversible encephalopathy syndrome. Arrows indicate common T2 fluid-attenuated inversion recovery-hyperintensities indicating focal areas of edema.
Consequently, this syndrome may be more prevalent not only because of increased awareness, but also because of a vast array of immunomodulating and more potent chemotherapies. Monoclonal antibodies, including rituximab and bevacizumab, as well as immunosuppressive agents, including cyclosporine, tacrolimus, and high-dose corticosteroid therapy, may cause PRES.17 PRES is most often observed in the setting of IV and IT methotrexate administration during induction in patients with acute lymphoblastic leukemia but other agents have also been implicated.15 PRES has also been reported in patients with neuroblastoma receiving anti-GD2 monoclonal antibody therapy.18
Treatment includes anti-epileptic drugs administration, cessation of the offending drug, reduction in blood pressure, and monitoring for seizures. Recently dextromethorphan has been used to decrease methotrexate neurotoxicity in some patients.19 Neurological deficits, particularly vision loss, may not be reversible.
Evaluation of seizures
Blood work should be ordered to identify a toxic-metabolic etiology including toxicology screen and metabolic panel to look for derangements of sodium, magnesium and calcium. Intracranial disease may disrupt the hypothalamic-pituitary axis, in which case an endocrinology consult is advised. Since systemic infections decrease the seizure threshold, blood cultures, urinalysis, urine culture, and a chest x-ray should be considered. Brain MRI and a lumbar puncture in this patient population are strongly recommended.
Routine and/or continuous video EEG may help characterize seizures, as well as, identify non-convulsive status epilepticus in patients who have persistently altered mental status after an initial seizure. Additionally, continuous video EEG is helpful in assessing the efficacy of antiepileptic treatment. Finally, other diagnoses, such as metabolic-induced encephalopathy, can be made based on characteristic EEG waveform morphologies.
Treatment of seizures
Treatment of underlying etiology of seizures
Correction of electrolyte disturbances, treatment of infections, evacuation of hemorrhage, and reduction of hypertension in the case of PRES may treat seizures. In some cases anti-epileptics may be required transiently while the underlying etiology is treated. Careful selection of anti-epileptic drugs is critical.10 Enzyme inducing anti-epileptic drugs may alter metabolism of chemotherapeutic agents, resulting in reduced efficacy or excessive toxicity. Valproic acid, which also has potential antitumor effect as a histone deacetylase inhibitor, may be used in patients over age 2 years with adequate hepatic function.20 Anti-epileptic drugs that are renally excreted, like levetiracetam, have few drug-drug interactions. Antiepileptic medications can exacerbate symptoms of fatigue, poor concentration, lethargy, and confusion, so slow up-titration is recommended to improve compliance. In particular, levetiracetam may cause behavioral disturbances which can sometimes be treated with pyridoxine.
Surgery
Tumor resection can abate seizures, and, in some children who have refractory epilepsy, surgery should be considered.
Altered mental status
Encephalopathy in children with cancer may occur because of new or worsening intracranial disease, PRES, seizures, and hydrocephalus. Other etiologies are discussed below.
Medications/therapies
Medications may act centrally or may induce toxic-metabolic derangements causing a secondary change in mental status. For example, intrathecal methotrexate can result in leukoencephalopathy while radiation somnolence syndrome is a well-recognized but poorly understood acute side effect of radiation therapy.21 Ifosfamide infusion may result in depressed consciousness and/or seizures. Treatments for ifosfamide toxicity include slowing down infusion rate, IV methylene blue, and thiamine.22
Wernicke's encephalopathy
Wernicke's encephalopathy is an important, treatable neurologic complication seen in oncology patients who may have compromised nutritional status. Dietary intake of thiamine is reduced due to poor oral intake in the setting of chronic anorexia and vomiting.16 MRI brain findings include increased signal intensity surrounding the third ventricle and periductal gray matter with fluid-attenuated inversion recovery changes in the thalamus. Nystagmus and abnormalities in extra-ocular movements may be observed. Pre-treatment thiamine level should be drawn, and then treatment with IV thiamine should begin.
Hyponatremia
Hyponatremia is a known complication of neurosurgical procedures and is associated with high morbidity.23 In a retrospective cohort study of children age 0 to 19 years who underwent aneurosurgical operation for an intracranial tumor, hyponatremia during admission occurred in 12% of patients. Low sodium was most commonly seen in younger children and those with obstructive hydrocephalus. This metabolic derangement resulted in altered mental status (41%) and seizures (21%). Overall, hyponatremia was independently associated with worse neurological outcome when adjusted for age and tumor factors.
Opsoclonus-myoclonus-ataxia syndrome
Opsoclonus-myoclonus-ataxia, a paraneoplastic syndrome is comprised of rapid, irregular, conjugate eye movements, myoclonus, rage attacks, and ataxia. Opsoclonus-myoclonus-ataxia occurs in 2-3% of patients with neuroblastoma, often preceding the diagnosis24. Meta-iodo-benzyl-guanidine imaging reveals neuroblastoma lesions in the adrenal gland or anywhere along the sympathetic chain. Patients with Opsoclonus-myoclonus-ataxia usually has favorable histology associated with low stage neuroblastoma, and surgery alone may be curative.25 CSF evaluation reveals B-cell clones,26 though lumbar puncture should be avoided in patients with bone marrow disease for fear of seeding the CSF.27 Unlike other paraneoplastic syndromes, no specific antibody has been identified in this syndrome but recently neurofilament light chain was noted in the CSF of patients with Opsoclonus-myoclonus-ataxia.28
Tumor resection may transiently increase symptoms, so administration of IVIG perioperatively is recommended. The mainstay of therapy is immunomodulation which may include: monthly intravenous immunoglobulin (1gm/kg/dose), intramuscular adrenocorticotropic hormone at 75 IU/m2/day, rituximab (or ofatumumab) at 375 mg/m2/week for 4 consecutive weeks,29 and/or dexamethasone pulse dosing.30 Patients with Opsoclonus-myoclonus-ataxia should not receive immunizations, as any stimulus to the immune system may exacerbate symptoms. Motor deficits typically resolve, but moderate cognitive and behavioral impairments tend to persist31.
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis
Another paraneoplastic syndrome, anti-NMDAR encephalitis results from immune-meditated production of antibodies to the NR1 subunit of the NMDA receptor. In adults, these antibodies are associated with ovarian teratomas and less commonly with small-cell lung cancer, Hodgkin's lymphoma, NB, breast cancer, and germ-cell tumor of the testes. The majority of pediatric patients, however, have no identifiable tumor.
A prodromal phase can include fever, headache, upper respiratory symptoms, nausea, vomiting, and diarrhea. Psychological changes are the prominent presenting symptom and include amnesia, personality changes, and hallucinations, which rapidly evolve into psychosis and bizarre behavior followed by a diminished level of consciousness. Patients develop catalepsy-like symptoms with dystonia and opisthotonus. Autonomic manifestations including cardiac arrhythmias, hyperthermia, hypertension, and central hypoventilation may be seen in children but are more common in adults. Children more commonly have enuresis and hypersomnia. Neurologic sequelae include refractory seizures, as well as aphasia and dyskinesias of the orofacial muscles, limbs, and trunk.
MR of the brain can be normal early on but later fluid-attenuated inversion recovery hyperintensity, primarily in the medial temporal lobes, but also in the cerebral or cerebellar cortex, can be seen. Initially EEG reveals diffuse delta activity with paroxysmal discharges, also known as an extreme delta brush pattern.32 Lymphocytic pleocytosis with or without an elevation in protein is seen in the CSF; serum and CSF reveal anti-N-methyd-D-Aspartate receptor antibodies, the defining feature of this condition. Treatment includes appropriate body imaging to identify a tumor. In pediatric patients if no tumor is identified, emphasis should be placed on treatment. Therapeutic interventions include pulse dosing of methylprednisolone and IVIG or plasmapheresis. Second line therapy includes rituximab and/or cyclophosphamide. Complete recovery is possible over months to years, but symptom relapses may occur. In this case, patients should be re-evaluated for the presence of tumor. 33
Posterior fossa syndrome
The majority of pediatric brain tumors are located in the posterior fossa. Posterior fossa syndrome (also known as cerebellar mutism), develops in 25-40% of patients who undergo surgical resection of these lesions.34 Clinically, patients present with varying degrees of mutism, hypotonia, pseudobulbar dysfunction, agitation, ataxia, inattention, vomiting, and incontinence typically within 12 to 48 hours of surgery.34 While the localization and cause of posterior fossa syndrome remains elusive, recent imaging studies including diffusion tensor imaging, suggest that injury to the bilateral superior cerebellar peduncles, particularly higher in the fourth ventricle, is associated with a greater risk of developing posterior fossa syndrome.34,35 In patients with severe posterior fossa syndrome, 66% continued to have language dysfunction at 1 year. Recovery from physical effects as well as emotional lability may take days to months and some patients may have persistent deficits for years.34,36 Zolpidem may improve speech in some patients with posterior fossa syndrome. 37
Evaluation for altered mental status
Standard work-up should include a complete blood count, renal panel, liver function tests, vitamin B1, vitamin B2, vitamin B12, thyroid studies, blood culture, urine toxicology and cultures. CSF studies should include; cell count, protein, glucose, and, if indicated, oligoclonal bands, bacterial and viral studies, cytology, flow cytometry, and paraneoplastic panel. Finally, neuro-imaging and EEG may be helpful.
Back Pain
The prevalence of back pain in adolescence is approximately 30%.38 Signs and symptoms suggesting more serious causes of back pain include: pain in children younger than age 11 years; constant pain, lasting weeks; pain that occurs spontaneously, at rest, or when lying supine; pain that interferes with activities of daily living; pain associated with limitation of movement; pain associated with neurological deficit; and pain in children diagnosed with cancer (Table 4). Of note, methotrexate or cytarabine-associated acute transverse myelitis presents as weakness and back pain starting 48 hours after infusion, but may be delayed for up to 2 weeks.
Table 4.
Etiologies of back pain resulting from spinal pathology
| Common pediatric cancers associated with spinal pathology | Less common pediatric cancers associated with spinal pathology | Treatment-induced spinal pathology | Other etiologies to consider |
|---|---|---|---|
| Lymphoma | Metastatic disease from solid tumors | Radiation-induced myelopathy | Osteomyelitis |
| Disseminated neuroblastoma | Leukemic expansion of the vertebrae | Radiation-induced meningioma | Spinal abscess |
| Ewing's sarcoma | Leptomeningeal disease | Granulocyte colony-stimulating factor agents causing diffuse bone pain | Post lumbar puncture epidural hematoma |
| Intrathecal chemotherapy-induced arachnoiditis | Referred pain from bowel obstruction | ||
| Methotrexate-associated acute transverse myelitis | Retroperitoneal tumor | ||
| Cytarabine-associated acute transverse myelitis | Pleural effusion | ||
| Long-term, high dose steroid induced epidural lipomatosis | Avascular necrosis of the hips in the setting of chronic steroid use | ||
| Musculoskeletal pain |
Evaluation of back pain
Back pain is more frequently observed in adult cancer patients, as the most common malignancies in adults (breast, lung, and prostate) can present with bony metastases. Nevertheless, pediatric cancer patients with back pain should receive a thorough workup to preemptively identify any lesions that could result in spinal cord compression, spinal instability, or cauda equina syndrome. The patient should be asked about constipation, urinary retention, and, if applicable, erectile dysfunction. Examination should include testing for Lhermitte's sign, straight leg raise, and evaluation for focal spine tenderness; neurologic exam should test strength, sensory level, reflexes, gait, and rectal tone (if patient is not neutropenic). MRI with and without contrast of the total spine is the preferred method of evaluating the complaint of back pain.
Treatment
Aggressive treatment at time of presentation should be pursued because of the high likelihood that neurological function may be restored. In fact, a majority of children have a relatively good prognosis with near full recovery after intervention for spinal cord compression. Treatment is variable but typically begins with high dose corticosteroids unless the spinal mass is the initial manifestation of cancer; steroids may completely abolish an as yet undiagnosed lymphoma, which cannot then be further characterized. Depending upon the type of cancer, chemotherapy, radiation, and/or surgery should be considered.
Sensory/neuromuscular junction/muscular complications
Peripheral neuropathy
Chemotherapy induced peripheral neuropathy is a well recognized syndrome which typically causes weakness and sensory loss distally39. Severity of chemotherapy induced peripheral neuropathy depends upon the dose and duration of treatment40. Diminished reflexes are usually the initial sign of neuropathy followed by dysesthesia/paresthesias in the feet then the hands. Loss of proprioception and vibration may also occur. Sensory disturbance may accompany motor weakness or be independent. Weakness can be demonstrated peripherally by wrist or foot drop but can also manifest as diplopia due to cranial neuropathies. In children, pain may be the first indication of a neuropathy. Chemotherapy induced peripheral neuropathy can improve with dose reduction or cessation of the offending medication, but the symptoms of neuropathy may continue regardless of intervention40. Agents that typically cause chemotherapy induced peripheral neuropathy include: vincristine, thalidomide, platins, taxanes, and bortezomib. Antibiotics like voriconazole may also cause neuropathy. Malnutrition associated vitamin B12 deficiency can contribute to the neuropathy. Current American Society of Clinical Oncology guidelines do not recommend any agents for use in prevention of chemotherapy induced peripheral neuropathy. Pressure palsies in the setting of cachexia should be considered, particularly in an isolated ulnar or peroneal neuropathy.41
Neuromuscular junction
Myasthenia gravis has been reported in patients who develop severe graft versus host disease after bone marrow transplant.42 Lambert–Eaton Myasthenic Syndrome has been reported in children diagnosed with neuroblastoma24.
Myopathy
Long-term steroid use may induce myopathy which manifests as proximal weakness which improves following a steroid taper. Critical illness myopathy can be seen in intensive care unit patients.
Evaluation of weakness and sensory changes
Neurologic examination and medication review can help distinguish among peripheral nerve, neuromuscular junction and muscle pathology. In some cases a Tensilon test, electromyography, and nerve conduction studies may help support the diagnosis.
Treatment
Treatment usually consists of dose reducing or completely eliminating the offending agent. Dysesthesia may be relieved long-term with gabapentin and pregabalin, or acutely with methadone. Neuromuscular junction disturbances may be treated with immunosuppressives and/or pyridostigmine. Physical and occupational therapy as well as ankle foot orthotics may help with weakness.
Other complications
Visual Complaints
Visual dysfunction can be the direct or indirect result of cancer and/or related treatments. Tumors, tumor-associated edema, paraneoplastic syndromes, and leptomeningeal disease, among other insults located at any point along the optic pathway from the retina/optic nerve to the occipital lobe, can result in decreased visual acuity or vision loss, whereas involvement of cranial III, IV, VI nuclei, their nerves, or the innervating extra-ocular muscles can result in diplopia. Increased intracranial pressure can result in bitemporal hemianopsia or diplopia. Medication side effects of opioids, steroids, antiepileptic medications, or chemotherapy can also cause visual disturbances. Evaluation by a neuro-ophthalmologist is recommended for a dilated funduscopic examination, formal visual field testing and/or optical computed tomography if indicated.
Hearing Loss
Acute hearing loss can be associated with cisplatin and carboplatin use as well as cranial external beam radiation therapy. Vitamin E may be protective.43
Conclusion
No neurological deficit in the pediatric ononcology patient is trivial and the threshold for a comprehensive evaluation should remain low. Importantly, a normal neurological examination in the setting of new subjective neurologic complaints should not automatically exclude underlying pathology. The oncology team evaluating these patients should possess a good understanding of the effects of cancer and treatments on the nervous system of pediatric patients. The spectrum of neurologic complications will continue to evolve alongside the advent of new, more targeted treatments for pediatric cancers.
Acknowledgements
We would like to thank Judy Lampron, Neurology editor, and Joe Olechnowicz, Pediatrics editor, at Memorial Sloan Kettering Cancer Center for editorial assistance.
Funding: Funding for this study was provided by: Maureen's Hoops for Hope and the MSKCC Summer Medical Student Fellowship Program sponsored by an NCI Cancer Education grant (R25) and NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest
The Authors declare that there is no conflict of interest.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-oncology. 2014 Oct;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeAngelis LM, Posner JB, Posner JB. Neurologic complications of cancer. 2nd ed. Oxford University Press; Oxford ; New York: 2009. [Google Scholar]
- 3.Roddy E, Mueller S. Late Effects of Treatment of Pediatric Central Nervous System Tumors. Journal of child neurology. 2015 Jun 4; doi: 10.1177/0883073815587944. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Arafeh I, Macleod S. Serious neurological disorders in children with chronic headache. Archives of disease in childhood. 2005 Sep;90(9):937–940. doi: 10.1136/adc.2004.067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullrich NJ. Neurologic Sequelae of Brain Tumors in Children. Journal of Child Neurology. 2009 Nov 1;24(11):1446–1454. doi: 10.1177/0883073809342491. 2009. [DOI] [PubMed] [Google Scholar]
- 6.McCrea N, Howells R. Fifteen minute consultation: headache in children under 5 years of age. Archives of disease in childhood. Education and practice edition. 2013 Oct;98(5):181–185. doi: 10.1136/archdischild-2012-303529. [DOI] [PubMed] [Google Scholar]
- 7.Nallasamy K, Singhi SC, Singhi P. Approach to headache in emergency department. Indian journal of pediatrics. 2012 Mar;79(3):376–380. doi: 10.1007/s12098-011-0570-2. [DOI] [PubMed] [Google Scholar]
- 8.Antunes NL. Acute neurologic complications in children with systemic cancer. Journal of child neurology. 2000 Nov;15(11):705–716. doi: 10.1177/088307380001501101. [DOI] [PubMed] [Google Scholar]
- 9.Ranta S, Tuckuviene R, Makipernaa A, et al. Cerebral sinus venous thromboses in children with acute lymphoblastic leukaemia - a multicentre study from the Nordic Society of Paediatric Haematology and Oncology. British journal of haematology. 2015 Feb;168(4):547–552. doi: 10.1111/bjh.13162. [DOI] [PubMed] [Google Scholar]
- 10.Wells EM, Gaillard WD, Packer RJ. Pediatric brain tumors and epilepsy. Seminars in pediatric neurology. 2012 Mar;19(1):3–8. doi: 10.1016/j.spen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Khan RBHD, Boop FA, Sanford RA, Merchant TE, Gajjar A, Kun LE. Seizures in children with primary brain tumors: Incidence and long-term outcome. Epilepsy Research. 2005;64:85–91. doi: 10.1016/j.eplepsyres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Ekici A, Yakut A, Kural N, Bor O, Yimenicioglu S, Carman KB. Nonconvulsive status epilepticus due to drug induced neurotoxicity in chronically ill children. Brain & development. 2012 Nov;34(10):824–828. doi: 10.1016/j.braindev.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Soni S, Skeens M, Termuhlen AM, Bajwa RP, Gross TG, Pai V. Levetiracetam for busulfan-induced seizure prophylaxis in children undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2012 Oct;59(4):762–764. doi: 10.1002/pbc.24126. [DOI] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Feb 20;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Im SA, Lee JW, et al. Predisposing factors of posterior reversible encephalopathy syndrome in acute childhood leukemia. Pediatric neurology. 2012 Dec;47(6):436–442. doi: 10.1016/j.pediatrneurol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Lim YJ, Kim HJ, Lee YJ, Seol IJ, Lee YH. Clinical features of encephalopathy in children with cancer requiring cranial magnetic resonance imaging. Pediatric neurology. 2011 Jun;44(6):433–438. doi: 10.1016/j.pediatrneurol.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Won SC, Kwon SY, Han JW, Choi SY, Lyu CJ. Posterior reversible encephalopathy syndrome in childhood with hematologic/oncologic diseases. J Pediatr Hematol Oncol. 2009 Jul;31(7):505–508. doi: 10.1097/MPH.0b013e3181a71868. [DOI] [PubMed] [Google Scholar]
- 18.Kushner BH, Modak S, Basu EM, Roberts SS, Kramer K, Cheung NK. Posterior reversible encephalopathy syndrome in neuroblastoma patients receiving anti-GD2 3F8 monoclonal antibody. Cancer. 2013 Aug 1;119(15):2789–2795. doi: 10.1002/cncr.28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afshar M, Birnbaum D, Golden C. Review of dextromethorphan administration in 18 patients with subacute methotrexate central nervous system toxicity. Pediatric neurology. 2014 Jun;50(6):625–629. doi: 10.1016/j.pediatrneurol.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhof M, Dielemans JCM, van Breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro-Oncology. 2013 Jul 1;15(7):961–967. doi: 10.1093/neuonc/not057. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballesteros-Zebadua P, Chavarria A, Celis MA, Paz C, Franco-Perez J. Radiation-induced neuroinflammation and radiation somnolence syndrome. CNS & neurological disorders drug targets. 2012 Nov 1;11(7):937–949. doi: 10.2174/1871527311201070937. [DOI] [PubMed] [Google Scholar]
- 22.Di Cataldo A, Astuto M, Rizzo G, Bertuna G, Russo G, Incorpora G. Neurotoxicity during ifosfamide treatment in children. Medical science monitor : international medical journal of experimental and clinical research. 2009 Jan;15(1):Cs22–25. [PubMed] [Google Scholar]
- 23.Williams CN, Belzer JS, Riva-Cambrin J, Presson AP, Bratton SL. The incidence of postoperative hyponatremia and associated neurological sequelae in children with intracranial neoplasms. Journal of neurosurgery. Pediatrics. 2014 Mar;13(3):283–290. doi: 10.3171/2013.12.PEDS13364. [DOI] [PubMed] [Google Scholar]
- 24.Khasraw M, Khakoo Y. Swaiman's Pediatric Neurology Principles and Practice. 2012:1388–1395. [Google Scholar]
- 25.Rudnick E, Khakoo Y, Antunes NL, et al. Opsoclonus-myoclonus-ataxia syndrome in neuroblastoma: clinical outcome and antineuronal antibodies-a report from the Children's Cancer Group Study. Med Pediatr Oncol. 2001 Jun;36(6):612–622. doi: 10.1002/mpo.1138. [DOI] [PubMed] [Google Scholar]
- 26.Pranzatelli MR, Tate ED, Swan JA, et al. B cell depletion therapy for new-onset opsoclonus-myoclonus. Movement Disorders. 2009;9999(9999):NA. doi: 10.1002/mds.22941. [DOI] [PubMed] [Google Scholar]
- 27.Kramer K, Kushner B, Heller G, Cheung N-KV. Neuroblastoma metastatic to the central nervous system. Cancer. 2001;91(8):1510–1519. [PubMed] [Google Scholar]
- 28.Pranzatelli MR, Tate ED, McGee NR, Verhulst SJ. CSF neurofilament light chain is elevated in OMS (decreasing with immunotherapy) and other pediatric neuroinflammatory disorders. Journal of Neuroimmunology. 2014;266(1–2):75–81. doi: 10.1016/j.jneuroim.2013.11.004. 1/15/ [DOI] [PubMed] [Google Scholar]
- 29.Pranzatelli MR, Tate ED, Shenoy S, Travelstead AL. Ofatumumab for a rituximab-allergic child with chronic-relapsing paraneoplastic opsoclonus–myoclonus. Pediatric Blood & Cancer. 2011:n/a–n/a. doi: 10.1002/pbc.23187. [DOI] [PubMed] [Google Scholar]
- 30.Rostasy K, Wilken B, Baumann M, et al. High dose pulsatile dexamethasone therapy in children with opsoclonus-myoclonus syndrome. Neuropediatrics. 2006 Oct;37(5):291–295. doi: 10.1055/s-2006-955931. [DOI] [PubMed] [Google Scholar]
- 31.Catsman-Berrevoets CE, Aarsen FK, van Hemsbergen ML, van Noesel MM, Hakvoort-Cammel FG, van den Heuvel-Eibrink MM. Improvement of neurological status and quality of life in children with opsoclonus myoclonus syndrome at long-term follow-up. Pediatr Blood Cancer. 2009 Dec;53(6):1048–1053. doi: 10.1002/pbc.22226. [DOI] [PubMed] [Google Scholar]
- 32.Nosadini M, Boniver C, Zuliani L, et al. Longitudinal Electroencephalographic (EEG) Findings in Pediatric Anti-N-Methyl-d-Aspartate (Anti-NMDA) Receptor Encephalitis: The Padua Experience. J Child Neurol. 2015 Feb;30(2):238–245. doi: 10.1177/0883073813515947. [DOI] [PubMed] [Google Scholar]
- 33.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009 Jul;66(1):11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006 Dec;105(6 Suppl):444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 35.Ojemann J, Partridge S, Poliakov A, et al. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst. 2013;29(11):2071–2077. doi: 10.1007/s00381-013-2205-6. 2013/11/01. [DOI] [PubMed] [Google Scholar]
- 36.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex. 2009 Oct 29; doi: 10.1016/j.cortex.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Shyu C, Burke K, Souweidane MM, et al. Novel use of zolpidem in cerebellar mutism syndrome. J Pediatr Hematol Oncol. 2011 Mar;33(2):148–149. doi: 10.1097/MPH.0b013e3182053a1a. [DOI] [PubMed] [Google Scholar]
- 38.Antunes NL. Back and neck pain in children with cancer. Pediatric neurology. 2002 Jul;27(1):46–48. doi: 10.1016/s0887-8994(02)00389-2. [DOI] [PubMed] [Google Scholar]
- 39.Ozyurek H, Turker H, Akbalik M, Bayrak AO, Ince H, Duru F. Pyridoxine and pyridostigmine treatment in vincristine-induced neuropathy. Pediatric hematology and oncology. 2007 Sep;24(6):447–452. doi: 10.1080/08880010701451327. [DOI] [PubMed] [Google Scholar]
- 40.Lee EQ, Arrillaga-Romany IC, Wen PY. Neurologic complications of cancer drug therapies. Continuum (Minneapolis, Minn.) 2012 Apr;18(2):355–365. doi: 10.1212/01.CON.0000413663.42798.64. [DOI] [PubMed] [Google Scholar]
- 41.Antunes NL. The spectrum of neurologic disease in children with systemic cancer. Pediatric neurology. 2001 Sep;25(3):227–235. doi: 10.1016/s0887-8994(01)00313-7. [DOI] [PubMed] [Google Scholar]
- 42.Unal S, Sag E, Kuskonmaz B, et al. Successful treatment of severe myasthenia gravis developed after allogeneic hematopoietic stem cell transplantation with plasma exchange and rituximab. Pediatr Blood Cancer. 2014 May;61(5):928–930. doi: 10.1002/pbc.24799. [DOI] [PubMed] [Google Scholar]
- 43.Pace A, Giannarelli D, Galie E, et al. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology. 2010 Mar 2;74(9):762–766. doi: 10.1212/WNL.0b013e3181d5279e. [DOI] [PubMed] [Google Scholar]
- 44.Lay CS-EC. Brain tumor headache. UpToDate. 2014 [Google Scholar]