Abstract
Spontaneous activity is known to be essential for the proper formation of sensory networks in the developing CNS. This activity can be produced by a variety of mechanisms including the presence of “pacemaker” neurons, which can be defined by their intrinsic ability to generate rhythmic bursts of action potential discharge. Recent work has identified pacemaker activity within lamina I of the neonatal rodent spinal cord that emerges from a complex interaction between voltage-dependent and voltage-independent (“leak”) ionic conductances, including an important modulatory role for the inward-rectifying K+ (Kir) channels. The available evidence suggests that lamina I pacemakers are glutamatergic and project extensively throughout the dorsal-ventral axis of the spinal cord, although the identity of their postsynaptic targets has yet to be elucidated. A better understanding of this connectivity could yield valuable insight into the role of the lamina I pacemaker population in the maturation of spinal circuitry underlying nociceptive processing and/or sensorimotor integration.
Keywords: rhythmic burst-firing, spinal cord, superficial dorsal horn, neonate
Neurons possessing the intrinsic ability to generate rhythmic bursts of activity, referred to as “pacemakers”, have recently been described in the developing superficial dorsal horn (SDH) of the rodent spinal cord (Li and Baccei 2011). Pacemaker activity was localized to lamina I of the SDH and largely restricted to the early postnatal period, which is characterized by significant anatomical and functional reorganization of the SDH network (Koch and Fitzgerald 2013). The clear importance of the SDH for nociceptive processing in the CNS, along with the observation that lamina I pacemakers are directly innervated by high-threshold sensory afferents (Li and Baccei 2011), lead one to hypothesize that these neurons may be important for the activity-dependent tuning of spinal nociceptive circuits (Beggs and others, 2002; Waldenstrom and others, 2003). However, many unanswered questions remain regarding the specific subtypes of lamina I neurons which comprise the pacemaker population, their connectivity within the developing spinal cord, and the ionic mechanisms governing the intrinsic burst-firing which distinguishes these cells from adjacent neurons within the SDH. This review will briefly summarize recent advances in these areas and discuss the potential role of pacemaker neurons in the maturation of sensorimotor pathways in the spinal cord.
Contribution of glutamatergic interneurons to pacemaker activity within lamina I of the neonatal dorsal horn
In many developing CNS networks, pacemaker neurons provide an endogenous source of excitation which facilitates the initial, activity-dependent formation of the circuit, which can then be refined by subsequent sensory experience (Blankenship and Feller 2010). Pacemaker neurons in the SDH appear to be glutamatergic in nature, as the majority of intrinsically bursting neurons recorded in the neonatal spinal cord slice preparation expresses the vesicular glutamate transporter vGluT2. In addition, intrinsic burst-firing was not observed in GABAergic neurons in the SDH of neonatal Gad-GFP mice (Li and Baccei 2011).
Numerous lines of evidence suggest that the majority of these pacemakers correspond to local circuit interneurons and/or propriospinal neurons: (1) the prevalence of pacemakers in the neonatal rat SDH (>25% of the lamina I population) far exceeds the number of ascending projection neurons reported in lamina I of the rodent spinal cord (Spike and others, 2003); (2) the average size of the pacemakers (as measured by membrane capacitance) is significantly smaller than the spino-parabrachial and spino-PAG projection neurons sampled in our prior studies (Li and Baccei 2012); and (3) targeted recordings from lamina I projection neurons identified by retrograde labeling with DiI failed to demonstrate rhythmic bursting in these populations during the first postnatal week (Li and Baccei 2011). Nonetheless, it must be noted that any spinal cord slice preparation will involve damage to the axonal and dendritic processes of dorsal horn neurons, with larger neurons particularly susceptible to injury during the slicing procedure. Therefore, we cannot exclude the possibility that ascending projection neurons do in fact exhibit pacemaker activity within the intact dorsal horn network during the neonatal period. This issue can be addressed using a recently developed infrared illumination technique to allow for visualized patch clamp recordings from lamina I neurons within the intact spinal cord in vitro (Szucs and others, 2009). Indeed, we have previously observed rhythmic bursting in a small number of lamina I projection neurons at later stages of postnatal development (Li and Baccei 2012).
Connectivity of pacemakers in the developing SDH
Consistent with a potential role in the maturation of spinal nociceptive circuits, pacemaker neurons within lamina I of the neonatal dorsal horn receive monosynaptic input from high-threshold primary afferents (Li and Baccei 2011), which presumably correspond to Aδ- and C-fiber nociceptors in vivo. While low-threshold Aβ-fiber inputs to the immature SDH have been demonstrated using in vitro patch clamp approaches (Li and Zhuo 1998; Park and others, 1999), we have yet to find evidence that the pacemaker population is directly innervated by Aβ-fibers early in life.
Interestingly, the axons of lamina I pacemakers are not confined to the superficial laminae of the developing spinal cord, as the majority of intrinsically bursting neurons sent projections to the deep dorsal horn as well as the ventral horn (Li and Baccei 2011), where the presence of vGLUT2-positive axonal boutons suggests the presence of functional synapses between pacemaker neurons and postsynaptic cells deeper in the spinal cord. The majority of pacemaker axons coursing through the deeper regions of the spinal cord appear relatively unbranched, which stands in contrast to the rich proliferation of axonal branches documented in local circuit interneurons within lamina I (Szucs and others, 2013). Recent evidence also suggests that pacemaker axons can cross the midline below the central canal in the anterior commissure (M.L. Baccei, unpublished observations). Notably, a fraction of ascending commissural interneurons are thought to reside in lamina I and send a relatively unbranched axon across the anterior commissure to innervate the contralateral ventral horn up to 5–6 segments in the rostral direction (Eide and others, 1999).
The direct projections of pacemaker axons to the deep dorsal and ventral horn suggest the possibility that this population might function, at least in part, as pre-motor interneurons which provide excitatory drive to developing motor pathways in the spinal cord. Indeed, recent work using monosynaptically-restricted viral vectors to elucidate the distribution of pre-motor interneurons in the developing rodent spinal cord revealed a small population located within the SDH (Tripodi and others, 2011). The injection of retrograde, trans-synaptic pseudorabies viruses encoding GFP and RFP into flexor and extensor muscles in the neonatal rodent (Jovanovic and others, 2010), coupled with the labeling of pacemaker neurons using patch clamp recordings in the intact spinal cord preparation (Szucs and others, 2009), will further identify the motor pathways engaged by rhythmically bursting lamina I neurons during early life. However, collectively the existing anatomical evidence suggests that the function of these pacemakers may extend beyond local signal processing in the SDH and could involve the modulation of sensorimotor integration within the developing spinal cord.
Ionic mechanisms underlying pacemaker activity in the immature SDH
As previously documented in pacemaker neurons within other regions of the CNS, calcium influx via voltage-gated Ca2+ channels (VGCCs) makes a critical contribution to the generation of rhythmic burst-firing in the neonatal SDH, in part by opening Ca2+-activated K+ channels that are responsible for the termination of the slow, plateau-like depolarization (Li and Baccei 2011). However, unlike bursting neurons in the thalamus or inferior olive (Huguenard 1996; Bal and McCormick 1997; Cueni and others, 2008), spinal pacemakers do not rely on calcium entry through the low-threshold T-type VGCCs (Li and Baccei 2011) in order to produce the initial membrane depolarization near the resting membrane potential (Vrest). This is perhaps unsurprising given that lamina I pacemakers exhibited an average Vrest between −55 and −65 mV, membrane potentials at which the vast majority of T-type VGCCs would be expected to be inactivated (Ryu and Randic 1990).
Instead, pacemaker activity in the spinal cord depends upon persistent, voltage-gated Na+ (NaP) channels (Tazerart and others, 2008; Li and Baccei 2011), which are activated in lamina I neurons at membrane potentials close to Vrest and are very slow to inactivate (Prescott and De Koninck 2005). Bath application of riluzole abolished the generation of the slow oscillations in lamina I pacemakers without directly interfering with action potential discharge, supporting the role for NaP currents in the initiation of the plateau-like depolarizations which underlie the rhythmic bursting activity (Li and Baccei 2011). Nonetheless, lamina I pacemakers do not express higher levels of NaP conductance compared to adjacent, non-bursting lamina I cells. They do, however, possess a significantly higher ratio of NaP conductance to “leak” membrane conductance, as reported previously for pacemaker neurons in the respiratory brain stem (Del Negro and others, 2002). The observation that high membrane resistance (Rm) is a distinguishing feature of pacemakers in the neonatal SDH is interesting in light of the fact that the average Rm across the lamina I population decreases sharply between postnatal (P) days 3 and 10 (Li and Baccei 2011), which may reflect an age-dependent enhancement in the dendritic arborizations of SDH neurons (Bicknell, Jr. and Beal 1984). This perfectly parallels the reduction in the prevalence of rhythmic burst-firing within lamina I, suggesting that pacemaker activity may be simply “turned off” at later stages of development as the neurons grow in size.
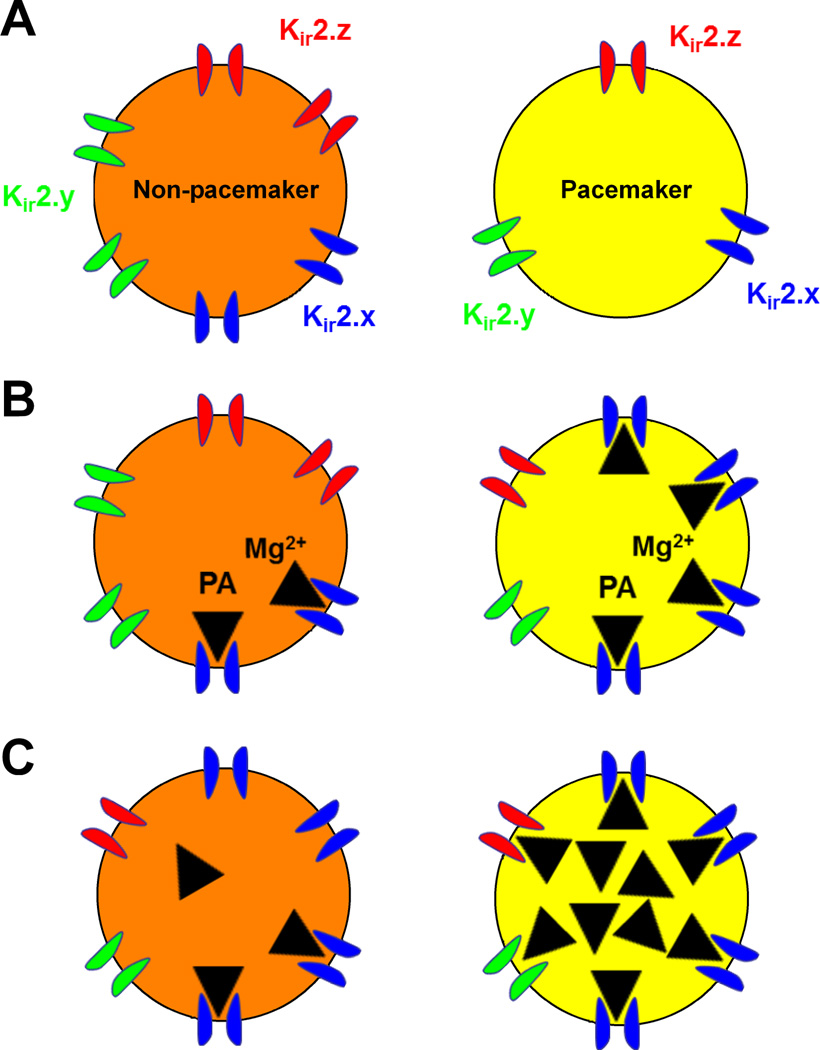
The ionic mechanisms which explain the higher Rm seen in the pacemaker population are not yet fully understood, and are likely complex given that any ion channel open at Vrest can contribute to the leak conductance of a neuron. However, our recent work suggests that inward-rectifying K+ (Kir) currents function as key regulators of pacemaker activity within the developing superficial dorsal horn. Rhythmically bursting cells within lamina I of the neonatal SDH expressed significantly lower Kir conductance compared to adjacent, non-bursting neurons (Li and others, 2013). While the reasons behind this difference in Kir levels remain unclear (Fig. 1), an enhanced degree of inward rectification in pacemakers suggests the presence of functionally distinct Kir2 channels in these neurons. A reduced Kir conductance likely contributes to the higher Rm and more depolarized Vrest which are features of the pacemaker population at early ages. Importantly, blocking Kir channels with external Ba2+ or elevated internal Mg2+ increased the prevalence of burst-firing within lamina I of the neonatal spinal cord (Li and others, 2013).
Figure 1.
Potential mechanisms underlying the reduced Kir conductance in pacemaker neurons of the immature superficial dorsal horn (SDH). (A) Pacemakers (yellow) may express similar Kir2 isoforms compared to adjacent, non-bursting neurons within lamina I (orange) but exhibit a lower density of Kir2 channels in the membrane. However, the greater degree of Kir inward rectification in pacemakers would seemingly argue against this scenario (Li and others, 2013). (B) Pacemakers may preferentially express Kir2 subunits that have a higher sensitivity to intracellular block by free Mg2+ or polyamines (PA) near the resting membrane potential (Hibino and others, 2010). (C) Intrinsically busting neurons could be distinguished by elevated internal concentrations of free Mg2+ or polyamines compared to non-pacemakers in the SDH, as the levels of these cations are known to be rapidly regulated by second messenger pathways (Romani 2007) and sensory experience (Aizenman and others, 2002) within the CNS.
This finding also indicates that intrinsic burst-firing is a latent property of a wider population of developing SDH neurons, and that cells can switch between pacemaker and non-pacemaker modes of activity. Similar conclusions were reached by Derjean et al. (2003) in their studies of deep dorsal horn neurons during adulthood, as endogenous rhythmic bursting could be unmasked in the majority of cells following the manipulation of metabotropic inputs (mGluR and GABABR) which resulted in a reduced G-protein-coupled Kir conductance (Derjean and others, 2003). Interestingly, recent work in the immature ventral horn has demonstrated that bursting activity is strongly regulated by the levels of extracellular Ca2+ and K+ via their effects on NaP channels (Brocard and others, 2013). Collectively, these results demonstrate that intrinsically bursting neurons are highly sensitive to their external and internal environments. As a result, one would predict that the level of pacemaker activity within developing spinal networks can be rapidly modulated based on sensory experience.
Potential role of lamina I pacemakers in the maturation of spinal circuitry
The functional importance of pacemaker neurons within the neonatal SDH for the development of central nociceptive pathways and/or spinal sensorimotor networks has yet to be elucidated. Ultimately, it will be important to be able to selectively modulate the activity of lamina I pacemakers in vivo, and subsequently examine the maturation of the nociceptive withdrawal reflex and other assays of pain sensitivity and motor behavior. Unfortunately, a specific marker of the pacemaker population in the SDH has yet to be identified, thus preventing the design of strategies to genetically manipulate their excitability in a selective manner. In contrast, the rhythmically bursting neurons in the parafacial region of the embryonic hindbrain can be identified by their expression of the Phox2b transcription factor, and have been shown to be essential for the generation of a normal respiratory rhythm (Thoby-Brisson and others, 2009).
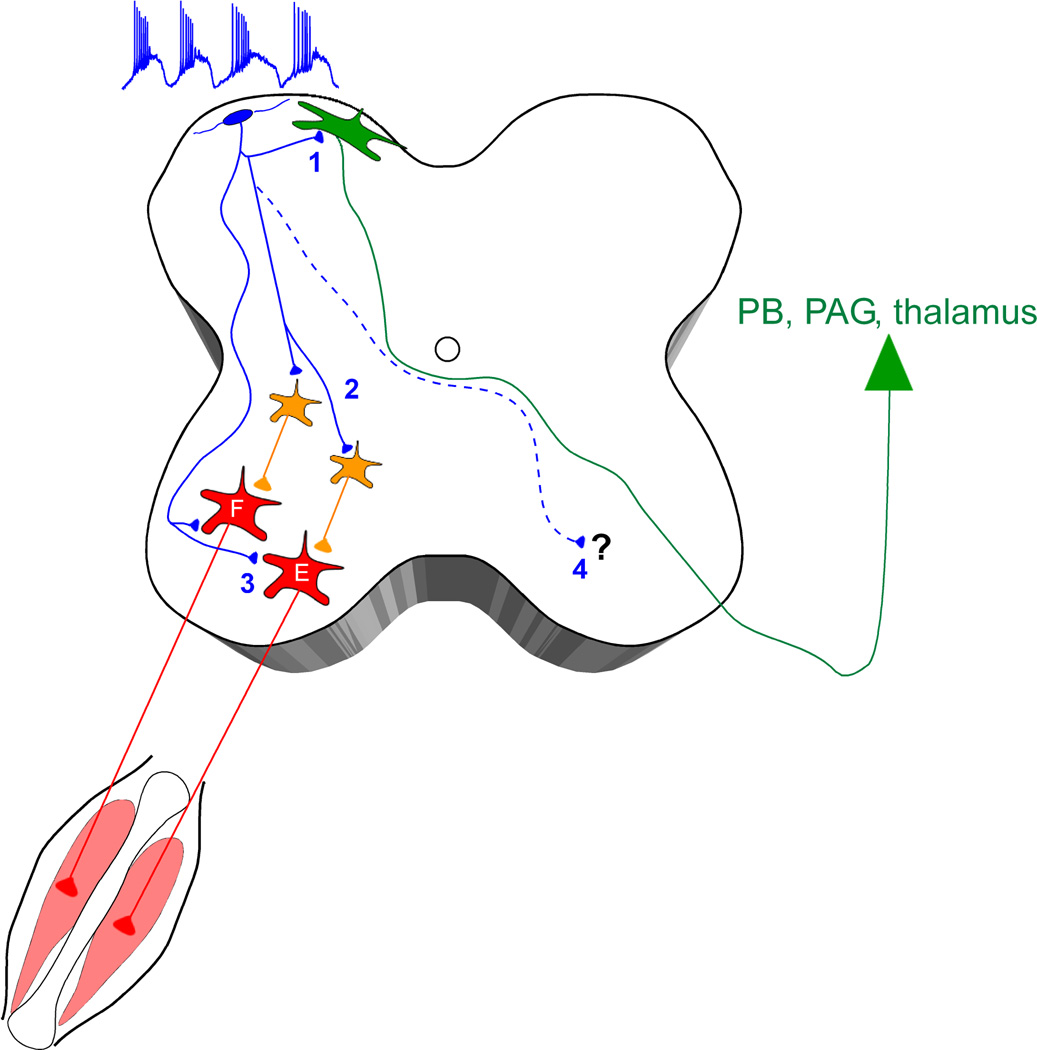
Despite the current absence of an identified marker of lamina I pacemaker neurons, clues as to their function may arise from a better understanding of their connectivity within the developing spinal cord. For example, if lamina I pacemakers are shown to make synaptic contacts with spinal motor pathways, in conjunction with our prior findings that they are directly innervated by high-threshold (i.e. Aδ- and C-fiber) sensory afferents (Li and Baccei 2011), this would support the notion that intrinsically bursting neurons may function to provide an endogenous drive to the developing circuits which underlie the nociceptive withdrawal reflex (NWR). Indeed, it has been demonstrated that spontaneous muscle twitches in the neonatal rodent are instrumental for the proper formation of sensorimotor networks in the spinal cord (Petersson and others, 2003), and are thought to provide the tactile feedback which drives the maturation of the NWR circuitry (Waldenstrom and others, 2009). The rhythmic burst-firing of lamina I pacemakers may help initiate this spontaneous muscle activity early in life via their excitatory synapses onto motor neurons and/or pre-motor interneurons within the deep dorsal or ventral horn (Fig. 2). In addition, limb withdrawal evoked by a noxious stimulus requires coordinated muscle activity on both sides of the body, as ipsilateral flexion must be accompanied by contralateral extension in order to maintain balance. Therefore, a population of pacemakers which project to the contralateral side (Fig. 2) could potentially facilitate the formation of the commissural networks which are important for left-right coordination during a behavioral response to actual or potential tissue damage.
Figure 2.
Lamina I pacemaker neurons may modulate the output of the neonatal spinal cord. Rhythmically bursting neurons (blue) send glutamatergic axonal projections throughout the dorsal-ventral axis of the immature spinal cord (Li and Baccei 2011). Collaterals which remain in the superficial laminae could contact lamina I projection neurons (1) to drive spontaneous activity in the nociceptive pathways which ascend to supraspinal sites including the parabrachial nucleus (PB), periaqueductal gray (PAG) and thalamus. Meanwhile, synaptic boutons in the deep dorsal horn could engage pre-motor interneurons (2) which are part of the flexor (F) or extensor (E) motor pathways in the spinal cord, and/or ascending projection neurons located in lamina V (not shown). The presence of pacemaker axons in the ventral horn raises the possibility that these rhythmically bursting neurons directly synapse onto motor neurons (3) and contribute to spontaneous muscle contractions which are common in the neonate. Finally, the final destination of pacemaker axons which cross in the anterior commissure remains unknown (4), but these contralateral fibers suggest that some lamina I pacemakers may correspond to ascending projection neurons and/or function as commissural interneurons within the developing spinal cord.
As mentioned above, there remains a distinct possibility that a fraction of lamina I pacemakers either directly correspond to ascending projection neurons or form glutamatergic synaptic contacts onto these cells (Fig. 2). If so, the rhythmic bursts of activity could be transmitted to the supraspinal targets of lamina I projection neurons, including the parabrachial nucleus, periaqueductal gray (PAG), and thalamus (Todd 2010). Indeed, pacemaker activity within other sensory networks is known to coordinate activity across the population of output neurons (McLaughlin and others, 2003; Zheng and others, 2006). Assuming that the formation of supraspinal nociceptive networks is dependent on neuronal activity, as has been demonstrated in the spinal cord (Beggs and others, 2002; Waldenstrom and others, 2003), lamina I pacemakers may thus provide the excitatory drive needed to shape the organization of fully mature pain circuits in the brain. This would provide a clear advantage compared to a reliance on modality-specific (i.e. noxious) sensory experience, as the presence of a population of spinal neurons which spontaneously generates rhythmic bursts of activity would remove the need for the animal to sustain repetitive tissue damage in various dermatomes in order to develop the nociceptive withdrawal reflexes which facilitate survival. In essence, pacemakers in the SDH could act as a surrogate for noxious stimulation during early postnatal development.
Conclusion
It is clear that much work needs to be done to delineate the functional significance of pacemaker activity in the SDH of the developing spinal cord. It seems likely that rhythmic burst-firing is not restricted to a single subpopulation of cells in the neonatal dorsal horn. As a result, there remains an exciting possibility that pacemaker neurons may contribute in multiple ways to the maturation of the highly complex circuitry which characterizes the spinal cord.
Acknowledgments
This work was supported by the National Institutes of Health (NS072202 to MLB). The author would like to thank Jie Li for feedback regarding this manuscript.
References
- Aizenman CD, Munoz-Elias G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h) J Neurophysiol. 1997;77:3145–3156. doi: 10.1152/jn.1997.77.6.3145. [DOI] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–1258. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- Bicknell HR, Jr, Beal JA. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol. 1984;226:508–522. doi: 10.1002/cne.902260406. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard F, Shevtsova NA, Bouhadfane M, Tazerart S, Heinemann U, Rybak IA, et al. Activity-dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron. 2013;77:1047–1054. doi: 10.1016/j.neuron.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol. 1999;403:332–345. [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Jovanovic K, Pastor AM, O'Donovan MJ. The use of PRV-Bartha to define premotor inputs to lumbar motoneurons in the neonatal spinal cord of the mouse. PLoS One. 2010;5:e11743. doi: 10.1371/journal.pone.0011743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SC, Fitzgerald M. Activity-dependent development of tactile and nociceptive spinal cord circuits. Ann N Y Acad Sci. 2013;1279:97–102. doi: 10.1111/nyas.12033. [DOI] [PubMed] [Google Scholar]
- Li J, Baccei ML. Pacemaker neurons within newborn spinal pain circuits. J Neurosci. 2011;31:9010–9022. doi: 10.1523/JNEUROSCI.6555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baccei ML. Developmental regulation of membrane excitability in rat spinal lamina I projection neurons. J Neurophysiol. 2012;107:2604–2614. doi: 10.1152/jn.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Blankenship ML, Baccei ML. Inward-rectifying potassium (Kir) channels regulate pacemaker activity in spinal nociceptive circuits during early life. J Neurosci. 2013;33:3352–3362. doi: 10.1523/JNEUROSCI.4365-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Park JS, Nakatsuka T, Nagata K, Higashi H, Yoshimura M. Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development. Brain Res Dev Brain Res. 1999;113:29–36. doi: 10.1016/s0165-3806(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenstrom A, Fahraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization. J Neurosci. 2005;25:4743–4754. doi: 10.1523/JNEUROSCI.0356-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. 2007;458:90–102. doi: 10.1016/j.abb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Ryu PD, Randic M. Low- and high-voltage-activated calcium currents in rat spinal dorsal horn neurons. J Neurophysiol. 1990;63:273–285. doi: 10.1152/jn.1990.63.2.273. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- Szucs P, Luz LL, Pinho R, Aguiar P, Antal Z, Tiong SY, et al. Axon diversity of lamina I local-circuit neurons in the lumbar spinal cord. J Comp Neurol. 2013;521:2719–2741. doi: 10.1002/cne.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs P, Pinto V, Safronov BV. Advanced technique of infrared LED imaging of unstained cells and intracellular structures in isolated spinal cord, brainstem, ganglia and cerebellum. J Neurosci Methods. 2009;177:369–380. doi: 10.1016/j.jneumeth.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Vinay L, Brocard F. The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci. 2008;28:8577–8589. doi: 10.1523/JNEUROSCI.1437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479:61–66. doi: 10.1038/nature10538. [DOI] [PubMed] [Google Scholar]
- Waldenstrom A, Christensson M, Schouenborg J. Spontaneous movements: Effect of denervation and relation to the adaptation of nociceptive withdrawal reflexes in the rat. Physiol Behav. 2009;98:532–536. doi: 10.1016/j.physbeh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Waldenstrom A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. J Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]