Abstract
Proteins are not equally digestible—their proteolytic susceptibility varies by their source and processing method. Incomplete digestion increases colonic microbial protein fermentation (putrefaction), which produces toxic metabolites that can induce inflammation in vitro and have been associated with inflammation in vivo. Individual humans differ in protein digestive capacity based on phenotypes, particularly disease states. To avoid putrefaction-induced intestinal inflammation, protein sources and processing methods must be tailored to the consumer’s digestive capacity. This review explores how food processing techniques alter protein digestibility and examines how physiological conditions alter digestive capacity. Possible solutions to improving digestive function or matching low digestive capacity with more digestible protein sources are explored. Beyond the ileal digestibility measurements of protein digestibility, less invasive, quicker and cheaper techniques for monitoring the extent of protein digestion and fermentation are needed to personalize protein nourishment. Biomarkers of protein digestive capacity and efficiency can be identified with the toolsets of peptidomics, metabolomics, microbial sequencing and multiplexed protein analysis of fecal and urine samples. By monitoring individual protein digestive function, the protein component of diets can be tailored via protein source and processing selection to match individual needs to minimize colonic putrefaction and, thus, optimize gut health.
Keywords: Digestibility, supplementation, pre-digestion, protease, putrefaction
INTRODUCTION
The majority of dietary proteins are fully degraded and absorbed in the small intestine: after a meal, proteins are denatured by acid and hydrolyzed by gastric pepsin in the stomach, further hydrolyzed by pancreatic proteases, subsequently degraded by small intestinal enterocyte membrane exopeptidases and absorbed across the small intestinal enterocytes into the bloodstream as individual AA for use in the body. However, proteins are not always digested in this way. High levels of protein in the diet lead to more protein surviving past the small intestine and into the large intestine. Some proteins are not easily digested (either inherently based on their source or due to processing conditions) and can survive intact or partially intact to the colon. Likewise, some humans have low proteolytic capacity, which also increases the survival of intact or partially intact proteins to the colon. Proteins that reach the colon no longer serve as a direct AA supply to the host: the colon does not secrete digestive proteases to break them down and contributes little to net AA absorption (Moughan, 2003; Fuller and Tomé, 2005). Therefore, any protein that arrives to the colon represents incomplete protein utilization.
Some of the protein arriving in the colon serves as an AA source for colonic microbes. Some of the protein serves as an energy source for resident protein fermentation (putrefaction)-capable microbial species such as Clostridium perfringens, Desulfovibrio, Peptostreptococcus, Acidaminococcus, Veillonella, Propionibacterium, Bacillus, Bacteroides and Staphylococcus (Macfarlane et al., 1986; Shen et al., 2010; An et al., 2014).
Increasing protein in the colon correlates with increased putrefactive bacteria and metabolites (Toden et al., 2007; Lubbs et al., 2009) and reduced carbohydrate-fermenting bacteria such as Eubacterium rectale, Roseburia spp. and Bifidobacterium spp. (Duncan et al., 2007). Unlike carbohydrate-based fiber fermentation in the colon, which is considered beneficial or benign, microbial protein putrefaction could be detrimental (Davila et al., 2013). In fermenting fiber, commensal microbes produce beneficial metabolites, including short-chain fatty acids (e.g., butyrate, which serves as the primary energy source for the colonic epithelium (Roediger, 1980; Roediger, 1982; Hamer et al., 2008)) and certain vitamins (LeBlanc et al., 2013). Beyond serving as an energy source, the short-chain fatty acids produced also lower the intraluminal pH, which inhibits the growth of some pathogens (Byrne and Dankert, 1979). Like fiber fermentation, putrefaction leads to some short-chain fatty acid production. However, unlike fiber fermentation, putrefactive bacteria also produce an array of metabolic byproducts including ammonia, sulfides, phenols (e.g. p-cresol), indoles and biogenic amines (Windey et al., 2012; Rist et al., 2013), which can, in vitro, reduce colonic epithelial cell viability (Pedersen et al., 2002), increase intestinal permeability (Ng and Tonzetich, 1984; Jowett et al., 2004; Hughes et al., 2008; McCall et al., 2009), provoke DNA damage (Attene-Ramos et al., 2006) and inhibit colonocyte cellular respiration and proliferation (Roediger et al., 1993; Leschelle et al., 2005; Medani et al., 2011; Andriamihaja et al., 2015). Increasing protein intake by humans from 15.4% to 23.8% of the diet for one week, while maintaining resistant carbohydrate intake, increased fecal and urinary markers of putrefaction—including ammonia, valeric acid, urinary p-cresol and volatile sulfur-containing metabolites (Geypens et al., 1997). Other studies also demonstrate that increased dietary protein correlates with increased ammonia production and other putrefactive products in the colon (Gibson et al., 1976; Macfarlane et al., 1986; Richardson et al., 2013). Increased putrefaction in the distal gut increases putrefactive metabolite production, which can be toxic to colonocytes (Cummings et al., 1979) and can induce inflammation (Lan et al., 2015). Indeed, prospective human and animal studies demonstrate that excess protein in the diet can lead to damage in the colon (increased relapse risk in ulcerative colitis in humans (Jowett et al., 2004), increased colonic DNA damage and thinned colonic mucus barrier in rats, depending on protein type (Toden et al., 2007), reduced brush border membrane height in rats (Andriamihaja et al., 2010), decreased telomere length and increased DNA breaks in colonic cells of rats fed red meat, which were reversed with addition of resistant starch (Toden et al., 2007; Toden et al., 2007; O’Callaghan et al., 2012) and increased risk of post-weaning diarrhea in piglets (Heo et al., 2009; Kim et al., 2011)). Though more in vivo studies are needed, the data suggests that protein overconsumption may interfere with optimal gut health, especially in individuals with lowered protein digestion capacity. Understanding the consequences of high protein diets on both healthy individuals and those with various disease states is necessary.
Whether protein reaching the colon and putrefaction causes damage likely depends on the amount of undigested protein and amount of putrefactive metabolites produced. The presence of low levels of putrefactive compounds is unlikely to be harmful—indeed, ammonia production has an important role in nitrogen recycling (Blachier et al., 2007; Bergen and Wu, 2009). Likewise, polyamine compounds, including putrescine and spermidine, are important for cellular growth and proliferation and are synthesized in cells but can also be absorbed from the gut lumen (Osborne and Seidel, 1990; Bardócz et al., 1998). Rather, it is when these levels become excessive that gut health may be affected. However, the concentrations of putrefactive compounds required for negatively impacting gut health in vivo remain unknown. Some protein enters the colon continually from healthy people consuming low protein diets. Endogenous proteins, including enzymes and proteins from sloughed off cells (e.g., mucins), enter the colon daily (Moughan et al., 2005). Between endogenous protein and dietary protein, approximately 12–18 g enter the colon each day, of which the majority come from proteins (50%) and peptides (20–30%) (Chacko and Cummings, 1988). The precise amount of additional dietary protein surviving to the large intestine to cause inflammation in the gut remains unknown and deserves further study.
The amount of putrefaction in the colon also depends on the amount of fiber in the diet. Most starch and small sugars are absorbed in the small intestine, but fiber survives to the colon, where it can serve as a carbon source for colonic microorganisms, most of which are saccharolytic (Macfarlane and Macfarlane, 2012). As carbohydrates are the preferred carbon source for microbes in the gut, protein putrefaction arises only becomes dominant when most of the dietary fiber has been fermented (Macfarlane and Macfarlane, 2012). Therefore, putrefaction becomes more likely the more distal in the large intestine. Consequently, adding fiber to diets can reduce putrefaction. Supplementing the diet with fiber lowers apparent putrefaction in the gut (Pieper et al., 2012; Windey et al., 2012; Pieper et al., 2014). Resistant starch supplementation in rats decreased the protein fermentation markers urinary nitrogen (Heijnen and Beynen, 1997) and p-cresol (Shen et al., 2010). In humans, adding resistant starch decreased fecal ammonia, p-cresol and total phenol concentrations (Birkett et al., 1996). The addition of fiber to a high protein diet in humans reduced fecal putrefactive metabolites (ammonia and putrescine) but did not return them to levels found in a low protein diet. This incomplete reduction of putrefactive metabolites may explain why the added fiber did not lower markers of mucosal inflammation (Pieper et al., 2012). The degree to which fiber prevents high protein-induced putrefaction in the gut and the degree to which reductions in putrefactive compounds reduce gut inflammation deserves further examination.
On an individual basis, monitoring the diet for the optimal amount of protein is challenging. Therefore, tests for markers of gut putrefaction could aid individuals in controlling their daily protein intake.
Beyond the amount of protein in the diet, two factors are important for controlling colonic putrefaction—the protein’s digestibility and the consumer’s digestive capacity. Dietary proteins are not all the same—they differ in digestibility based on source and processing methods. Likewise, consumers differ in their ability to digest proteins based on disease state, age and other phenotypes. To avoid the consequences of incomplete protein digestion in the small intestine, the protein component of diets should be tailored to match specific digestive phenotypes, particularly for various disease states. This review discusses the effects of incomplete protein digestion on overall health, how protein source and processing methods lead to differences in protein digestibility and how individuals differ in their digestive capacity. The review examines potential solutions for improving protein digestion, ranging from consumption of common pre-digested foods (e.g., yogurt), to protease pre-treatments of proteins to protease and hormonal supplementation. The analytical toolsets—including peptidomics, metabolomics, microbial sequencing and protein biomarker testing—needed to determine an individual’s digestive capacity are identified. How this knowledge can be applied to design tailored protein nutrition to optimize gut health is clarified.
PROTEINS VARY IN DIGESTIBILITY
Proteins vary in digestibility based on their three-dimensional structure and chemical modifications. Digestibility differs across protein sources (e.g., plant vs. animal proteins) and processing methods (e.g., various heat treatments).
Chemical and Physical Protein Structure Influence Protein Digestibility
Dietary proteins vary greatly in their susceptibility to digestive processes in the human gut due to size, charge, AA sequence, tertiary structure and post-translational modifications, especially glycosylation (Sareneva et al., 1995; Yu et al., 2007) and phosphorylation (Sareneva et al., 1995; Yu et al., 2007; Boutrou et al., 2010; Dupont et al., 2010). Generally, globular and compact proteins like β-lactoglobulin (Reddy et al., 1988) and compact storage proteins like phaseolin (a protein found in kidney beans) (Venkatachalam and Sathe, 2003), wheat gluten (Mitea et al., 2008) and soy protein glycinin (Nielsen et al., 1988) are less susceptible to hydrolysis in the gut. Other proteins with looser structure, like native milk β-casein, are easily hydrolyzed into AA via endogenous proteases (Lindberg et al., 1997; Lindberg et al., 1998; Dupont et al., 2010; Dupont et al., 2010). Glycosylation of a protein can decrease digestibility through steric hindrance of digestive protease activity (Bernard et al., 1983; Semino et al., 1985). Likewise, phosphorylation lowers protein digestibility: compared with non-phosphorylated β-casein (1–25), the phosphorylated version is highly resistant to in vitro digestion by brush border peptidases, likely also through steric hindrance (Boutrou et al., 2010).
Variation in Protein Digestibility with Protein Source
Protein digestibility varies depending on the source, and the digestibility of animal proteins (meat and dairy proteins) is higher (typically >90%) than that of plant proteins (70–90%). In early weaned-piglets, plant sources of protein such as soybean meal were lower in digestibility compared with animal proteins such as whey and fish (Yun et al., 2005). Milk casein proteins are slightly more digestible than meat proteins (Gilbert et al., 2011). In rats fed diets with different proteins (milk casein, fish meal and soy protein), fish meal and soy protein increased fecal putrefactive metabolites (indole, phenol and hydrogen sulfide) compared with milk casein (An et al., 2014). These findings suggested that more soy and fish meal protein survived intact to the colon and increased putrefaction. These different proteins also altered the microbiome, possibly due to increased putrefaction (An et al., 2014).
Differences in protein digestibility among sources derive partially from matrix effects. Within a food source, other components alter the overall digestibility of the proteins. Some plant foods contain anti-nutritional factors that decrease protein digestibility. Legumes, cereals, potatoes and tomatoes contain inhibitors that reduce protein digestibility by blocking trypsin, pepsin and other gut proteases (Savelkoul et al., 1992; Liener, 1994; Friedman and Brandon, 2001). Cereal grains and legume seeds contain tannins (polyphenols) that bind strongly to dietary proteins and digestive enzymes, thus inhibiting protein digestion (Jansman, 1993; Jansman et al., 1994). Nuts, seeds and grains contain phytic acid (Lott et al., 2002), which chelates minerals such as calcium and zinc. As these minerals are necessary cofactors for digestive enzymes (e.g., alkaline phosphatase, carboxypeptidases and aminopeptidases), phytic acid in foods reduces overall protein digestibility (Ryden and Selvendran, 1993). Many legumes and alliums contain saponins, which form part of the plant’s defense system (Francis et al., 2002). These saponins reduce protein digestibility by forming saponin-protein complexes (Potter et al., 1993). Likewise, many plant proteins are surrounded by complex carbohydrates (non-starch polysaccharides or fiber)—often as cell wall components—that can impede enzyme access to the proteins (Duodu et al., 2003). The abundance of anti-nutritional factors and complex carbohydrates in plant protein sources likely explains their overall lower digestibility than that of typical animal proteins.
PROCESSING TECHNIQUES ALTER PROTEIN DIGESTIBILITY
Various processing techniques alter food proteins digestibility (Table 1), and thereby influence protein survival to the colon. Understanding how these processing techniques alter protein digestibility is essential for individualizing and optimizing protein nutrition. Common food processing techniques, including heat treatments, extrusion, spray drying, fermentation, homogenization, high pressure processing and enzymatic/chemical hydrolysis, can alter protein structure, anti-nutritional factors and complex carbohydrates that can in turn alter protein digestibility (Table 1).
Table 1.
Effects of food source processing on protein modifications
| Increases protein digestibility | Decreases protein digestibility | ||||||
|---|---|---|---|---|---|---|---|
| Processing technique |
Protein denaturati on |
Protein degradati on |
Deactivati on of anti- nutritional factors |
Remova l of anti- nutrition al factors |
Maillar d reactio ns |
Formati on of cross- links |
Protein aggregati on |
| Heat (lower temp, short time) |
X | X | |||||
| Heat (higher temp, longer time) |
X | X | X | ||||
| Extrusion* | X | X | X | X | X | ||
| High pressure processing |
X | X | X | ||||
| Fermentation | X | X | |||||
| Enzymatic hydrolysis |
X | X | |||||
| Soaking | X | ||||||
| Dehulling | X | ||||||
| Germination | X | X | X | ||||
| Alkaline/heat treatment (lysinoalanine) |
X | X | X | ||||
| Transglutamin ase |
X | ||||||
| Heat (lower temp, short time) |
X | X | |||||
Digestibility changes depend on protein source, temperatures, times and other variables
Heat Treatment
The effects of heat treatment depend on the temperature and time. Moderate temperature and short times typically denature proteins and deactivate anti-nutritional factors, which increases their digestibility (Lassé et al., 2015). Heat pre-treatment of egg whites increases ileal protein digestibility in humans from a low value of 51.3% for raw eggs to 90.9% (Evenepoel et al., 1998). The investigators suggest that the improvement in digestibility results from protein denaturation and deactivation of egg protein protease inhibitors. Lower digestibility of raw egg protein compared with cooked egg proteins was also shown in humans via an increase in fecal 15N excretion and urinary isotopically-labelled protein putrefaction end-products (p-cresol, phenol) (Evenepoel et al., 1999). Heat treatment of legumes increases their overall digestibility by denaturing the endogenous trypsin inhibitors (Kakade and Evans, 1966). Heating legume seeds (Jack beans) in water for 1 hour at 100 °C reduced trypsin inhibitor concentration by 48.4% (Babar et al., 1988). A number of heat treatments (boiling, autoclaving, microwave cooking and roasting) can deactivate anti-nutritional factors in legumes and seeds and improve in vitro digestibility (Embaby, 2010).
High temperatures and long heating times also denature proteins and destroy anti-nutritional factors, which improves digestibility, but can also cause chemical changes and render proteins insoluble, which reduces their digestibility (deWit and Klarenbeek, 1984). Some reported chemical changes that proteins undergo when exposed to heat include racemization (complete racemization took 8 hours at 151 °C or 2 hours at 185 °C) (Spies and Chambers, 1949), hydrolysis (occurs at 160 °C for 45–60 minutes, at 170 °C for 30–45 minutes and at 180 °C for 30 minutes) (Csapá et al., 1997), formation of oxidation-induced cross links, including disulfide and dityrosine bridges (Duodu et al., 2003; Santé-Lhoutellier et al., 2008), and deamidation (complete deamidation occurs at 121 °C for 3 hours) (Qiu et al., 2013). Heating foods to high temperatures also initiates non-enzymatic browning (the Maillard reaction) where the amino group of AA and the carbonyl group of reducing sugars react to form a complex array of products including melanoidins and aromatic compounds. For example, lysine reacts with reducing sugars to produce lactulosyl-lysine and fructosyl-lysine (Hurrell, 1990). The Maillard reaction negatively impacts protein nutritional quality because it decreases the bioavailability of AA, especially lysine, through chemical modification (Mottu and Mauron, 1967; Erbersdobler and Hupe, 1991). The combination of sugars with AA in Maillard reactions can also make protein digestion and absorption difficult or impossible for the host (Seiquer et al., 2006; Mills et al., 2008). Moreover, some Maillard reaction products, including DL-2-formyl-5-(hydroxymethyl)pyrrole-l-norleucine, directly inhibit the activities of proteases such as carboxypeptidase A and aminopeptidase N (Oeste et al., 1987). Overall, high heat treatments for extended times can decrease digestibility. Heating casein at 180 °C for 1 hour decreased protein digestibility and increased protein fermentation markers (fecal ammonium; urinary phenol, cresol and indol-3-ol) in rats (Corpet et al., 1995). Likewise, cooking meat protein at 100 °C for 45 minutes oxidized proteins, thus inducing disulfide and dityrosine bridge formation, protein aggregation and decreased protein in vitro digestibility (Santé-Lhoutellier et al., 2008).
Other types of heat treatments, including spray drying, pasteurization and ultra-high-temperature treatment, drum drying and in-can sterilization, also alter protein digestibility. With each processing step that involves heat, the degree of denaturation and chemical modifications (hence, changes to digestibility) depend on the temperature, heating time and moisture content. Even when these variables are constant, these effects vary with protein source.
Fermentation
For millennia, humans have altered foods with bacteria and fungi. Lactic acid bacteria used to make kefir and yogurt partially digest milk proteins in addition to fermenting lactose (Adolfsson et al., 2004). Lactic acid bacteria produce many extracellular serine proteases which can hydrolyze the milk proteins (Pritchard and Coolbear, 1993), increasing their digestibility. Commercially, cheese proteins are partially pre-digested by the bacteria and mold used for ripening/ageing (De Angelis Curtis et al., 2000). This microbial proteolytic activity can improve protein digestibility. As compared with milk, lactic acid bacteria-fermented milks (kefir, yogurt and sour milk) were more digestible using in vitro pepsin digestibility assays of free amino-nitrogen and growth rate monitoring in rats (Vass et al., 1983). In other studies, in vitro digestibility of yogurt was greater than that of non-fermented milk (Breslaw and Kleyn, 1973). Fermentation of milk to yogurt also improved digestibility and feed efficiency in rats (Lee et al., 1988).
Fermented soybean-based foods such as miso and soy sauce (fermented by the fungi Aspergillus oryzae) have higher protein digestibility than unfermented soybeans. Aspergillus oryzae secretes proteases that break down proteins in fermented soy (Chancharoonpong et al., 2012). When fed to piglets, Aspergillus oryzae-fermented soybean meal had higher protein fecal digestibility than unfermented soybean meal (Feng et al., 2007). The authors attributed this improved digestibility to the inactivation of soybean’s trypsin inhibitors and degradation of other proteins (Feng et al., 2007).
Alkali fermentation is applied to create numerous traditional foods in Southeast Asia and Africa (Parkouda et al., 2009). Typically, these fermentations rely on Bacillus subtilis, which not only ferments carbohydrates but produces proteases that degrade the food proteins into peptides and AA (Steinkraus, 2002; Hong et al., 2004). Bacillus subtilis also degrades AA and releases ammonia, which increases the pH—hence ―alkali fermentation‖ (Parkouda et al., 2009). Soybeans are fermented by Bacillus subtilis to produce Japanese natto (Wang and Fung, 1996). The degradation of proteins in alkali fermentations likely means that these proteins have increased digestibility. Bacterial protein degradation also can degrade trypsin inhibitors, thus improving overall protein digestibility (Hong et al., 2004).
Fermentation of rye flour with lactic acid bacteria sourdough cultures hydrolyzes prolamins (a group of plant storage proteins high in proline and similar to gluten). This fermentation approach could reduce reactivity in humans with celiac disease by degrading prolamin epitopes that may contaminate a gluten-free diet (De Angelis et al., 2006). Similarly, a 24-hour lactic acid bacterial fermentation of wheat flour degraded gliadin and did not increase intestinal permeability in celiac patients (Di Cagno et al., 2004). Indeed, specific Lactobacillus species with the ability to degrade the immunotoxic peptides related to celiac responses have been identified (Duar et al., 2015). A 48-hour fermentation of wheat flour with three Enterococcus strains and Rhizopus oryzae degraded 98% of the gluten protein (M’hir et al., 2009). Fermentation approaches not only reduces the allergenicity of some proteins but also improves overall protein digestibility.
Future personalization of protein digestibility to protein digestive capacity can take advantage of naturally pre-digested foods to match the protein digestibility requirements of individuals with lower digestive capacity.
Hydrolysates
Proteins can be hydrolyzed, either extensively or partially, via treatment with proteases, acid or alkali (Clemente, 2000). Acid and alkali hydrolyses are difficult to control and they can modify/destroy AA, which lowers the nutritional quality of a product. As proteases do not alter the AA, they are preferred for making hydrolysates. Common protein sources used for preparing nutritional hydrolysates are bovine milk and soybeans (Chiang et al., 1999). Hydrolyzed foods have a variety of uses, including in infant formulas, nutritional supplements for the elderly and sports nutrition products.
Other Processing
A variety of other processing methods, including soaking, germination, dehulling, pressure cooking, high pressure processing, extrusion, transglutaminase treatment and alkaline treatment, alter protein digestibility.
Soaking uncooked beans improves protein digestibility (Barampama and Simard, 1994). In one study, soaking mung beans improved in vitro protein digestion 4% in 6 hours and 21% in 18 hours by lowering anti-nutritional factors (phytic acid, saponins and polyphenols) in the legume seed (Kataria et al., 1989). The investigators hypothesized that these anti-nutritional factors leached out during the soaking; however, mechanistic evidence for this hypothesis was not presented.
Germination of seeds after soaking further improves their overall protein digestibility. In vitro protein digestibility of cowpeas after 12 hours of soaking followed by 48 hours of germination improved 7.7% compared with digestibility after 12 hours of soaking only (Preet and Punia, 2000). Overall, germination significantly improved the in vitro protein digestibility of legume seeds compared with that of the raw seeds (14–18%) (Ghavidel and Prakash, 2007). The increased protein digestibility after germination of legumes may result from reduction in anti-nutritional factors such as phytic acid, tannins (polyphenols) and saponins and degradation of storage proteins (Kataria et al., 1989). Decreases in these anti-nutritional factors may result from leaching into the soaking medium through simple diffusion (Khokhar and Chauhan, 1986). During extended germination for 2–8 days, plant proteases are activated and begin to degrade plant storage proteins, which also increases digestibility (Shastry and John, 1991).
Dehulling of seeds also improves digestibility. Dehulling after germination improved the in vitro digestibility of various legume seeds 3–5% compared with digestibility of germinated-only seeds (Ghavidel and Prakash, 2007). Dehulling improved protein digestibility by reducing concentrations of anti-nutritional factors, including phytic acid, tannins and dietary fiber, present in the seed coat.
High pressure processing can either increase or decrease protein digestibility. Treatment of β-lactoglobulin at 400 MPa for 30 minutes at 50 °C denatured the protein, which improved its in vitro pepsin digestibility (Chicón et al., 2008). Similarly, with treatment at 600 MPa and 800 MPa for 10 minutes at 20 °C, in vitro pepsin digestibility of β-lactoglobulin was greatly improved (Zeece et al., 2008). However, the effect of high pressure processing on digestibility varies with the protein source and type. For instance, red kidney beans treated at 400 MPa and above for 20 minutes at 25 °C had significantly lower trypsin digestibility than untreated beans (Yin et al., 2008). Yin et al. hypothesized that protein aggregation reduced digestibility.
Depending on the protein source and conditions, extrusion can increase or decrease protein digestibility. Extrusion increased in vitro digestibility for cereal grains, depending on the temperature and moisture content (Dahlin and Lorenz, 1993). This improved digestibility is likely due to denaturation of proteins with heat and degradation of antiproteases. Extrusion at high temperatures (> 180 °C), high shear (>100 rpm) and low moisture (<15 %) causes Maillard reactions, leading to reduced digestibility (Camire et al., 1990). Extrusion at high moisture and lower temperatures (85–135 °C) causes cross-link formation and protein aggregation in soybean protein isolates (Chen et al., 2011), which may also reduce digestibility.
Transglutaminases are used frequently in dairy product manufacturing, meat processing and producing wheat-based baked goods (Kieliszek and Misiewicz, 2014). Transglutaminases create covalent cross-links between proteins (Mahmoud and Savello, 1993), which improves the protein product functional properties, including increasing solubility, water-binding capacity, viscosity, elasticity and gelation. Transglutaminase treatments of food may, however, reduce protein digestibility. One study reported that transglutaminase-treated β-casein was more resistant to pepsin degradation than non-cross-linked β-casein (Monogioudi et al., 2011). However, in another study, transglutaminase digestibility of cross-linked casein was similar to that of normal casein in an in vitro assay simulating gastric and small intestinal conditions (Havenaar et al., 2013). Differences in these results may be due to the degree of cross-linking. Phaseolin, the major storage protein in the common bean (Phaseolus vulgaris), had decreased digestibility in in vitro pepsin and trypsin assays after transglutamination (Mariniello et al., 2007). The investigators predicted that the transglutaminated phaseolin would survive to the colon intact.
Alkaline treatment is often used in food processing to improve protein solubility (Maga, 1984). Alkali solutions are used to extract proteins from soybeans, cereal grains, oilseeds, peanuts and milk (sodium caseinate) (Friedman, 1999). Alkaline treatment with heating causes β-elimination. β-Elimination allows formation of a variety of intramolecular and intermolecular cross-links, which can reduce protein digestibility. These cross-links include lysinoalanine (LAL), lysinomethylalanine (Walter et al., 1994), lanthionine (Aymard et al., 1978) and histidinoalanine (Friedman, 2005). A complete review of the cross-links caused by alkali and heat treatment is outside out the scope of this paper, but was thoroughly reviewed previously (Gerrard, 2002). LAL formation results in essential AA losses (such a lysine, cysteine and threonine) and reduced the protein digestibility due to the formation of cross-links, which inhibit proteolysis (Maga, 1984). LAL began to appear at pH 9 and increased as pH increased (Friedman et al., 1984). But when temperature increased, LAL was produced at lower pH—LAL formation occurred at pH 5 when casein was heated to 100 °C (Sternberg and Kim, 1977). The combination of alkali and heat is particularly problematic for digestibility. Heating at 65 °C for 24 hours at pH 10.5–11.5 caused LAL formation and racemization, resulting in 13–18% decreases in ileal digestibility for casein, β-lactoglobulin and wheat proteins in minipigs (Vrese et al., 2000). Digestibility of alkali and heat-treated caseins and soy proteins is also reduced in rats (Sarwar et al., 1999).
Typical meat processing steps (e.g., mincing, salting, irradiation and aging) can also influence protein digestibility. During these processes, protein can be oxidized via free radical chain-reactions (typically induced by oxidized lipids). This oxidation causes the formation of carbonyl derivatives and disulfide protein cross-links (Mercier et al., 1998; Renerre et al., 1999; Rowe et al., 2004; Rowe et al., 2004). Oxidation-induced cross-links can lead to protein aggregation, which can decrease protein susceptibility to proteolytic degradation (Morzel et al., 2006; Santé-Lhoutellier et al., 2008; Baron et al., 2009). However, increases in oxidation do not always decrease protein digestibility. For example, refrigerated storage for 7 days significantly increases carbonyl group formation in meat protein, but this storage time has little effect on protein digestibility in either pepsin or trypsin and α-chymotrypsin in vitro digestion assays (Santé-Lhoutellier et al., 2008). Further research is needed to fully understand the consequences of oxidation on protein digestibility.
CONSUMERS DIFFER IN DIGESTIVE CAPACITY
Beyond variations in basic protein requirements, consumers vary in their ability to digest protein, particularly across disease states. Host proteolytic capacity depends on a number of facets of gut health and function, including the concentration and activity of endogenous luminal proteases (partially determined by certain gastrointestinal hormones such as gastrin and cholecystokinin (CCK)) and brush border peptidases, luminal pH and transit time. Across conditions, variations in these factors lead to differences in overall proteolytic capacity. Lowered protein digestion can be observed with techniques beyond traditional protein digestibility measurements such as examining the residual peptides, microbiota, microbial metabolites and protein biomarkers.
Variations in Protein Digestion Capacity with Disease Conditions with impaired gastric digestion
Both protein entering the stomach and a gastric pH >4 stimulate G-cells to release gastrin (Figure 1A). Gastrin stimulates enterochromaffin-like (ECL) cells to release histamine, and histamine induces parietal cells to release gastric acid (Waldum et al., 2014). Gastric acid activates pepsinogen, denatures proteins and protects against pathogens (Martinsen et al., 2005; Beasley et al., 2015) (Figure 1A). Hypo-or achlorhydria (low or absent gastric acid, respectively) can result from a variety of conditions, including atrophic gastritis (Strickland and Mackay, 1973), gastrectomy (Hoya et al., 2009), acid suppression therapy (e.g., proton pump inhibitors, antacid medication) (Schubert, 2014) and Helicobacter pylori infection (Schubert, 2014). Though the lowered gastric acid production increases infection risk (Martinsen et al., 2005), the consequences of low acidity in protein digestion remain poorly understood.
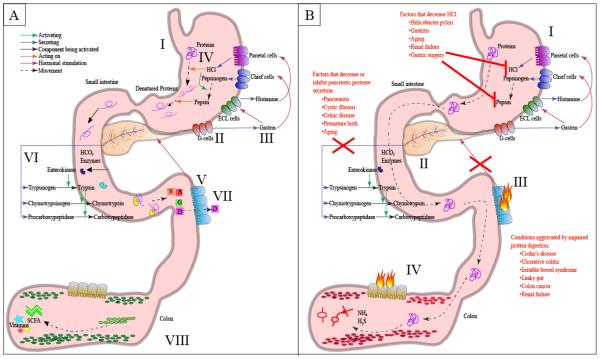
Fig 1.
Diagram of the effects of protein maldigestion and putrefaction in the gut. A) Healthy protein digestion: I. Dietary protein enters the stomach. II. Protein in the stomach stimulates G-cells secrete gastrin. III. Gastrin stimulates enterochromaffin-like (ECL) cells, which releases histamine that stimulates parietal cells. Gastrin also stimulates chief cells to release pepsinogen. IV. HCl denatures the proteins and activates pepsinogen into pepsin. Pepsin begins cleaving proteins into smaller peptides. V. Cholecystokinin (CCK) and secretin released by the small intestine and stimulates the pancreas to release digestive enzymes and HCO3. VI. The pancreas releases HCO3 and enzymes (trypsinogen, chymotrypsinogen, procarboxypeptidase, etc.). Enterokinase (released by intestinal cells) activates trypsin, and trypsin activates chymotrypsinogen and procarboxypeptidase. VII. Amino acids and di-and tripeptides get absorbed into the enterocytes primarily in the jejunum. VIII. Primarily carbohydrates reach the colon and undergo fermentation releasing vitamins and short-chain fatty acids (SCFA). B) Incomplete protein digestion and pathogenesis: I. Decreased HCl limits protein denaturation and pepsin activation. II. Pancreatitis and pancreatic insufficiency result in decreased secretion of digestive enzymes. III. Inflammation in the intestine can impair CCK release and thus lower pancreatic secretion of proteases. IV. Protein escapes digestion and reaches the colon, which can promote microbial putrefaction. Colonic putrefaction produces toxic metabolites such as hydrogen sulfide, ammonia and p-cresol.
Medications such as antacids, proton pump inhibitors and H2 blockers reduce gastric acidity and may negatively impact protein digestion (Figure 1B). Low gastric pH (between pH 1.8–3.5) allows for optimum gastric pepsin activity and food protein denaturation (Freeman and Kim, 1978). As the acidity of the stomach decreases, peptic activity and protein denaturation diminishes. Pepsin activity is reduced to less than 5% with pH >5 (Koop, 1992), and these medications can elevate gastric pH to around pH 5.0 for 11 hours (Prichard et al., 1985). Ten percent of the middle-aged population uses antacids for dyspeptic disorders (i.e., heartburn) (Furu and Straume, 1999).
Initial studies measuring fecal nitrogen digestibility suggested that gastric digestion has a limited effect on protein assimilation since patients with little or no gastric function have adequate digestibility (Bradley et al., 1975; Freeman and Kim, 1978; Erickson and Kim, 1990; Rinaldi Schinkel et al., 2006; Skroubis et al., 2006). However, unlike the preferred ileal nitrogen digestibility, fecal nitrogen digestibility discounts the effect of colonic protein fermentation, which can provide falsely high digestibility values (Evenepoel et al., 1998). Evenepoel et al. took a different approach, using a 13C-egg white breath test to evaluate the influence of acid suppression therapy (Omeprazole) on overall protein assimilation in humans. After one month of Omeprazole usage, protein assimilation was impaired. Moreover, urinary phenol and p-cresol, markers of colonic protein fermentation of tyrosine and tryptophan, respectively (Blachier et al., 2007), were increased. Therefore, antacid use can decrease overall protein digestion and assimilation and increase colonic microbial putrefaction (Evenepoel et al., 1998).
Decreased digestion capacity with antacid intake occurs in animal models with common allergenic proteins. Normal pH 2.0 gastric conditions quickly degrade the fish protein parvalbumin. However, mice treated with antacids (gastric pH 5.0) were unable to digest parvalbumin as demonstrated by detection with a parvalbumin specific IgE antibody (Untersmayr et al., 2003). Decreased digestibility with antacid intake in mice also occurs for other proteins, including cod protein (Untersmayr et al., 2005), egg ovalbumin (Diesner et al., 2008) and hazelnut proteins (Schöll et al., 2005). In humans, several dietary antigens (including those from peanut, walnut, almond, potato, tomato, celery, carrot, orange, wheat flour and rye flour) become less digestible after antacid intake, as demonstrated by increased dietary protein-specific IgE antibodies detected in the blood after 3 months of antacid treatment, suggesting increased survival of immune-stimulating epitopes within the intestine (Untersmayr et al., 2005).
Diseases can also induce hypochlorhydria, and typically result in a cyclical relationship with initial low gastric acid progressing to hypergastrinemia and eventual long-term hypochlorhydria. Renal failure, gastritis, gastric ulcers, gastric cancer and H. pylori infection are all characterized by hypo-or achlorhydria along with hypergastrinemia (Strid et al., 2002) (Figure 1B). In renal failure, 70–79% of patients experience gastrointestinal complications, many of which can alter protein digestion (Shirazian and Radhakrishnan, 2010). Renal failure leads to uremia—the build-up of nitrogenous amino acid metabolism end products in the blood—which alters gastrointestinal function and impairs protein digestion (Bammens et al., 2003; Sirich, 2015). In uremia, excess urea leaks into the stomach and is metabolized by H. pylori into ammonia, thereby increasing gastric pH (el Nujumi et al., 1992; Malyszko et al., 1995; Bammens et al., 2003; Bammens et al., 2004). As discussed above, increased gastric pH limits protein denaturation and pepsin activation and thus reduces protein degradation (Evenepoel et al., 1998). Uremia is also associated with hypergastrinemia (Kang, 1993), the build-up of gastrin in the blood. This excess gastrin may be caused by the inability of kidneys to excrete gastrin (Hällgren et al., 1978) and/or increased G-cell stimulation by the H. pylori-induced increased pH (Chuang et al., 2004). Gastrin is a trophic hormone, and excessive amounts, as seen in renal failure and other conditions (e.g., atrophic gastritis, H. pylori infection and acid suppression therapy) results in ECL hyperplasia, which potentially leads to ECL neoplasia and further hypochlorhydria (Freston et al., 1995; Waldum et al., 2014). As a result, renal failure seems to impair protein assimilation, which is supported by various studies (Ala-Kaila et al., 1989; Bammens et al., 2003; Meijers and Evenepoel, 2011).
Gastric bypass and other weight-loss surgeries also can reduce gastric digestion by removing or bypassing the stomach and/or the upper small intestine (Figure 1B). Bariatric surgeries, particularly the Roux-en-Y gastric bypass (RYGB) procedure, are becoming increasingly common as a treatment for obesity, but may lead to impaired protein digestion. The RYGB procedure bypasses most of the stomach and part of the small intestine, and the shrunken stomach produces less pepsinogen and HCl (Smith et al., 1993; Behrns et al., 1994; Sundbom et al., 2003). The lower HCl production also means that there is lower pepsin activity (as low pH is required for pepsinogen activation). RYGB typically causes hormonal changes, including increased anorectic peptide YY (Suzuki et al., 2005) and glucagon-like peptide-1 (Bose et al., 2010), which may slow intestinal motility. As RYGB impairs the gastric phase of protein digestion and slows intestinal motility, undigested protein may remain longer in the colon, representing a source for increased putrefaction. Indeed, RYGB-operated rats compared with sham-surgery controls had higher concentrations of 1H NMR-identified putrefactive metabolites in stool (e.g., putrescine, tyramine, uracil and gamma-aminobutyric acid) and urine (e.g., p-cresol sulfate, p-cresyl glucuronide, 5-aminovalerate, phenylacetylglycine, p-hydroxyphenylacetate, indoxyl sulphate) (Li et al., 2011). From a microbial perspective, the RYGB procedure in humans, rats and mice causes a common dramatic shift in the gut microbiome, significant increases in typically putrefactive Gammaproteobacteria and decreases in typically fermentative Firmicutes (Zhang et al., 2009; Li et al., 2011; Liou et al., 2013). Further research is needed on how the microbiome and microbial metabolism influence the immediate and long-term health of patients who undergo bariatric surgery.
Conditions with impaired intestinal digestion
A few disorders, including chronic pancreatitis and cystic fibrosis, can cause near complete loss of pancreatic enzymes (Keller and Layer, 2005) (Figure 1B). Cystic fibrosis is caused by a mutation that inhibits the transport of chloride outside the cell, which limits the lungs ability to fully hydrate its mucus secretions (Ferrone et al., 2007). As a result, a thick mucus accumulates on the cells of the respiratory tract and pancreas and limits cell function (Tucker et al., 2003). Exocrine pancreatic insufficiency is observed in 85–90% of cystic fibrosis patients and causes decreased digestive capacity, increased risk for diarrhea, protein malnutrition, decreased anabolism and increased putrefaction (Engelen et al., 2014). In chronic pancreatitis (induced primarily by gallstones, alcohol abuse, medications, renal failure and surgery complications), enzyme activation of trypsin within the pancreatic cells leads to auto-digestion of the gland and local inflammation, causing reduced pancreatic function and reduced enzyme secretion (Greenberger, 1999). In chronic pancreatitis patients, 30–40% have concurrent pancreatic insufficiency (Bruno et al., 1995). Pancreatic insufficiency or poor protein digestion are also caused by variety of rare genetic conditions, including enterokinase deficiency (Canani and Terrin, 2011), trypsinogen deficiency, Shwachman–Diamond syndrome, Johanson–Blizzard syndrome and Pearson syndrome (Guarino et al., 2012).
Other variations in proteolytic capacity are less dramatic. Milder cases of pancreatic insufficiency (i.e., insufficient release of pancreatic proteases, assessed by fecal elastase-1 (E1) concentration (< 200 μg/g stool)) are common in gastrointestinal disorders such as celiac disease (Carroccio et al., 1991; Carroccio et al., 1994; Nousia-Arvanitakis et al., 1999; Evans et al., 2010), Crohn’s disease and ulcerative colitis (UC) (Maconi et al., 2008), giardiasis and cow milk-related enteropathy (Walkowiak and Herzig, 2001) and irritable bowel syndrome (Leeds et al., 2010) (Figure 1B). Mucosal damage and villous atrophy, as seen in most of these conditions, can decrease CCK release from I cells in the crypts and villi of the duodenum and jejunum, which results in pancreatic insufficiency. Pancreatic sufficiency returns to normal once villi and mucosa are restored (Nousia-Arvanitakis et al., 1999; Nousia-Arvanitakis et al., 2006). In celiac patients, pancreatic exocrine function does not completely return to normal until at least 12 months on a gluten-free diet (Walkowiak and Herzig, 2001).
Pancreatic insufficiency can also be induced by enteric pathogen colonization leading to acute enteritis in adults (Salvatore et al., 2003). Low E1 activity also occurs in some diabetics (Rathmann et al., 2001; Larger et al., 2012). In insulin-dependent (type II) diabetes mellitus, pancreas atrophy decreases pancreatic enzyme secretion (including proteases) and bicarbonate secretion (Silva et al., 1993). Pancreatic insufficiency associated with several diseases such as diabetes, celiac disease and cystic fibrosis was extensively reviewed by others (Keller and Layer, 2005).
Pancreatitis and/or pancreatic insufficiency are associated with inflammatory bowel disease (IBD) (i.e., UC and Crohn’s disease). Pancreatitis can occur prior to IBD onset or after IBD is established (Barthet et al., 1999). In IBD patients, the leading causes of pancreatitis include gallstones (Fraquelli et al., 2001; Parente et al., 2007), IBD medications (Bermejo et al., 2008) and alcohol use (Moolsintong et al., 2005; Pitchumoni et al., 2010). Some IBD patients experience idiopathic pancreatitis (i.e., not linked to a known cause) (Seyrig et al., 1985). These associations suggest that IBD may increase risk of pancreatitis. The prevalence of pancreatitis in IBD is likely higher than recognized, as pancreatitis can be present prior to overt symptoms (Barthet et al., 1999; Herrlinger and Stange, 2000). Some UC patients with no pancreatitis symptoms had pancreatic duct abnormalities as imaged via magnetic resonance cholangiopancreatography (Toda et al., 2005). IBD patients without pancreatitis may instead have pancreatic insufficiency. Exocrine pancreatic insufficiency was found in both Crohn’s disease and UC patients, as measured by the fecal elastase test (insufficiency defined as < 200 µg/ml) (Maconi et al., 2008; Burkovskaia et al., 2010).
IBD is characterized by intestinal inflammation. Crohn’s disease involves non-continuous inflammation throughout the intestine, with inflammation reaching the deeper tissues layers, which results in strictures, fistulas and abscesses. Inflammation of UC begins in the rectum and continuously spreads up the colon, with the inflammation limited to the superficial mucosa and submucosa layers (Khor et al., 2011). The development of IBD has been related to genetic (Khor et al., 2011), environmental and microbial factors (Ho et al., 2015); however, the exact etiology of these diseases remains unknown. Putrefactive metabolism of sulfur-containing amino acid compounds and inorganic sulfates to release hydrogen sulfide by sulfate-reducing microbes is strongly associated with the progression of UC (Rowan et al., 2009; Khalil et al., 2014). Hydrogen sulfide can permeate cell membranes and induce DNA damage, inhibit cytochrome C oxidase activity (essential for ATP generation) and impede butyrate production in colonocytes in vitro (Rowan et al., 2009). When compared with healthy control biopsies, colonic mucosal biopsies of UC patients displayed increased sulfate-reducing bacteria (Rowan et al., 2010). Fecal samples from UC patient had lower microbial diversity and proportionally higher sulfate-reducing bacteria counts compared with healthy control samples (Khalil et al., 2014). In addition to the increased sulfate-reducing bacteria counts, IBD disease patients display other characteristic shifts in the microbiota, including decreased Bacteroides and Firmicutes (typically associated with fermentation), and increased Gammaproteobacteria (associated with putrefaction), which could contribute to the progression of these diseases (Linskens et al., 2001; Frank et al., 2007; Walker et al., 2011; Kostic et al., 2014).
Variation in Protein Digestion with Nutritional Status
Chronic malnutrition may reduce protein degradation. Severely undernourished patients had lower pentagastrin-stimulated gastric acid output, and secretion of CCK-stimulated trypsin and other non-protease pancreatic enzymes compared with controls (Winter et al., 2000). Severely undernourished patients also secreted significantly less gastric acid and trypsin in response to both CCK and enteral feeding stimulation (Winter et al., 2001). After 2 weeks to 3 months of nutritional support, non-protease pancreatic enzyme concentrations returned to normal, whereas trypsin and gastric acid concentrations remained low (Winter et al., 2000; Winter et al., 2001). This lack of recovery likely corresponds to longer term gastric parietal and pancreatic acinar cell dysfunction (Winter et al., 2001). However, because many of the undernourished patients in these test groups had coincident gastrointestinal disease, including Crohn’s disease, small bowel disease and short bowel, more research is needed to determine whether undernourishment, in the absence of gastrointestinal disease, causes lower gastric acid and trypsin production.
ENZYME AND HORMONE SUPPLEMENTATION, AND USE OF HYDROLYSATES FOR HUMANS WITH PROTEOLYTIC LIMITATIONS
For optimal human health, the ability to digest protein must be matched to the digestibility of a protein. Therefore, personalizing protein nourishment would mean recommending groups with lower digestive capacity to consume foods that are more digestible. As mentioned above, many plant proteins are difficult to digest due to structure and the presence of anti-nutritional factors. Therefore, animal proteins, including milk proteins, which are more digestible, could be recommended over plant proteins. For consumption of vegetable proteins, a preparation method that improves their digestibility could also be recommended (i.e., fermentation or cooking to appropriate temperatures). Beyond dietary advice, individuals with low digestive function could be recommended specific enzyme or hormone supplementation or specific hydrolysate products. Nutritional products already available that could be applied for personalization of protein digestibility are discussed below.
Enzyme Supplementation for Humans
Enzyme supplementation of human diets has a long history for a few clinical conditions such as chronic pancreatitis and cystic fibrosis. Both diseases can cause pancreatic exocrine enzyme secretion insufficiency, which causes chronic nutrient malabsorption. Therefore, supplementation with the deficient enzyme(s), sometimes called enzyme therapy, is an important treatment modality for these conditions (Pairent and Howard, 1975; Fieker et al., 2011).
Enzyme supplementation with lipases, glycosidases and proteases is used extensively in patients with chronic pancreatitis (Greenberger, 1999). Enzyme supplements effectively increase digestion (primarily of lipids, as steatorrhea is a major problem) and decrease malabsorption (Valerio et al., 1981). Increasing the dose of proteases in these supplements enhanced protein absorption in patients with chronic pancreatitis (Morrison et al., 1992). More research is needed to determine the effectiveness of enzyme supplementation treatments to enhance protein digestion in patients with chronic pancreatitis.
Enzyme therapy is also commonly used for patients with cystic fibrosis. Pancreatic enzyme supplementation improves protein digestion (Engelen et al., 2014), which prevents protein malnutrition and putrefaction. Supplementation with pancreatic enzymes (Creon) increased protein digestibility in patients with cystic fibrosis from about 47% at baseline to a maximum of 90% of the digestion rate of healthy control patients (Engelen et al., 2014).
Enzyme replacement therapy is often used to treat individuals with rare genetic conditions that cause pancreatic insufficiency or poor protein digestion. Enterokinase supplements are provided to patients with enterokinase deficiency, which allows the normal activation of trypsin and improves overall pancreatic protease activity in the small intestine (Tarlow et al., 1970; Haworth et al., 1971).
Enzyme supplementation may also be beneficial in cases of less severe pancreatic enzyme insufficiency. Celiac patients have a higher rate of pancreatic insufficiency than healthy controls (Leeds et al., 2007), most likely due to allergy-induced dysfunction. Patients with celiac disease, pancreatic insufficiency and persistent diarrhea, and who consumed an ostensibly gluten-free diet experienced improved stool frequency when supplemented with pancreatic enzymes (Leeds et al., 2007). This finding suggests that the enzyme supplements improved protein digestion, but no data on protein digestibility were provided.
Supplemental proteases may also be useful for the elimination of allergic sensitization or allergic response. A variety of enzymes are under investigation for enhancing gluten degradation to prevent flare-ups in patients with celiac disease (Stepniak et al., 2006; Tye-Din et al., 2010). Increased protein digestibility and thus decreased putrefaction could have additional benefits for reducing gut inflammation and the sequelae of the allergic disease.
Protease supplementation is also applied to treat humans for kinesiology research. Specific protease supplementation protocols accelerated muscle recovery after strenuous exercises (Miller et al., 2004; Beck et al., 2007; Buford et al., 2009). These findings may indicate that protein digestibility was improved; however, the reported studies lacked direct measurements of protein digestibility. Further examination is necessary to determine whether the means by which protease supplementation aids muscle recovery is by enhancing protein digestion and AA absorption.
Numerous companies produce non-prescription supplements containing proteases, lipases and glycosidases, and market them for general use in healthy individuals. Beyond improving digestibility, these supplements are marketed for a wide range of mostly unsubstantiated benefits, including enhanced blood flow, improved immune response, improved detoxification (e.g., Protease 375K by Transformation Enzymes), tissue repair activation, improved joint health and support of optimal viscosity of nasal mucus (e.g., Best Proteolytic Enzymes 90VC). To date, none of these supplements have been clinically validated in the general population. Many companies do not provide information describing the exact proteases included in the supplements that they promote, leading to questions of enzyme activity in vivo. Beyond uses for pancreatitis and cystic fibrosis, allergy elimination and muscle recovery, no clinical trials on the effects on protein digestibility of protease supplementation in the general population were identified in a search of the literature. Future work needs to explore the effectiveness of protease supplementation for minor cases of poor protein digestibility.
Digestive Hormone Supplementation
CCK is a peptide hormone released from inclusion (I) cells in the crypts and villi of the duodenum and jejunum in response to protein or fat in the proximal small intestine (Little et al., 2005). CCK is released into the bloodstream, and through binding to the CCK-A receptors on vagal afferent neurons (Jensen, 2002), has a number of physiological effects, including slowing gastric emptying, suppressing gastric acid secretion, suppressing energy intake and stimulating pancreatic fluid secretion (Nousia-Arvanitakis et al., 2006). Damage to the proximal intestinal mucosa (sloughing of epithelial cells, flattening of epithelium), e.g., in Crohn’s disease and cow milk protein enteropathy, can impair CCK production and thereby reduce pancreatic enzyme secretion (Nousia-Arvanitakis et al., 2006). Potentially, in individuals with inflammatory conditions that suppress CCK production, injection with CCK could return pancreatic enzyme secretion to normal. Not only would this method enhance overall protein digestion, it could also lower putrefaction and inflammation and prevent further digestion impairment. Healthy humans injected with 2.5 pmol CCK/kg/h demonstrated a 280% increase in trypsin secretion (Kerstens et al., 1985). If CCK is used as a therapy, the dose must be kept in a normal biological range. Injections of 10 times the amount of CCK required to generate the maximal secretory response induced pancreatitis in rats (Saluja et al., 2007). Overstimulation of the pancreas with CCK leads to activation of protease zymogens, which can damage acinar cells and initiate pancreatitis. Lower doses of CCK (less than 0.1 µM) injected into rats did not activate zymogen activation nor damage cells (Grady et al., 1998).
Enzyme Hydrolysates for Humans with Limited Proteolytic Capacity
Enzyme hydrolysates can be used to prevent allergic reactions to food proteins, such as the use of hypoallergenic hydrolyzed infant formulas to prevent cow milk protein allergy flare-ups. Beyond their use to prevent allergic responses, hydrolysates can also be used to help improve digestion, generally in humans with low digestive capacity.
The most common use of non-fermented partially hydrolyzed foods for humans is in hypoallergenic infant formulas. To reduce epitope exposure, infants are often given partially or extensively hydrolyzed protein formulas after they develop bovine milk protein allergies. Infants without allergies but at high risk for developing them (due to family history) are often fed hydrolyzed formulas rather than traditional formula as a preventative measure. These formulas successfully reduce exposure to allergenic protein epitopes. Compared with regular infant formulas, both partially and extensively hydrolyzed formulas reduced atopic severity in infants with milk allergies (Chandra, 1997; Greer et al., 2008; von Berg et al., 2008) and lowered rates or milk protein allergy development in infants with high familial allergy risk (Osborn and Sinn, 2006; Alexander and Cabana, 2010; Szajewska and Horvath, 2010). The ―partially hydrolyzed‖ formulas typically consist of peptides between 8 and 20 kDa (Wahn, 1993), whereas ―extensively hydrolyzed‖ formulas consist of peptides under 5 kDa—peptides in most commonly available extensively hydrolyzed products are below 1.2 kDa (Maldonado et al., 1998). Extensively hydrolyzed formula may reduce the risk for milk allergy more than partially hydrolyzed formula (Halken et al., 2000). Though use of hydrolysates in formula targets allergy elimination, this approach also likely increases protein digestibility. However, there is little evidence that the extent of AA uptake is greater for hydrolysates.
For non-infants, protein hydrolysates are used in energy drinks, sports nutrition beverages and foods, geriatric products and weight control diets (Clemente, 2000). In sports beverages, protein hydrolysates are added because di-and tripeptides are absorbed more rapidly than proteins or free AA (Di Pasquale, 2008). The hypothesis behind this formulation is that the increased absorption rate will improve muscle recovery (Manninen, 2004), but this concept remains unverified. Consumption of a casein protein hydrolysate compared with intact casein by elderly men accelerated gut AA absorption (Koopman et al., 2009). This finding implies improved digestibility, but there is no evidence of overall increased AA absorption.
Feeding free AA to patients with cystic fibrosis can prevent the consequences of protein malnutrition (Engelen et al., 2013) and likely reduce putrefaction in the gut. Likewise, hydrolyzed proteins are used by humans with acute and chronic liver disease, short bowel syndrome, Crohn’s disease, pancreatitis and UC (Clemente, 2000). In Crohn’s disease patients, feeding either protein hydrolysates or elemental AA-based diets induced remission and reduced intestinal inflammation (Mansfield et al., 1995). Feeding elemental AA was as effective as corticosteroid treatment in causing remission in Crohn’s disease patients (O'moráin et al., 1984; Saverymuttu et al., 1985; Hunt et al., 1989).
Why Complete Protein Hydrolysis is Problematic
Elemental diets containing free AA in lieu of intact proteins, such as in hypoallergenic infant formulas, can be detrimental to gut health. Feeding total hydrolysates impedes the development of the exocrine pancreas and its ability to produce adequate proteases. Kinouchi et al. (Kinouchi et al., 2012) showed that large intact proteins were required during the suckling period for proper pancreatic digestive function to develop in rats. After a gastric application of extensively hydrolyzed milk proteins in rats, there was no enhancement of pancreatic enzyme secretion, as is found with intact proteins. Rats fed extensively hydrolyzed milk proteins had lower pancreas weight and stock of pancreatic enzymes. When intact proteins were reintroduced to the diet to restimulate pancreatic protease production, pancreatic secretions did not recover to normal levels. Therefore, partial hydrolysis or protease supplementation are likely better interventions than extensive hydrolysis as they maintain stimulatory signals for pancreatic function. Based on the study with rats, one can speculate that consumption of extensively hydrolyzed proteins may set patients up for lower digestive function in the long-term, although more research is needed in this area.
A partially hydrolyzed protein diet yields better results than an extensively hydrolyzed protein or an elemental AA diet on a variety of metrics. Compared with an elemental AA diet, an oligopeptide-based diet produced a substantial trophic effect on the small intestine, including increased bowel weight, increased mucosal weight, and increased DNA content (Zaloga et al., 1991). Feeding of a milk protein hydrolysate (with 50% of peptides <5 AA in length) versus a free AA mixture also increased the amount and rate of AA absorption (Rerat et al., 1988).
The findings described above provide further evidence that dietary proteins must be personalized congruently with changes in health and digestive capacity. Personalizing proteins for optimal digestibility will require continual monitoring of intestinal function to gradually transition individuals through appropriate dietary regimens. Some individuals may first need to control intestinal inflammation through consumption of extensively hydrolyzed protein. As the intestine is repaired and immune balance is restored, these individuals can then begin to consume partial protein hydrolysates and eventually intact proteins to return to full digestive capacity. Based on the reviewed information, we hypothesize that by monitoring protein digestion as individuals progress from consuming hydrolyzed proteins to increasingly difficult-to-digest protein components, optimal gut health can be obtained.
HOW MUCH PROTEIN IS TOO MUCH?
Though the metabolic consequences of excess protein intake (e.g., aminoacidemia, hyperammonia) have been investigated (Bilsborough and Mann, 2006), the physiological consequences of excess protein entering the colon and undergoing putrefaction remain poorly understood. High protein intake by healthy humans clearly increases protein metabolizing bacterial counts in the colon and putrefactive metabolites, and many of these metabolites are toxic in in vitro assays (Geypens et al., 1997; Evenepoel et al., 1999). However, direct links between the concentrations of these toxic metabolites in stool and urine, and numbers of protein metabolizing bacteria in stool to specific health changes (e.g., inflammation, tissue damage) remain unknown.
Human participants who consumed a maintenance low protein diet (13% protein) excreted significantly fewer toxic metabolites (N-nitroso compounds) in the fecal samples than those who consumed either a high protein diet (29% protein) (Russell et al., 2011); however, how these diets altered health (e.g., tissue damage, inflammation) was not reported. In another study with human subjects, consumption of a high protein diet (27% of kcal, 124 g/day) induced excretion of more p-cresol compared with consumption of a low protein diet (12% of kcal, 50 g/day) (Windey et al., 2012). However, fecal genotoxicity (measured by Comet Assay) and cytotoxicity (measured by WST-1 Assay) did not differ between the two groups of subjects. The investigators concluded that protein fermentation did not induce gut toxicity in healthy human subjects. The assays used for the measurements do not represent a comprehensive analysis of gut health metrics. Measurements of inflammation (inflammatory cytokines, tissue histology) would be useful to determine how specific levels of putrefactive metabolites in the intestinal tract affect gut health. Further investigation is warranted to determine how changes in gut microbiota and microbial metabolism impact health outcomes.
CONCLUSIONS: POSSIBLE SOLUTIONS AND NEW DIRECTIONS
Proteins differ in their digestibility and consumers differ in their ability to digest, particularly across disease state. Incomplete protein digestion stimulates the growth of putrefactive colonic microbes and the production of toxic metabolites. Therefore, it is clear that the future of optimizing nutrition must include monitoring individual protein digestive capacity, especially in diseases that reduce digestive capacity. As measuring digestibility with the gold standard approach, true ileal digestibility, is impossible for a personalization approach due to its invasive nature, alternative approaches to monitor digestion must be developed—i.e., identification of specific metabolites, microbes and protein biomarkers as indices of protein digestibility. Based on the assessed digestive capacity of an individual, protein source, processing treatment, enzymes or hormonal supplementation can be tailored to that individual. Continual monitoring of these markers of digestion and gut health will be essential to determine how digestive capacity changes with these interventions.
Further research is needed to explore the incorporation of supplemental enzymes and hormones as part of a personalized diet to improve digestive capacity in humans with digestive problems. As post-translational modifications (i.e., glycosylation) and processing-induced protein chemical alterations (i.e., Maillard reaction products) can increase resistance to digestion, enzymatic removal of these modifications or enzyme supplementation of humans should be explored for their ability to increase overall protein digestibility.
Perhaps the most important advance needed is better techniques for simple, quick and cost-effective analysis of the degree of protein digestion occurring in the gut, the growth of protein putrefactive bacteria and the production of inflammatory metabolites. Simply monitoring these markers frequently will allow determination of which individuals are at risk for incomplete protein digestion and need to have tailored personalized protein diets.
So, what are the toolsets available to make such personalized determination of protein digestibility? The most useful tools include peptidomics, metabolomics, genetic sequencing and multiplexed enzyme-linked immunosorbent assays (ELISAs). Protein digestion can be measured with extreme precision with peptidomics (Dallas et al., 2013; Dallas et al., 2015), putrefactive metabolites can be quickly measured via metabolomics, increases in putrefactive bacteria can be measured with next-generation sequencing technologies and markers of inflammation in the gut can be measured by multiplexed ELISAs (measuring proteins like calprotectin, calgranulins, lactoferrin, defensins, osteoprotegerin) (Pang et al., 2014). Personalization of protein digestion will mean that these technologies must be available in simple, quick, single-platform formats, such as breath tests for putrefactive metabolites, that are easily interpreted by the consumer. Enabling consumers to quickly observe a measurement of the degree of putrefaction in their gastrointestinal tract will empower them to make important changes to their diet and behaviors. Individuals with chronic pancreatitis are treated with enzyme supplementation, but supplementation is often inadequate and clinical guidelines to monitor treatment effectiveness are lacking (Fieker et al., 2011). With tools to observe their own personal digestion, patients can determine what changes need to be made to their enzyme supplementation doses or to their diets.
Designing strategies for specific protein sources to meet the personalized needs of consumers will be essential to optimize nutrition, particularly across disease states. The insights gained from this research will have profound implications for agricultural processing, food formulations and the value of foods within the marketplace.
ACKNOWLEDGEMENTS
The authors thank C. J. Dillard for editing this manuscript and Aashish Bhandari for literature searching assistance. All authors read and approved the final manuscript. This project was funded in part by the K99/R00 Pathway to Independence Career Award, Eunice Kennedy Shriver Institute of Child Health & Development of the National Institutes of Health (K99HD079561) (D.C. Dallas).
REFERENCES
- Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr. 2004;80(2):245–256. doi: 10.1093/ajcn/80.2.245. [DOI] [PubMed] [Google Scholar]
- Ala-Kaila K, Kekki M, Paronen I, Paakkala T. Serum gastrin in chronic renal failure: its relation to acid secretion, G-cell density, and upper gastrointestinal findings. Scand J Gastroentero. 1989;24(8):939–948. doi: 10.3109/00365528909089238. [DOI] [PubMed] [Google Scholar]
- Alexander DD, Cabana MD. Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a meta-analysis. J Pediatr Gastroenterol Nutr. 2010;50(4):422–430. doi: 10.1097/MPG.0b013e3181cea52b. [DOI] [PubMed] [Google Scholar]
- An C, Kuda T, Yazaki T, Takahashi H, Kimura B. Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl Microbiol Biotechnol. 2014;98(6):2779–2787. doi: 10.1007/s00253-013-5271-5. [DOI] [PubMed] [Google Scholar]
- Andriamihaja M, Davila A-M, Eklou-Lawson M, Petit N, Delpal S, Allek F, Blais A, Delteil C, Tomé D, Blachier F. Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1030–G1037. doi: 10.1152/ajpgi.00149.2010. [DOI] [PubMed] [Google Scholar]
- Andriamihaja M, Lan A, Beaumont M, Audebert M, Wong X, Yamada K, Yin Y, Tomé D, Carrasco-Pozo C, Gotteland M, Kong X, Blachier F. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radical Biology and Medicine. 2015;85:219–227. doi: 10.1016/j.freeradbiomed.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4(1):9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- Aymard C, Cuq J, Cheftel J. Formation of lysino-alanine and lanthionine in various food proteins, heated at neutral or alkaline pH. Food Chem. 1978;3(1):1–5. [Google Scholar]
- Babar V, Chavan J, Kadam S. Effects of heat treatments and germination on trypsin inhibitor activity and polyphenols in jack bean (Canavalia ensiformis L. DC) Plant Foods Hum Nutr. 1988;38(4):319–324. doi: 10.1007/BF01091729. [DOI] [PubMed] [Google Scholar]
- Ball R, Aherne F. Influence of dietary nutrient density, level of feed intake and weaning age on young pigs. II. Apparent nutrient digestibility and incidence and severity of diarrhea. Can J Anim Sci. 1987;67(4):1105–1115. [Google Scholar]
- Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Impairment of small intestinal protein assimilation in patients with end-stage renal disease: extending the malnutrition-inflammation-atherosclerosis concept. Am J Clin Nutr. 2004;80(6):1536–1543. doi: 10.1093/ajcn/80.6.1536. [DOI] [PubMed] [Google Scholar]
- Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003;64(6):2196–2203. doi: 10.1046/j.1523-1755.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- Barampama Z, Simard RE. Oligosaccharides, antinutritional factors, and protein digestibility of dry beans as affected by processing. J Food Sci. 1994;59(4):833–838. [Google Scholar]
- Bardócz S, Hughes EL, Grant G, Brown DS, Duguid TJ, Pusztai A. Uptake, inter-organ distribution and metabolism of dietary putrescine in the rat. The Journal of Nutritional Biochemistry. 1998;9(6):332–338. [Google Scholar]
- Baron CP, Hyldig G, Jacobsen C. Does feed composition affect oxidation of rainbow trout (Oncorhynchus mykiss) during frozen storage? J Agric Food Chem. 2009;57(10):4185–4194. doi: 10.1021/jf803552h. [DOI] [PubMed] [Google Scholar]
- Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, Mambrini P, Saint-Paul MC, Delmont JP, Salducci J, Grimaud JC, Sahel J. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141–2148. doi: 10.1111/j.1572-0241.1999.01287.x. [DOI] [PubMed] [Google Scholar]
- Beasley DE, Koltz AM, Lambert JE, Fierer N, Dunn RR. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PloS one. 2015;10(7):e0134116. doi: 10.1371/journal.pone.0134116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck TW, Housh TJ, Johnson GO, Schmidt RJ, Housh DJ, Coburn JW, Malek MH, Mielke M. Effects of a protease supplement on eccentric exercise-induced markers of delayed-onset muscle soreness and muscle damage. J Strength Cond Res. 2007;21(3):661–667. doi: 10.1519/R-21016.1. [DOI] [PubMed] [Google Scholar]
- Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci. 1994;39(2):315–320. doi: 10.1007/BF02090203. [DOI] [PubMed] [Google Scholar]
- Bergen WG, Wu G. Intestinal Nitrogen Recycling and Utilization in Health and Disease. J Nutr. 2009;139(5):821–825. doi: 10.3945/jn.109.104497. [DOI] [PubMed] [Google Scholar]
- Bermejo F, Lopez-Sanroman A, Taxonera C, Gisbert JP, Perez-Calle JL, Vera I, Menchen L, Martin-Arranz MD, Opio V, Carneros JA, Van-Domselaar M, Mendoza JL, Luna M, Lopez P, Calvo M, Algaba A. Acute pancreatitis in inflammatory bowel disease, with special reference to azathioprine-induced pancreatitis. Aliment Pharmacol Ther. 2008;28(5):623–628. doi: 10.1111/j.1365-2036.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- Bernard B, Newton S, Olden K. Effect of size and location of the oligosaccharide chain on protease degradation of bovine pancreatic ribonuclease. J Biol Chem. 1983;258(20):12198–12202. [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PloS one. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsborough S, Mann N. A review of issues of dietary protein intake in humans. Int J Sport Nutr Exerc Metab. 2006;16(2):129–152. doi: 10.1123/ijsnem.16.2.129. [DOI] [PubMed] [Google Scholar]
- Birkett A, Muir J, Phillips J, Jones G, O'Dea K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr. 1996;63(5):766–772. doi: 10.1093/ajcn/63.5.766. [DOI] [PubMed] [Google Scholar]
- Blachier F, Mariotti F, Huneau JF, Tome D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. 2007;33(4):547–562. doi: 10.1007/s00726-006-0477-9. [DOI] [PubMed] [Google Scholar]
- Bose M, Machineni S, Oliván B, Teixeira J, McGinty JJ, Bawa B, Koshy N, Colarusso A, Laferrère B. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity. 2010;18(6):1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry G, Jamin A, Chatelais L, Gras-Le Guen C, Michel C, Le Huërou-Luron I. Dietary protein excess during neonatal life alters colonic microbiota and mucosal response to inflammatory mediators later in life in female pigs. J Nutr. 2013;143(8):1225–1232. doi: 10.3945/jn.113.175828. [DOI] [PubMed] [Google Scholar]
- Boudry G, Morise A, Seve B, Le Huërou-Luron I. Effect of milk formula protein content on intestinal barrier function in a porcine model of LBW neonates. Pediatr Res. 2011;69(1):4–9. doi: 10.1203/PDR.0b013e3181fc9d13. [DOI] [PubMed] [Google Scholar]
- Boutrou R, Coirre E, Jardin J, Léonil J. l. Phosphorylation and coordination bond of mineral inhibit the hydrolysis of the β-casein (1− 25) peptide by intestinal brush-border membrane enzymes. J Agric Food Chem. 2010;58(13):7955–7961. doi: 10.1021/jf100568r. [DOI] [PubMed] [Google Scholar]
- Bradley EL, Isaacs J, Hersh T, Davidson ED, Millikan W. Nutritional consequences of total gastrectomy. Ann Surg. 1975;182(4):415–429. doi: 10.1097/00000658-197510000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslaw ES, Kleyn DH. In vitro digestibility of protein in yogurt at various stages of processing. J Food Sci. 1973;38(6):1016–1021. [Google Scholar]
- Bruno MJ, Haverkort EB, Tytgat GN, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol. 1995;90(9):1383–1393. [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Redd LL, Hudson GM, Shelmadine BD, Willoughby DS. Protease supplementation improves muscle function after eccentric exercise. Med Sci Sports Exerc. 2009;41(10):1908–1914. doi: 10.1249/MSS.0b013e3181a518f0. [DOI] [PubMed] [Google Scholar]
- Burkovskaia VA, Beloborodova EI, Glinskaia ON, Markidonova AA, Naumova EL, Akimova LA, Kvach EA, Kaksht AE. Exocrine function of the pancreas in patients with ulcerative colitis. Klin Med (Mosk) 2010;88(1):46–49. [PubMed] [Google Scholar]
- Byrne B, Dankert J. Volatile fatty acids and aerobic flora in the gastrointestinal tract of mice under various conditions. Infect Immun. 1979;23(3):559–563. doi: 10.1128/iai.23.3.559-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camire ME, Camire A, Krumhar K. Chemical and nutritional changes in foods during extrusion. Crit Rev Food Sci Nutr. 1990;29(1):35–57. doi: 10.1080/10408399009527513. [DOI] [PubMed] [Google Scholar]
- Canani RB, Terrin G. Recent progress in congenital diarrheal disorders. Curr Gastroenterol Rep. 2011;13(3):257–264. doi: 10.1007/s11894-011-0188-6. [DOI] [PubMed] [Google Scholar]
- Carroccio A, Iacono G, Montalto G, Cavataio F, Di Marco C, Balsamo V, Notarbartolo A. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. 1991;32(7):796–799. doi: 10.1136/gut.32.7.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroccio A, Iacono G, Montalto G, Cavataio F, Lorello D, Soresi M, Di Martino D, Notarbartolo A. Pancreatic insufficiency in celiac disease is not dependent on nutritional status. Dig Dis Sci. 1994;39(10):2235–2242. doi: 10.1007/BF02090377. [DOI] [PubMed] [Google Scholar]
- Chacko A, Cummings JH. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut. 1988;29(6):809–815. doi: 10.1136/gut.29.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancharoonpong C, Hsieh P-C, Sheu S-C. Enzyme production and growth of Aspergillus oryzae s. on soybean koji fermentation. APCBEE Procedia. 2012;2(0):57–61. [Google Scholar]
- Chandra RK. Five-year follow-up of high-risk infants with family history of allergy who were exclusively breast-fed or fed partial whey hydrolysate, soy, and conventional cow's milk formulas. J Pediatr Gastroenterol Nutr. 1997;24(4):380–388. doi: 10.1097/00005176-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Chen FL, Wei YM, Zhang B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. Lebenson Wiss Technol. 2011;44(4):957–962. [Google Scholar]
- Chiang W-D, Shih C-J, Chu Y-H. Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem. 1999;65(2):189–194. [Google Scholar]
- Chicón R, Belloque J, Alonso E, López-Fandiño R. Immunoreactivity and digestibility of high-pressure-treated whey proteins. Int Dairy J. 2008;18(4):367–376. [Google Scholar]
- Chow J, Panasevich MR, Alexander D, Vester Boler BM, Rossoni Serao MC, Faber TA, Bauer LL, Fahey GC. Fecal metabolomics of healthy breast-fed versus formula-fed infants before and during in vitro batch culture fermentation. J Proteome Res. 2014;13(5):2534–2542. doi: 10.1021/pr500011w. [DOI] [PubMed] [Google Scholar]
- Chuang CH, Sheu BS, Yang HB, Kao AW, Cheng HC, Yao WJ. Hypergastrinemia after Helicobacter pylori infection is associated with bacterial load and related inflammation of the oxyntic corpus mucosa. J Gastroenterol Hepatol. 2004;19(9):988–993. doi: 10.1111/j.1440-1746.2004.03416.x. [DOI] [PubMed] [Google Scholar]
- Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Technol. 2000;11(7):254–262. [Google Scholar]
- Collino S, Montoliu I, Martin F-PJ, Scherer M, Mari D, Salvioli S, Bucci L, Ostan R, Monti D, Biagi E. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PloS one. 2013;8(3):e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet DE, Yin Y, Zhang XM, Remesy C, Stamp D, Medline A, Thompson L, Bruce WR, Archer MC. Colonic protein fermentation and promotion of colon carcinogenesis by thermolyzed casein. Nutr Cancer. 1995;23(3):271–281. doi: 10.1080/01635589509514381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csapá J, Csapó-Kiss Z, Wágner L, Tálos T, Martin TG, Folestad S, Tivesten A, Némethy S. Hydrolysis of proteins performed at high temperatures and for short times with reduced racemization, in order to determine the enantiomers of D-and L-amino acids. Analytica chimica acta. 1997;339(1):99–107. [Google Scholar]
- Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr. 1979;32(10):2094–2101. doi: 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- Dahlin K, Lorenz K. Protein digestibility of extruded cereal grains. Food Chem. 1993;48(1):13–18. [Google Scholar]
- Dallas D, Guerrero A, Khaldi N, Castillo P, Martin W, Smilowitz J, Bevins CL, Barile D, German JB, Lebrilla CB. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J Proteome Res. 2013;12(5):2295–2304. doi: 10.1021/pr400212z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas DC, Guerrero A, Parker EA, Robinson RC, Gan J, German JB, Barile D, Lebrilla CB. Current peptidomics: Applications, purification, identification, quantification and functional analysis. Proteomics. 2015;15:1026–1038. doi: 10.1002/pmic.201400310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas DC, Underwood MA, Zivkovic AM, German JB. Digestion of protein in premature and term infants. J Nutr Disord Ther. 2012;2(3):112–121. doi: 10.4172/2161-0509.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila A-M, Blachier F, Gotteland M, Andriamihaja M, Benetti P-H, Sanz Y, Tomé D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol Res. 2013;68(1):95–107. doi: 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- De Angelis Curtis S, Curini R, Delfini M, Brosio E, D'Ascenzo F, Bocca B. Amino acid profile in the ripening of Grana Padano cheese: a NMR study. Food Chem. 2000;71(4):495–502. [Google Scholar]
- De Angelis M, Coda R, Silano M, Minervini F, Rizzello CG, Di Cagno R, Vicentini O, De Vincenzi M, Gobbetti M. Fermentation by selected sourdough lactic acid bacteria to decrease coeliac intolerance to rye flour. J Cereal Sci. 2006;43(3):301–314. [Google Scholar]
- deWit JN, Klarenbeek G. Effects of various heat treatments on structure and solubility of whey proteins. J Dairy Sci. 1984;67(11):2701–2710. [Google Scholar]
- Di Cagno R, De Angelis M, Auricchio S, Greco L, Clarke C, De Vincenzi M, Giovannini C, D'Archivio M, Landolfo F, Parrilli G. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl Environ Microbiol. 2004;70(2):1088–1096. doi: 10.1128/AEM.70.2.1088-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale MG. Amino acids and proteins for the athlete: The anabolic edge. CRC Press; Boca Raton, FL: 2008. Protein foods versus protein and amino acid supplements; pp. 185–196. [Google Scholar]
- Diesner S, Knittelfelder R, Krishnamurthy D, Pali-Schöll I, Gajdzik L, Jensen-Jarolim E, Untersmayr E. Dose-dependent food allergy induction against ovalbumin under acid-suppression: a murine food allergy model. Immunol Lett. 2008;121(1):45–51. doi: 10.1016/j.imlet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duar R, Clark K, Patil P, Hernández C, Brüning S, Burkey T, Madayiputhiya N, Taylor S, Walter J. Identification and characterization of intestinal lactobacilli strains capable of degrading immunotoxic peptides present in gluten. J Appl Microbiol. 2015;118(2):515–527. doi: 10.1111/jam.12687. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73(4):1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duodu KG, Taylor JRN, Belton PS, Hamaker BR. Factors affecting sorghum protein digestibility. J Cereal Sci. 2003;38(2):117–131. [Google Scholar]
- Dupont D, Mandalari G, Molle D, Jardin J, Léonil J, Faulks RM, Wickham MS, Clare Mills E, Mackie AR. Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol Nutr Food Res. 2010;54(6):767–780. doi: 10.1002/mnfr.200900142. [DOI] [PubMed] [Google Scholar]
- Dupont D, Mandalari G, Mollé D, Jardin J, Rolet-Répécaud O, Duboz G, Léonil J, Mills CE, Mackie AR. Food processing increases casein resistance to simulated infant digestion. Mol Nutr Food Res. 2010;54(11):1677–1689. doi: 10.1002/mnfr.200900582. [DOI] [PubMed] [Google Scholar]
- el Nujumi AM, Rowe PA, Dahill S, Dorrian CA, Neithercut WD, McColl KE. Role of ammonia in the pathogenesis of the gastritis, hypergastrinaemia, and hyperpepsinogenaemia I caused by Helicobacter pylori infection. Gut. 1992;33(12):1612–1616. doi: 10.1136/gut.33.12.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embaby HE-S. Effect of heat treatments on certain antinutrients and in vitro protein digestibility of peanut and sesame seeds. Food Sci Technol Res. 2010;17(1):31–38. [Google Scholar]
- Engelen MP, Com G, Deutz NE. Protein is an important but undervalued macronutrient in the nutritional care of patients with cystic fibrosis. Curr Opin Clin Nutr Metab Care. 2014;17(6):515–520. doi: 10.1097/MCO.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen MPKJ, Com G, Anderson PJ, Deutz NEP. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr. 2014;33(6):1024–1032. doi: 10.1016/j.clnu.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen MPKJ, Com G, Wolfe RR, Deutz NEP. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. J Cyst Fibros. 2013;12(5):445–453. doi: 10.1016/j.jcf.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbersdobler H, Hupe A. Determination of lysine damage and calculation of lysine bio-availability in several processed foods. Z Ernahrungswiss. 1991;30(1):46–49. doi: 10.1007/BF01910731. [DOI] [PubMed] [Google Scholar]
- Erickson RH, Kim YS. Digestion and absorption of dietary protein. Annu Rev Med. 1990;41(1):133–139. doi: 10.1146/annurev.me.41.020190.001025. [DOI] [PubMed] [Google Scholar]
- Evans KE, Leeds JS, Morley S, Sanders DS. Pancreatic insufficiency in adult celiac disease: do patients require long-term enzyme supplementation? Dig Dis Sci. 2010;55(10):2999–3004. doi: 10.1007/s10620-010-1261-y. [DOI] [PubMed] [Google Scholar]
- Evenepoel P, Claus D, Geypens B, Hiele M, Geboes K, Rutgeerts P, Ghoos Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am J Physiol. 1999;277(5 Pt 1):G935–943. doi: 10.1152/ajpgi.1999.277.5.G935. [DOI] [PubMed] [Google Scholar]
- Evenepoel P, Claus D, Geypens B, Maes B, Hiele M, Rutgeerts P, Ghoos Y. Evidence for impaired assimilation and increased colonic fermentation of protein, related to gastric acid suppression therapy. Aliment Pharmacol Ther. 1998;12(10):1011–1019. doi: 10.1046/j.1365-2036.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- Evenepoel P, Geypens B, Luypaerts A, Hiele M, Ghoos Y, Rutgeerts P. Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J Nutr. 1998;128(10):1716–1722. doi: 10.1093/jn/128.10.1716. [DOI] [PubMed] [Google Scholar]
- Feng J, Liu X, Xu Z, Lu Y, Liu Y. The effect of Aspergillus oryzae fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim Feed Sci Technol. 2007;134(3):295–303. [Google Scholar]
- Ferrone M, Raimondo M, Scolapio JS. Pancreatic enzyme pharmacotherapy. Pharmacotherapy. 2007;27(6):910–920. doi: 10.1592/phco.27.6.910. [DOI] [PubMed] [Google Scholar]
- Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol. 2011;4:55–73. doi: 10.2147/CEG.S17634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88(06):587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Frank DN, Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraquelli M, Losco A, Visentin S, Cesana BM, Pometta R, Colli A, Conte D. Gallstone disease and related risk factors in patients with Crohn's disease: analysis of 330 consecutive cases. Arch Intern Med. 2001;161(18):2201–2204. doi: 10.1001/archinte.161.18.2201. [DOI] [PubMed] [Google Scholar]
- Freeman HJ, Kim YS. Digestion and absorption of protein. Annu Rev Med. 1978;29(1):99–116. doi: 10.1146/annurev.me.29.020178.000531. [DOI] [PubMed] [Google Scholar]
- Freston JW, Borch K, Brand SJ, Carlsson E, Creutzfeldt W, Håkanson R, Olbe L, Solcia E, Walsh JH, Wolfe MM. Effects of hypochlorhydria and hypergastrinemia on structure and function of gastrointestinal cells. Dig Dis Sci. 1995;40(2):50S–62S. doi: 10.1007/BF02214871. [DOI] [PubMed] [Google Scholar]
- Friedman M. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. J Agric Food Chem. 1999;47(4):1295–1319. doi: 10.1021/jf981000+. [DOI] [PubMed] [Google Scholar]
- Friedman M. Biological effects of Maillard browning products that may affect acrylamide safety in food. In: Friedman M, Mottram DS, editors. Chemistry and Safety of Acrylamide in Food. Springer; New York, NY: 2005. pp. 135–156. [DOI] [PubMed] [Google Scholar]
- Friedman M, Brandon DL. Nutritional and health benefits of soy proteins. J Agric Food Chem. 2001;49(3):1069–1086. doi: 10.1021/jf0009246. [DOI] [PubMed] [Google Scholar]
- Friedman M, Levin CE, Noma AT. Factors governing lysinoalanine formation in soy proteins. J Food Sci. 1984;49(5):1282–1288. [Google Scholar]
- Fuller MF, Tomé D. In vivo determination of amino acid bioavailability in humans and model animals. J AOAC Int. 2005;88(3):923–934. [PubMed] [Google Scholar]
- Furu K, Straume B. Use of antacids in a general population: the impact of health-related variables, lifestyle and sociodemographic characteristics. J Clin Epidemiol. 1999;52(6):509–516. doi: 10.1016/s0895-4356(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Gerrard JA. Protein–protein crosslinking in food: methods, consequences, applications. Trends Food Sci Technol. 2002;13(12):391–399. [Google Scholar]
- Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, Peeters M, Rutgeerts P, Ghoos Y. Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut. 1997;41(1):70–76. doi: 10.1136/gut.41.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavidel RA, Prakash J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. Lebenson Wiss Technol. 2007;40(7):1292–1299. [Google Scholar]
- Gibson JA, Sladen GE, Dawson AM. Protein absorption and ammonia production: the effects of dietary protein and removal of the colon. Br J Nutr. 1976;35(1):61–65. doi: 10.1079/bjn19760009. [DOI] [PubMed] [Google Scholar]
- Gilani GS, Sepehr E. Protein digestibility and quality in products containing antinutritional factors are adversely affected by old age in rats. J Nutr. 2003;133(1):220–225. doi: 10.1093/jn/133.1.220. [DOI] [PubMed] [Google Scholar]
- Gilbert J-A, Bendsen N, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis. 2011;21:B16–B31. doi: 10.1016/j.numecd.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Grady T, Mah’Moud M, Otani T, Rhee S, Lerch M, Gorelick F. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol Gastrointest Liver Physiol. 1998;275(5):G1010–G1017. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- Greenberger NJ. Enzymatic therapy in patients with chronic pancreatitis. Gastroenterol Clin North Am. 1999;28(3):687–693. doi: 10.1016/s0889-8553(05)70081-9. [DOI] [PubMed] [Google Scholar]
- Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- Guarino A, Lo Vecchio A, Berni Canani R. Chronic diarrhoea in children. Best Pract Res Clin Gastroenterol. 2012;26(5):649–661. doi: 10.1016/j.bpg.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Gullo L, Simoni P, Migliori M, Lucrezio L, Bassi M, Frau F, Lorenzo Costa P, Nestico V. A study of pancreatic function among subjects over ninety years of age. Pancreatology. 2009;9(3):240–244. doi: 10.1159/000212090. [DOI] [PubMed] [Google Scholar]
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Christensen AEF, Hansen LG, Kier SR, Lassen K, Lintrup M, Mortensen S, Ibsen KK, Østerballe O, Høst A. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention:A prospective, randomized study. Pediatr Allergy Immunol. 2000;11(3):149–161. doi: 10.1034/j.1399-3038.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Hällgren R, Karlsson FA, Lundqvist G. Serum level of immunoreactive gastrin: influence of kidney function. Gut. 1978;19(3):207–213. doi: 10.1136/gut.19.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost F, BRUMMER RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Harmsen H, Wildeboer-Veloo A, Raangs G, Wagendorp A, Klijn N, Bindels J, Welling G. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30(1):61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- Havenaar R, de Jong A, Koenen ME, van Bilsen J, Janssen AM, Labij E, Westerbeek HJM. Digestibility of transglutaminase cross-linked caseinate versus native caseinate in an in vitro multicompartmental model simulating young child and adult gastrointestinal conditions. J Agric Food Chem. 2013;61(31):7636–7644. doi: 10.1021/jf402824u. [DOI] [PubMed] [Google Scholar]
- Haworth J, Gourley B, Hadorn B, Sumida C. Malabsorption and growth failure due to intestinal enterokinase deficiency. J Pediatr. 1971;78(3):481–490. doi: 10.1016/s0022-3476(71)80231-7. [DOI] [PubMed] [Google Scholar]
- Heijnen M-LA, Beynen AC. Consumption of retrograded (RS3) but not uncooked (RS2) resistant starch shifts nitrogen excretion from urine to feces in cannulated piglets. J Nutr. 1997;127(9):1828–1832. doi: 10.1093/jn/127.9.1828. [DOI] [PubMed] [Google Scholar]
- Heo J, Kim J, Hansen CF, Mullan B, Hampson D, Pluske J. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of. J Anim Sci. 2009;87(9):2833–2843. doi: 10.2527/jas.2008-1274. [DOI] [PubMed] [Google Scholar]
- Herrlinger K, Stange E. The pancreas and inflammatory bowel diseases. Int J Pancreatol. 2000;27(3):171–179. doi: 10.1385/IJGC:27:3:171. [DOI] [PubMed] [Google Scholar]
- Herzig K-H, Purhonen A-K, Räsänen KM, Idziak J, Juvonen P, Phillps R, Walkowiak J. Fecal pancreatic elastase-1 levels in older individuals without known gastrointestinal diseases or diabetes mellitus. BMC geriatrics. 2011;11(4):1–5. doi: 10.1186/1471-2318-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho G-T, Boyapati R, Satsangi J. Ulcerative colitis. Medicine. 2015;43(5):276–281. [Google Scholar]
- Hong K-J, Lee C-H, Kim SW. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 2004;7(4):430–435. doi: 10.1089/jmf.2004.7.430. [DOI] [PubMed] [Google Scholar]
- Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39(8):647–651. doi: 10.1007/s00595-009-3964-2. [DOI] [PubMed] [Google Scholar]
- Htoo J, Araiza B, Sauer W, Rademacher M, Zhang Y, Cervantes M, Zijlstra R. Effect of dietary protein content on ileal amino acid digestibility, growth performance, and formation of microbial metabolites in ileal and cecal digesta of early-weaned pigs. J Anim Sci. 2007;85(12):3303–3312. doi: 10.2527/jas.2007-0105. [DOI] [PubMed] [Google Scholar]
- Hughes R, Kurth MJ, McGilligan V, McGlynn H, Rowland I. Effect of colonic bacterial metabolites on Caco-2 cell paracellular permeability in vitro. Nutr Cancer. 2008;60(2):259–266. doi: 10.1080/01635580701649644. [DOI] [PubMed] [Google Scholar]
- Hunt J, Payne-James J, Palmer K, Kumar P, Clark M, Farthing M, Misiewicz J, Silk D. A randomised controlled trial of elemental diet and prednisolone as primary therapy in acute exacerbations of Crohn's disease. Gastroenterology. 1989;96:A224. [Google Scholar]
- Hurrell R. Influence of the Maillard reaction on the nutritional value of foods. In: Finot P, Aeschbacher H, Hurrell R, Liardon R, editors. The Maillard reaction in food processing, human nutrition and physiology. Birkhauser; Berlin, Germany: 1990. pp. 245–258. [Google Scholar]
- Jansman A. Tannins in feedstuffs for simple-stomached animals. Nutr Res Rev. 1993;6(01):209–236. doi: 10.1079/NRR19930013. [DOI] [PubMed] [Google Scholar]
- Jansman A, Enting H, Verstegen M, Huisman J. Effect of condensed tannins in hulls of faba beans (Vicia faba L.) on the activities of trypsin (EC 2.4. 21.4) and chymotrypsin (EC 2.4. 21.1) in digesta collected from the small intestine of pigs. Br J Nutr. 1994;71(04):627–641. doi: 10.1079/bjn19940168. [DOI] [PubMed] [Google Scholar]
- Jeaurond E, Rademacher M, Pluske J, Zhu C, De Lange C. Impact of feeding fermentable proteins and carbohydrates on growth performance, gut health and gastrointestinal function of newly weaned pigs. Can J Anim Sci. 2008;88(2):271–281. [Google Scholar]
- Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol Toxicol. 2002;91(6):333–350. doi: 10.1034/j.1600-0773.2002.910611.x. [DOI] [PubMed] [Google Scholar]
- Jost R, Maire JC, Maynard F, Secretin MC. Aspects of whey protein usage in infant nutrition, a brief review. Int J Food Sci Technol. 1999;34(5-6):533–542. [Google Scholar]
- Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, Welfare MR. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53(10):1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya M, Iwata N, Toda Y, Nakae Y, Kondo T. Small bowel transit time and colonic fermentation in young and elderly women. J Gastroenterol. 1997;32(4):453–456. doi: 10.1007/BF02934082. [DOI] [PubMed] [Google Scholar]
- Kakade M, Evans RJ. Chemical and enzymatic determinations of available lysine in raw and heated navy beans (Phaseolus vulgaris) Can J Biochem. 1966;44(5):648–650. doi: 10.1139/o66-078. [DOI] [PubMed] [Google Scholar]
- Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38(2):257–268. doi: 10.1007/BF01307542. [DOI] [PubMed] [Google Scholar]
- Kataria A, Chauhan B, Punia D. Antinutrients and protein digestibility (in vitro) of mungbean as affected by domestic processing and cooking. Food Chem. 1989;32(1):9–17. [Google Scholar]
- Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(suppl 6):1–28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstens PJSM, Lamers CBHW, Jansen JBMJ, de Jong AJL, Hessels M, Hafkenscheid JCM. Physiological plasma concentrations of cholecystokinin stimulate pancreatic enzyme secretion and gallbladder contraction in man. Life Sci. 1985;36(6):565–569. doi: 10.1016/0024-3205(85)90638-1. [DOI] [PubMed] [Google Scholar]
- Khalil NA, Walton GE, Gibson GR, Tuohy KM, Andrews SC. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int J Food Sci Nutr. 2014;65(1):79–88. doi: 10.3109/09637486.2013.825700. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Chauhan B. Antinutritional factors in moth bean (Vigna aconitifolia): varietal differences and effects of methods of domestic processing and cooking. J Food Sci. 1986;51(3):591–594. [Google Scholar]
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Misiewicz A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol (Praha) 2014;59(3):241–250. doi: 10.1007/s12223-013-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Heo J, Mullan B, Pluske J. Efficacy of a reduced protein diet on clinical expression of post-weaning diarrhoea and life-time performance after experimental challenge with an enterotoxigenic strain of Escherichia coli. Anim Feed Sci Technol. 2011;170(3):222–230. [Google Scholar]
- Kinouchi T, Koyama S, Harada E, Yajima T. Large molecule protein feeding during the suckling period is required for the development of pancreatic digestive functions in rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(12):R1268–R1276. doi: 10.1152/ajpregu.00064.2012. [DOI] [PubMed] [Google Scholar]
- Koop H. Review article: metabolic consequences of long-term inhibition of acid secretion by omeprazole. Aliment Pharmacol Ther. 1992;6(4):399–406. doi: 10.1111/j.1365-2036.1992.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90(1):106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan A, Andriamihaja M, Blouin J-M, Liu X, Descatoire V, de Maredsous CD, Davila A-M, Walker F, Tomé D, Blachier F. High-protein diet differently modifies intestinal goblet cell characteristics and mucosal cytokine expression in ileum and colon. The Journal of nutritional biochemistry. 2015;26(1):91–98. doi: 10.1016/j.jnutbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobecourt E, Boitard C. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29(8):1047–1054. doi: 10.1111/j.1464-5491.2012.03597.x. [DOI] [PubMed] [Google Scholar]
- Lassé M, Deb-Choudhury S, Haines S, Larsen N, Gerrard JA, Dyer JM. The impact of pH, salt concentration and heat on digestibility and amino acid modification in egg white protein. J Food Compost Anal. 2015;38:42–48. [Google Scholar]
- Laugier R, Bernard JP, Berthezene P, Dupuy P. Changes in pancreatic exocrine secretion with age: pancreatic exocrine secretion does decrease in the elderly. Digestion. 1991;50(3-4):202–211. doi: 10.1159/000200762. [DOI] [PubMed] [Google Scholar]
- Lebenthal E, Antonowicz I, Shwachman H. The interrelationship of enterokinase and trypsin activities in intractable diarrhea of infancy, celiac disease, and intravenous alimentation. Pediatrics. 1975;56(4):585–591. [PubMed] [Google Scholar]
- LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Lee H, Friend BA, Shahani KM. Factors affecting the protein quality of yogurt and acidophilus milk. J Dairy Sci. 1988;71(12):3203–3213. [Google Scholar]
- Leeds J, Hopper A, Hurlstone D, Edwards S, McAlindon M, Lobo A, Donnelly M, Morley S, Sanders D. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. 2007;25(3):265–271. doi: 10.1111/j.1365-2036.2006.03206.x. [DOI] [PubMed] [Google Scholar]
- Leeds JS, Hopper AD, Sidhu R, Simmonette A, Azadbakht N, Hoggard N, Morley S, Sanders DS. Some patients with irritable bowel syndrome may have exocrine pancreatic insufficiency. Clin Gastroenterol Hepatol. 2010;8(5):433–438. doi: 10.1016/j.cgh.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Leschelle X, Goubern M, Andriamihaja M, Blottière HM, Couplan E, Gonzalez-Barroso M.-d.-M., Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta. 2005;1725(2):201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK, Holmes E. Metabolic surgery profoundly influences gut microbial–host metabolic cross-talk. Gut. 2011;60(9):1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liener IE. Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr. 1994;34(1):31–67. doi: 10.1080/10408399409527649. [DOI] [PubMed] [Google Scholar]
- Lindberg T, Engberg S, Jakobsson I, Lönnerdal B. Digestion of proteins in human milk, human milk fortifier, and preterm formula in infant rhesus monkeys. J Pediatr Gastroenterol Nutr. 1997;24(5):537–543. doi: 10.1097/00005176-199705000-00009. [DOI] [PubMed] [Google Scholar]
- Lindberg T, Engberg S, Sĵberg L, Lönnerdal B. In vitro digestion of proteins in human milk fortifiers and in preterm formula. J Pediatr Gastroenterol Nutr. 1998;27(1):30. doi: 10.1097/00005176-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Linskens RK, Huijsdens XW, Savelkoul PHM, Vandenbroucke-Grauls CMJE, Meuwissen SGM. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroentero. 2001;36(234):29–40. doi: 10.1080/003655201753265082. [DOI] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano J-M, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes Rev. 2005;6(4):297–306. doi: 10.1111/j.1467-789X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- Lott JNA, Ockenden I, Radoy V, Batten GD. A global estimate of phytic acid and phosphorous in crop grains, seeds and fruits. In: Reddy NR, Sathe SK, editors. Food Phytates. CRC Press; Boca Raton, Fl: 2002. pp. 7–24. [Google Scholar]
- Lubbs DC, Vester BM, Fastinger ND, Swanson KS. Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J Anim Physiol Anim Nutr (Berl) 2009;93(1):113–121. doi: 10.1111/j.1439-0396.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- M’hir S, Rizzello CG, Di Cagno R, Cassone A, Hamdi M. Use of selected enterococci and Rhizopus oryzae proteases to hydrolyse wheat proteins responsible for celiac disease. J Appl Microbiol. 2009;106(2):421–431. doi: 10.1111/j.1365-2672.2008.04008.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane G, Cummings J, Allison C. Protein degradation by human intestinal bacteria. Microbiology. 1986;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95(1):50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- Maconi G, Dominici R, Molteni M, Ardizzone S, Bosani M, Ferrara E, Gallus S, Panteghini M, Bianchi Porro G. Prevalence of pancreatic insufficiency in inflammatory bowel diseases. Assessment by fecal elastase-1. Dig Dis Sci. 2008;53(1):262–270. doi: 10.1007/s10620-007-9852-y. [DOI] [PubMed] [Google Scholar]
- Maga JA. Lysinoalanine in foods. J Agric Food Chem. 1984;32(5):955–964. [Google Scholar]
- Mahmoud R, Savello PA. Solubility and hydrolyzability of films produced by transglutaminase catalytic crosslinking of whey protein. J Dairy Sci. 1993;76(1):29–35. [Google Scholar]
- Maldonado J, Gil A, Narbona E, Molina JA. Special formulas in infant nutrition: a review. Early Hum Dev. 1998;53:S23–S32. doi: 10.1016/s0378-3782(98)00062-0. [DOI] [PubMed] [Google Scholar]
- Malyszko J, Sosnowski S, Mazerska M, Raimer J, Romatowski J, Stasiewicz J, Kemona A, Mysliwiec M. Gastric and pancreatic functions in haemodialyzed patients. Int Urol Nephrol. 1995;27(4):471–478. doi: 10.1007/BF02550086. [DOI] [PubMed] [Google Scholar]
- Manninen AH. Protein hydrolysates in sports and exercise: A brief review. J Sports Sci Med. 2004;3(2):60–63. [PMC free article] [PubMed] [Google Scholar]
- Mansfield JC, Giaffer MH, Holdsworth CD. Controlled trial of oligopeptide versus amino acid diet in treatment of active Crohn's disease. Gut. 1995;36(1):60–66. doi: 10.1136/gut.36.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariniello L, Giosafatto CVL, Di Pierro P, Sorrentino A, Porta R. Synthesis and resistance to in vitro proteolysis of transglutaminase cross-linked phaseolin, the major storage protein from Phaseolus vulgaris. J Agric Food Chem. 2007;55(12):4717–4721. doi: 10.1021/jf0637269. [DOI] [PubMed] [Google Scholar]
- Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96(2):94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- McCall IC, Betanzos A, Weber DA, Nava P, Miller GW, Parkos CA. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharm. 2009;241(1):61–70. doi: 10.1016/j.taap.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medani M, Collins D, Docherty NG, Baird AW, O'Connell PR, Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17(7):1620–1625. doi: 10.1002/ibd.21528. [DOI] [PubMed] [Google Scholar]
- Meijers BK, Evenepoel P. The gut–kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. 2011;26(3):759–761. doi: 10.1093/ndt/gfq818. [DOI] [PubMed] [Google Scholar]
- Mercier Y, Gatellier P, Viau M, Remignon H, Renerre M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998;48(3):301–318. doi: 10.1016/s0309-1740(97)00113-7. [DOI] [PubMed] [Google Scholar]
- Miller PC, Bailey SP, Barnes ME, Derr SJ, Hall EE. The effects of protease supplementation on skeletal muscle function and DOMS following downhill running. J Sports Sci. 2004;22(4):365–372. doi: 10.1080/02640410310001641584. [DOI] [PubMed] [Google Scholar]
- Mills DJS, Tuohy KM, Booth J, Buck M, Crabbe MJC, Gibson GR, Ames JM. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. J Appl Microbiol. 2008;105(3):706–714. doi: 10.1111/j.1365-2672.2008.03783.x. [DOI] [PubMed] [Google Scholar]
- Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57(1):25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- Monogioudi E, Faccio G, Lille M, Poutanen K, Buchert J, Mattinen M-L. Effect of enzymatic cross-linking of β-casein on proteolysis by pepsin. Food Hydrocoll. 2011;25(1):71–81. [Google Scholar]
- Moolsintong P, Loftus EV, Chari ST, Egan LJ, Tremaine WJ, Sandborn WJ. Acute pancreatitis in patients with Crohn's disease: clinical features and outcomes. Inflamm Bowel Dis. 2005;11(12):1080–1084. doi: 10.1097/01.mib.0000186485.30623.ad. [DOI] [PubMed] [Google Scholar]
- Morrison G, Morrison JM, Redmond AOB, Byers CA, McCracken KJ, Dodge JA, Guilfords SA, Bowden MW. Comparison between a standard pancreatic supplement and a high enzyme preparation in cystic fibrosis. Aliment Pharmacol Ther. 1992;6(5):549–555. doi: 10.1111/j.1365-2036.1992.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Morzel M, Gatellier P, Sayd T, Renerre M, Laville E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006;73(3):536–543. doi: 10.1016/j.meatsci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Mottu F, Mauron J. The differential determination of lysine in heated milk II. Comparison of the in vitro methods with the biological evaluation. J Sci Food Agric. 1967;18(2):57–62. doi: 10.1002/jsfa.2740180205. [DOI] [PubMed] [Google Scholar]
- Moughan PJ. Amino acid availability: aspects of chemical analysis and bioassay methodology. Nutr Res Rev. 2003;16(02):127–141. doi: 10.1079/NRR200365. [DOI] [PubMed] [Google Scholar]
- Moughan PJ, Butts CA, Rowan AM, Deglaire A. Dietary peptides increase endogenous amino acid losses from the gut in adults. Am J Clin Nutr. 2005;81(6):1359–1365. doi: 10.1093/ajcn/81.6.1359. [DOI] [PubMed] [Google Scholar]
- Ng W, Tonzetich J. Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J Dent Res. 1984;63(7):994–997. doi: 10.1177/00220345840630071701. [DOI] [PubMed] [Google Scholar]
- Nielsen SS, Deshpande SS, Hermodson MA, Scott MP. Comparative digestibility of legume storage proteins. J Agric Food Chem. 1988;36(5):896–902. [Google Scholar]
- Nissler K, Von Katte I, Huebner A, Henker J. Pancreatic elastase 1 in feces of preterm and term infants. J Pediatr Gastroenterol Nutr. 2001;33(1):28–31. doi: 10.1097/00005176-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Nousia-Arvanitakis S, Fotoulaki M, Tendzidou K, Vassilaki C, Agguridaki C, Karamouzis M. Subclinical exocrine pancreatic dysfunction resulting from decreased cholecystokinin secretion in the presence of intestinal villous atrophy. J Pediatr Gastroenterol Nutr. 2006;43(3):307–312. doi: 10.1097/01.mpg.0000228098.66583.85. [DOI] [PubMed] [Google Scholar]
- Nousia-Arvanitakis S, Karagiozoglou-Lamboudes T, Aggouridaki C, Malaka-Lambrellis E, Galli-Tsinopoulou A, Xefteri M. Influence of jejunal morphology changes on exocrine pancreatic function in celiac disease. J Pediatr Gastroenterol Nutr. 1999;29(1):81–85. doi: 10.1097/00005176-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Nyachoti C, Omogbenigun F, Rademacher M, Blank G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J Anim Sci. 2006;84(1):125–134. doi: 10.2527/2006.841125x. [DOI] [PubMed] [Google Scholar]
- O'moráin C, Segal A, Levi A. Elemental diet as primary treatment of acute Crohn's disease: a controlled trial. BMJ. 1984;288(6434):1859–1862. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan NJ, Toden S, Bird AR, Topping DL, Fenech M, Conlon MA. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin Nutr. 2012;31(1):60–64. doi: 10.1016/j.clnu.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Oeste RE, Miller R, Sjoestroem H, Noren O. Effect of Maillard reaction products on protein digestion. Studies on pure compounds. J Agric Food Chem. 1987;35(6):938–942. [Google Scholar]
- Osborn DA, Sinn JK. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Libr. 2006:1–144. doi: 10.1002/14651858.CD003664.pub3. [DOI] [PubMed] [Google Scholar]
- Osborne DL, Seidel ER. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am J Physiol Gastrointest Liver Physiol. 1990;258(4):G576–G584. doi: 10.1152/ajpgi.1990.258.4.G576. [DOI] [PubMed] [Google Scholar]
- Pairent FW, Howard JM. Pancreatic exocrine insufficiency: IV. the enzyme content of commercial pancreatic supplements. Arch Surg. 1975;110(6):739–741. doi: 10.1001/archsurg.1975.01360120057010. [DOI] [PubMed] [Google Scholar]
- Pang T, Leach ST, Katz T, Day AS, Ooi CY. Fecal biomarkers of intestinal health and disease in children. Front Pediatr. 2014;2(6):1–12. doi: 10.3389/fped.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente F, Pastore L, Bargiggia S, Cucino C, Greco S, Molteni M, Ardizzone S, Porro GB, Sampietro GM, Giorgi R, Moretti R, Gallus S. Incidence and risk factors for gallstones in patients with inflammatory bowel disease: a large case-control study. Hepatology. 2007;45(5):1267–1274. doi: 10.1002/hep.21537. [DOI] [PubMed] [Google Scholar]
- Parkouda C, Nielsen DS, Azokpota P, Ivette Irène Ouoba L, Amoa-Awua WK, Thorsen L, Hounhouigan JD, Jensen JS, Tano-Debrah K, Diawara B, Jakobsen M. The microbiology of alkaline-fermentation of indigenous seeds used as food condiments in Africa and Asia. Crit Rev Microbiol. 2009;35(2):139–156. doi: 10.1080/10408410902793056. [DOI] [PubMed] [Google Scholar]
- Pedersen G, Brynskov J, Saermark T. Phenol toxicity and conjugation in human colonic epithelial cells. Scand J Gastroentero. 2002;37(1):74–79. doi: 10.1080/003655202753387392. [DOI] [PubMed] [Google Scholar]
- Pieper R, Boudry C, Bindelle J, Vahjen W, Zentek J. Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Archives of animal nutrition. 2014;68(4):263–280. doi: 10.1080/1745039X.2014.932962. [DOI] [PubMed] [Google Scholar]
- Pieper R, Kröger S, Richter JF, Wang J, Martin L, Bindelle J, Htoo JK, von Smolinski D, Vahjen W, Zentek J. Fermentable fiber ameliorates fermentable protein-induced changes in microbial ecology, but not the mucosal response, in the colon of piglets. J Nutr. 2012;142(4):661–667. doi: 10.3945/jn.111.156190. [DOI] [PubMed] [Google Scholar]
- Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44(4):246–253. doi: 10.1097/MCG.0b013e3181cadbe1. [DOI] [PubMed] [Google Scholar]
- Potter SM, Jimenez-Flores R, Pollack J, Lone TA, Berber-Jimenez MD. Protein-saponin interaction and its influence on blood lipids. J Agric Food Chem. 1993;41(8):1287–1291. [Google Scholar]
- Preet K, Punia D. Antinutrients and digestibility (in vitro) of soaked, dehulled and germinated cowpeas. Nutr Health. 2000;14(2):109–117. doi: 10.1177/026010600001400203. [DOI] [PubMed] [Google Scholar]
- Prichard PJ, Yeomans ND, Mihaly GW, Jones DB, Buckle PJ, Smallwood RA, Louis WJ. Omeprazole: a study of its inhibition of gastric pH and oral pharmacokinetics after morning or evening dosage. Gastroenterology. 1985;88(1):64–69. doi: 10.1016/s0016-5085(85)80133-5. [DOI] [PubMed] [Google Scholar]
- Pritchard GG, Coolbear T. The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiology Reviews. 1993;12(1-3):179–206. doi: 10.1111/j.1574-6976.1993.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Qiu C, Sun W, Cui C, Zhao M. Effect of citric acid deamidation on in vitro digestibility and antioxidant properties of wheat gluten. Food Chem. 2013;141(3):2772–2778. doi: 10.1016/j.foodchem.2013.05.072. [DOI] [PubMed] [Google Scholar]
- Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, O'Toole PW, Brigidi P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 2013;5(12) doi: 10.18632/aging.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmann W, Haastert B, Icks A, Giani G, Hennings S, Mitchell J, Curran S, Wareham N. Low faecal elastase 1 concentrations in type 2 diabetes mellitus. Scand J Gastroentero. 2001;36(10):1056–1061. doi: 10.1080/003655201750422657. [DOI] [PubMed] [Google Scholar]
- Reddy IM, Kella NK, Kinsella JE. Structural and conformational basis of the resistance of beta-lactoglobulin to peptic and chymotryptic digestion. J Agric Food Chem. 1988;36(4):737–741. [Google Scholar]
- Renerre M, Poncet K, Mercier Y, Gatellier P, Métro B. Influence of dietary fat and vitamin E on antioxidant status of muscles of turkey. J Agric Food Chem. 1999;47(1):237–244. doi: 10.1021/jf9805000. [DOI] [PubMed] [Google Scholar]
- Rerat A, Nunes CS, Mendy F, Roger L. Amino acid absorption and production of pancreatic hormones in non-anaesthetized pigs after duodenal infusions of a milk enzymic hydrolysate or of free amino acids. Br J Nutr. 1988;60(1):121–136. doi: 10.1079/bjn19880082. [DOI] [PubMed] [Google Scholar]
- Richardson A, McKain N, Wallace RJ. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013;13(1):6. doi: 10.1186/1471-2180-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi Schinkel E, Pettine SM, Adams E, Harris M. Impact of varying levels of protein intake on protein status indicators after gastric bypass in patients with multiple complications requiring nutritional support. Obes Surg. 2006;16(1):24–30. doi: 10.1381/096089206775222168. [DOI] [PubMed] [Google Scholar]
- Rist VTS, Weiss E, Eklund M, Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal. 2013;7(07):1067–1078. doi: 10.1017/S1751731113000062. [DOI] [PubMed] [Google Scholar]
- Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83(2):424–429. [PubMed] [Google Scholar]
- Roediger WE, Duncan A, Kapaniris O, Millard S. Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis? Clin Sci (Lond) 1993;85(5):623–627. doi: 10.1042/cs0850623. [DOI] [PubMed] [Google Scholar]
- Rothenbacher D, Löw M, Hardt PD, Klör H-U, Ziegler H, Brenner H. Prevalence and determinants of exocrine pancreatic insufficiency among older adults: Results of a population-based study. Scand J Gastroentero. 2005;40(6):697–704. doi: 10.1080/00365520510023116. [DOI] [PubMed] [Google Scholar]
- Rowan F, Docherty NG, Murphy M, Murphy B, Coffey JC, O’Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53(11):1530–1536. doi: 10.1007/DCR.0b013e3181f1e620. [DOI] [PubMed] [Google Scholar]
- Rowan FE, Docherty NG, Coffey JC, O'Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br J Surg. 2009;96(2):151–158. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- Rowe L, Maddock K, Lonergan SM, Huff-Lonergan E. Influence of early postmortem protein oxidation on beef quality. J Anim Sci. 2004;82(3):785–793. doi: 10.2527/2004.823785x. [DOI] [PubMed] [Google Scholar]
- Rowe LJ, Maddock K, Lonergan SM, Huff-Lonergan E. Oxidative environments decrease tenderization of beef steaks through inactivation of μ-calpain. J Anim Sci. 2004;82(11):3254–3266. doi: 10.2527/2004.82113254x. [DOI] [PubMed] [Google Scholar]
- Russell RM. Factors in aging that effect the bioavailability of nutrients. J Nutr. 2001;131(4):1359S–1361S. doi: 10.1093/jn/131.4.1359S. [DOI] [PubMed] [Google Scholar]
- Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, Duncan G, Johnstone AM, Lobley GE, Wallace RJ, Duthie GG, Flint HJ. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- Ryden P, Selvendran RR. Encyclopedia of food science, food technology, and nutrition. In: Macrae R, Robinson RK, editors. Encyclopedia of food science, food technology, and nutrition. Academic Press; San Diego, CA: 1993. pp. 3582–3587. [Google Scholar]
- Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreat ic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- Salvatore S, Finazzi S, Barassi A, Verzelletti M, Tosi A, Melzi d'Eril GV, Nespoli L. Low fecal elastase: potentially related to transient small bowel damage resulting from enteric pathogens. J Pediatr Gastroenterol Nutr. 2003;36(3):392–396. doi: 10.1097/00005176-200303000-00018. [DOI] [PubMed] [Google Scholar]
- Santé-Lhoutellier V, Astruc T, Marinova P, Greve E, Gatellier P. Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins. J Agric Food Chem. 2008;56(4):1488–1494. doi: 10.1021/jf072999g. [DOI] [PubMed] [Google Scholar]
- Santé-Lhoutellier V, Engel E, Aubry L, Gatellier P. Effect of animal (lamb) diet and meat storage on myofibrillar protein oxidation and in vitro digestibility. Meat Sci. 2008;79(4):777–783. doi: 10.1016/j.meatsci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Sareneva T, Pirhonen J, Cantell K, Julkunen I. N-glycosylation of human interferon-gamma: glycans at Asn-25 are critical for protease resistance. Biochem J. 1995;308(1):9–14. doi: 10.1042/bj3080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar G, L’Abbé M, Trick K, Botting H, Ma C. Influence of feeding alkaline/heat processed proteins on growth and protein and mineral status of rats. In: Jackson LS, Knize MG, Morgan JN, editors. Impact of Processing on Food Safety. Springer; New York, NY: 1999. pp. 161–177. [Google Scholar]
- Savelkoul FHMG, Van Der Poel AFB, Tamminga S. The presence and inactivation of trypsin inhibitors, tannins, lectins and amylase inhibitors in legume seeds during germination. A review. Plant Foods Hum Nutr. 1992;42(1):71–85. doi: 10.1007/BF02196074. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S, Hodgson H, Chadwick V. Controlled trial comparing prednisolone with an elemental diet plus non-absorbable antibiotics in active Crohn's disease. Gut. 1985;26(10):994–998. doi: 10.1136/gut.26.10.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, Scheiner O, Jensen-Jarolim E. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81(1):154–160. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2014;30(6):578–582. doi: 10.1097/MOG.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Seiquer I, Díaz-Alguacil J, Delgado-Andrade C, López-Frías M, Muñoz Hoyos A, Galdó G, Navarro MP. Diets rich in Maillard reaction products affect protein digestibility in adolescent males aged 11–14 y. Am J Clin Nutr. 2006;83(5):1082–1088. doi: 10.1093/ajcn/83.5.1082. [DOI] [PubMed] [Google Scholar]
- Semino GA, Restani P, Cerletti P. Effect of bound carbohydrate on the action of trypsin on lupine seed glycoproteins. J Agric Food Chem. 1985;33(2):196–199. [Google Scholar]
- Seyrig J-A, Jian R, Modigliani R, Golfain D, Florent C, Messing B, Bitoun A. Idiopathic pancreatitis associated with inflammatory bowel disease. Dig Dis Sci. 1985;30(12):1121–1126. doi: 10.1007/BF01314044. [DOI] [PubMed] [Google Scholar]
- Shastry M, John E. Biochemical changes and in-vitro protein digestibility of the endosperm of germinating dolichos lablab. J Sci Food Agric. 1991;55(4):529–538. [Google Scholar]
- Shen Q, Chen YA, Tuohy KM. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe. 2010;16(6):572–577. doi: 10.1016/j.anaerobe.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Shirazian S, Radhakrishnan J. Gastrointestinal disorders and renal failure: exploring the connection. Nat Rev Nephrol. 2010;6(8):480–492. doi: 10.1038/nrneph.2010.84. [DOI] [PubMed] [Google Scholar]
- Silva MER, Vezozzo DP, Ursich MJM, Rocha DM, Cerri GG, Wajchenberg BL. Ultrasonographic abnormalities of the pancreas in IDDM and NIDDM patients. Diabetes Care. 1993;16(9):1296–1297. doi: 10.2337/diacare.16.9.1296. [DOI] [PubMed] [Google Scholar]
- Sirich TL. Dietary protein and fiber in end stage renal disease. Seminars in dialysis. 2015;28(1):75–80. doi: 10.1111/sdi.12315. [DOI] [PubMed] [Google Scholar]
- Skroubis G, Anesidis S, Kehagias I, Mead N, Vagenas K, Kalfarentzos F. Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion in a non-superobese population: prospective comparison of the efficacy and the incidence of metabolic deficiencies. Obes Surg. 2006;16(4):488–495. doi: 10.1381/096089206776327251. [DOI] [PubMed] [Google Scholar]
- Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 1993;218(1):91–96. doi: 10.1097/00000658-199307000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies JR, Chambers D. Chemical determination of tryptophan in proteins. Anal Chem. 1949;21(10):1249–1266. doi: 10.1021/ac60256a004. [DOI] [PubMed] [Google Scholar]
- Steinkraus KH. Fermentations in world food processing. Compr Rev Food Sci Food Saf. 2002;1(1):23–32. doi: 10.1111/j.1541-4337.2002.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Stepniak D, Spaenij-Dekking L, Mitea C, Moester M, de Ru A, Baak-Pablo R, van Veelen P, Edens L, Koning F. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G621–G629. doi: 10.1152/ajpgi.00034.2006. [DOI] [PubMed] [Google Scholar]
- Sternberg M, Kim C. Protein Crosslinking. Springer; New York, NY: 1977. Lysinoalanine formation in protein food ingredients; pp. 73–84. [DOI] [PubMed] [Google Scholar]
- Strickland RG, Mackay IR. A reappraisal of the nature and significance of chronic atrophic gastritis. Digest Dis Sci. 1973;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Strid H, Simren M, Johansson AC, Svedlund J, Samuelsson O, Bjornsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17(8):1434–1439. doi: 10.1093/ndt/17.8.1434. [DOI] [PubMed] [Google Scholar]
- Sundbom M, Maårdh E, Maårdh S, Ohrvall M, Gustavsson S. Reduction in serum pepsinogen I after roux-en-Y gastric bypass. J Gastrointest Surg. 2003;7(4):529–535. doi: 10.1016/s1091-255x(03)00063-5. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138(2):283–290. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Horvath A. Meta-analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Curr Med Res Opin. 2010;26(2):423–437. doi: 10.1185/03007990903510317. [DOI] [PubMed] [Google Scholar]
- Tarlow M, Hadorn B, Arthurton M, Lloyd JK. Intestinal enterokinase deficiency a newly-recognized disorder of protein digestion. Arch Dis Child. 1970;45(243):651–655. doi: 10.1136/adc.45.243.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N, Akahane M, Kiryu S, Matsubara Y, Yamaji Y, Okamoto M, Minagawa N, Ohgi K, Komatsu Y, Yahagi N, Yoshida H, Kawabe T, Ohtomo K, Omata M. Pancreas duct abnormalities in patients with ulcerative colitis. A magnetic resonance pancreatography study. Inflamm Bowel Dis. 2005;11(10):903–908. doi: 10.1097/01.mib.0000183419.17563.17. [DOI] [PubMed] [Google Scholar]
- Toden S, Bird AR, Topping DL, Conlon MA. Differential effects of dietary whey, casein and soya on colonic DNA damage and large bowel SCFA in rats fed diets low and high in resistant starch. Br J Nutr. 2007;97(3):535–543. doi: 10.1017/S0007114507336817. [DOI] [PubMed] [Google Scholar]
- Toden S, Bird AR, Topping DL, Conlon MA. Dose-dependent reduction of dietary protein-induced colonocyte DNA damage by resistant starch in rats correlates more highly with caecal butyrate than with other short chain fatty acids. Cancer biology & therapy. 2007;6(2):253–258. doi: 10.4161/cbt.6.2.3627. [DOI] [PubMed] [Google Scholar]
- Toden S, Bird AR, Topping DL, Conlon MA. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: attenuation by high-amylose maize starch. Carcinogenesis. 2007;28(11):2355–2362. doi: 10.1093/carcin/bgm216. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Spock A, Spicer SS, Shelburne JD, Bradford W. Inspissation of pancreatic zymogen material in cystic fibrosis. Ultrastruct Pathol. 2003;27(5):323–335. [PubMed] [Google Scholar]
- Tye-Din JA, Anderson RP, Ffrench RA, Brown GJ, Hodsman P, Siegel M, Botwick W, Shreeniwas R. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin Immunol. 2010;134(3):289–295. doi: 10.1016/j.clim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, Riemer AB, Ankersmit HJ, Scheiner O, Boltz-Nitulescu G. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB journal. 2005;19(6):656–658. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- Untersmayr E, Poulsen LK, Platzer MH, Pedersen MH, Boltz-Nitulescu G, Skov PS, Jensen-Jarolim E. The effects of gastric digestion on codfish allergenicity. J Allergy Clin Immunol. 2005;115(2):377–382. doi: 10.1016/j.jaci.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E, Walter F, Riemer A, Kraml G, Kinaciyan T, Spitzauer S. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112(3):616–623. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- Valerio D, Whyte EHA, Schlamm HT, Ruggiero JA, Blackburn GL. Clinical effectiveness of a pancreatic enzyme supplement. JPEN J Parenter Enteral Nutr. 1981;5(2):110–114. doi: 10.1177/0148607181005002110. [DOI] [PubMed] [Google Scholar]
- Vass A, Szakaly S, Schmidt P. Experimental study of the nutritional biological characters of fermented milks. Acta Med Hung. 1983;41(2-3):157–161. [PubMed] [Google Scholar]
- Vellas B, Balas D, Moreau J, Bouisson M, Senegas-Balas F, Guidet M, Ribet A. Exocrine pancreatic secretion in the elderly. Int J Pancreatol. 1988;3(6):497–502. doi: 10.1007/BF02788208. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M, Sathe SK. Phaseolin in vitro pepsin digestibility: role of acids and phenolic compounds. J Agric Food Chem. 2003;51(11):3466–3472. doi: 10.1021/jf026105y. [DOI] [PubMed] [Google Scholar]
- von Berg A, Filipiak-Pittroff B, Krämer U, Link E, Bollrath C, Brockow I, Koletzko S, Grübl A, Heinrich J, Wichmann H-E. Preventive effect of hydrolyzed infant formulas persists until age 6 years: long-term results from the German Infant Nutritional Intervention Study (GINI) J Allergy Clin Immunol. 2008;121(6):1442–1447. doi: 10.1016/j.jaci.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Vrese M. d., Frik R, Roos N, Hagemeister H. Protein-bound D-amino acids, and to a lesser extent lysinoalanine, decrease true ileal protein digestibility in minipigs as determined with 15N-labeling. J Nutr. 2000;130(8):2026–2031. doi: 10.1093/jn/130.8.2026. [DOI] [PubMed] [Google Scholar]
- Wahn U. Role of hydrolysates in prophylactic and therapeutic diets for food allergy. In: De Weck A, Sampson H, editors. Intestinal Immunology and Food Allergy, Nestle Nutrition Workshop Series. Vol. 34. ROWEN PRESS; 1993. pp. 289–289. [Google Scholar]
- Waldum HL, Hauso Ø, Fossmark R. The regulation of gastric acid secretion – clinical perspectives. Acta Physiologica. 2014;210(2):239–256. doi: 10.1111/apha.12208. [DOI] [PubMed] [Google Scholar]
- Walker A, Sanderson J, Churcher C, Parkes G, Hudspith B, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11(1):7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkowiak J, Herzig KH. Fecal elastase-1 is decreased in villous atrophy regardless of the underlying disease. Eur J Clin Invest. 2001;31(5):425–430. doi: 10.1046/j.1365-2362.2001.00822.x. [DOI] [PubMed] [Google Scholar]
- Walter AW, Henle T, Haeßner R, Klostermeyer H. Studies on the formation of lysinomethylalanine and histidinomethylalanine in milk products. Z Lebensm Unters Forsch. 1994;199(3):243–247. doi: 10.1007/BF01193454. [DOI] [PubMed] [Google Scholar]
- Wang J, Fung DYC. Alkaline-fermented foods: a review with emphasis on Pidan fermentation. Crit Rev Microbiol. 1996;22(2):101–138. doi: 10.3109/10408419609106457. [DOI] [PubMed] [Google Scholar]
- Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56(1):184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- Winter TA, Lemmer ER, O'Keefe SJ, Ogden JM. The effect of severe undernutrition, and subsequent refeeding on digestive function in human patients. Eur J Gastroenterol Hepatol. 2000;12(2):191–196. doi: 10.1097/00042737-200012020-00010. [DOI] [PubMed] [Google Scholar]
- Winter TA, Marks T, Callanan M, O’Keefe SJ, Bridger S. Impaired pancreatic secretion in severely malnourished patients is a consequence of primary pancreatic dysfunction. Nutrition. 2001;17(3):230–235. doi: 10.1016/s0899-9007(00)00575-x. [DOI] [PubMed] [Google Scholar]
- Yin S-W, Tang C-H, Wen Q-B, Yang X-Q, Li L. Functional properties and in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: Effect of high-pressure treatment. Food Chem. 2008;110(4):938–945. doi: 10.1016/j.foodchem.2008.02.090. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Fournier J, Gilar M, Gebler JC. Identification of N-linked glycosylation sites using glycoprotein digestion with pronase prior to MALDI tandem time-of-flight mass spectrometry. Anal Chem. 2007;79(4):1731–1738. doi: 10.1021/ac0616052. [DOI] [PubMed] [Google Scholar]
- Yun J, Kwon I, Lohakare J, Choi J, Yong J, Zheng J, Cho W, Chae B. Comparative efficacy of plant and animal protein sources on the growth performance, nutrient digestibility, morphology and caecal microbiology of early-weaned pigs. Asian-Aust J Anim Sci. 2005;18:1285–1293. [Google Scholar]
- Zaloga GP, Ward KA, Prielipp RC. Effect of enteral diets on whole body and gut growth in unstressed rats. JPEN J Parenter Enteral Nutr. 1991;15(1):42–47. doi: 10.1177/014860719101500142. [DOI] [PubMed] [Google Scholar]
- Zeece M, Huppertz T, Kelly A. Effect of high-pressure treatment on in-vitro digestibility of β-lactoglobulin. Innov Food Sci Emerg Technol. 2008;9(1):62–69. [Google Scholar]
- Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]