Abstract
Alzheimer’s disease (AD), the most common form of dementia in the elderly, is characterized by the presence of extracellular plaques composed of amyloid β (Aβ) peptides and intracellular tau aggregates. The plaques are surrounded by microglia, the brain’s resident immune cells, which likely participate in the clearance of Aβ by phagocytosis. The microglia that are associated with plaques display an abnormal amoeboid morphology and do not respond to tissue damage, in contrast to microglia in healthy brains. Here, we used time lapse confocal microscopy to perform a detailed real time examination of microglial motility in acute hippocampal brain slices from the 5xFAD mouse model of AD, which was crossed to Cx3cr1GFP/GFP mice to achieve microglia-specific GFP expression for visualization. During baseline conditions, microglia around plaques appeared hypermotile, moving the processes that were pointing away from plaques at higher speed than microglia not associated with plaques. Yet, neither plaque-associated, nor plaque-free microglia were able to extend processes towards sites of modest mechanical damage. Application of the selective adenosine A2A receptor antagonist preladenant, which restores microglial response to cellular damage in a mouse model of Parkinson’s disease, reduced the hypermotility of plaque-associated microglia, but did not restore motility towards damaged cells in slices from 5xFAD mice. Our results suggest that process hypermotility and resistance to A2A antagonism during response to tissue damage may represent unique functional phenotypes of plaque-associated microglia that impair their ability to function properly in the AD brain.
Keywords: Alzheimer’s disease, microglia, neuroinflammation, motility, imaging, adenosine A2A receptors
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease and the leading cause of dementia in the elderly. Patients affected by AD display cognitive deficits such as impaired memory and difficulties with daily functions. Some of the pathological features associated with the disease are brain shrinkage and the presence of two types of abnormal protein aggregates: tau protein aggregated in intraneuronal neurofibrillary tangles and amyloid β (Aβ) peptides aggregated in extracellular plaques (Spires-Jones and Hyman, 2014).
Another prominent feature of AD pathology is the presence of an inflammatory response, including increased levels of inflammatory mediators and activated microglia (Akiyama et al., 2000; Heneka et al., 2010), the brain’s resident immune cells. Interestingly, the greatest inflammatory reaction is observed around Aβ plaques, which are often completely surrounded by microglia with activated morphology in both humans (Itagaki et al., 1994; Perlmutter et al., 1992; Sheng et al., 1997) and mouse models of the disease (Frautschy et al., 1998).
Despite being considered immune cells, the two most prominent features of microglia in the healthy brain are their highly ramified morphology and motility. Microglia have small cell bodies that give rise to several primary processes that branch to generate multiple secondary and tertiary processes. These processes constantly move back-and-forth, appearing to sample the brain parenchyma and interacting with synapses (Davalos et al., 2005; Nimmerjahn et al., 2005; Wake et al., 2009). When cell damage occurs, microglia extend their processes to surround the damaged area (Davalos et al., 2005). This response is mediated by ATP release at sites of damage and activation of P2Y12 receptors on microglia (Haynes et al., 2006). In the context of AD, microglia exposed to Aβ oligomers may release ATP to enhance migration to Aβ plaques (Kim et al., 2012). However, when microglia become activated by toll-like receptor activation or Aβ peptides, they downregulate their P2Y12 receptors and upregulate adenosine A2A receptors at the mRNA level in vitro (Orr et al., 2009). A2A receptor activation by the ATP breakdown product adenosine leads to process retraction by activated microglia in vitro (Orr et al., 2009), and impairs microglial responses to tissue damage in models of systemic inflammation and Parkinson’s disease (PD) (Gyoneva et al., 2014a; Gyoneva et al., 2014b).
A2A receptors may also play a role in AD pathogenesis. Consumption of caffeine, a nonselective adenosine receptor antagonist, has been linked to a lower risk for developing AD (Eskelinen and Kivipelto, 2010; Santos et al., 2010), as well as reduced neuronal loss, lowered Aβ levels and improved cognition in animal models of AD (Arendash et al., 2009; Canas et al., 2009; Cao et al., 2009). Caffeine also blocks the actions of adenosine on microglial motility (Gyoneva et al., 2014a), raising the possibility that this contributes to its role in AD. Microglia in a mouse model of AD show a reduced response to tissue damage by laser ablation (Krabbe et al., 2013), although the involvement of A2A receptors in modulating microglial motility has not been evaluated in models of AD.
We hypothesized that adenosine A2A receptors will modulate the motility of microglia and microglial response to tissue damage in Alzheimer’s disease. In this study, we used an ex vivo preparation of acute brain slices to evaluate the motility of microglia and response to damage in the 5xFAD mouse model of AD. Moreover, we examined the contribution of A2A receptors to this response by perfusing the slices with the A2A receptor–selective antagonist preladenant. We found that plaque-associated microglia have a unique motility response, consistent with a functional difference between plaque-associated and plaque-free microglia. These data suggest that microglial function is altered in AD, raising the possibility that cell surface receptors that restore microglial function could serve as a therapeutic target.
Experimental Procedures
Animals and acute slice preparation
All procedures involving the use of animals were reviewed and approved by the Emory University Institutional Animal Care and Use Committee. Two mouse stains were used here: 5xFAD mice [obtained from Dr. Doug Feinstein (University of Illinois, Chicago)] and Cx3cr1GFP/GFP mice (purchased from The Jackson Laboratories); both strains are on the C57Bl/6 background. 5xFAD mice carry five mutations associated with familial AD [Swedish (K670N, M671L), Florida (I716V) and London (V717I) mutations in amyloid precursor protein (APP) and M146L and L286V mutations in presenilin 1 (PS1)] and develop several features of AD seen in humans such as Aβ plaques – which appear as early as 2 months of age in the mice (Oakley et al., 2006). To facilitate the visualization of microglia in 5xFAD mice, hemizygous 5xFAD mice (hAPPK670N, M671L, I716V/0:hPS1M146L, L286V/0) were mated to Cx3cr1GFP/GFP mice (Jung et al., 2000). The hAPPK670N, M671L, I716V/0:hPS1M146L, L286V/0: Cx3cr1GFP/+ offspring develop AD-like pathology and express GFP in microglia in the brain; these mice are herein referred to 5xFAD:mg-GFP. The Cx3cr1GFP/+ offspring that do not carry mutant APP and PS1 were used as non-transgenic (non-Tg) littermate controls.
Coronal slices containing the hippocampus were prepared at 200 µm thickness from a total of 7 5xFAD:mgGFP and 5 non-Tg mice as previously described (Gyoneva et al., 2014b; Gyoneva and Traynelis, 2013). All mice were 2–4 months old, which for the 5xFAD mice represents the initial stages of plaque deposition and inflammation, with plaques surrounded by amoeboid microglia; however, no significant neuronal death is observed at this age (Oakley et al., 2006). To visualize amyloid plaques, 24 hr before slicing both 5xFAD and non-Tg mice were injected i.p. with 10 mg/kg Methoxy-X04 [4% v/v Methoxy-X04 (Tocris), 7.7% v/v KolliphorEL (Sigma-Aldrich), 88.3% v/v PBS] as described (Hefendehl et al., 2011)]. Methoxy-X04 has been extensively used to study microglial dynamics in AD models and does not appear to affect the motility of microglia not associated with plaques (Bolmont et al., 2008; Koeningsknecht-Talboo et al., 2008; Klunk et al., 2002; Krabbe et al., 2013; Meyer-Luehmann et al., 2008). While we cannot preclude plaque-bound Methoxy-X04 affects microglial motility, we believe that the likelihood of this is very low.
Slices were prepared and maintained for 1–5 hours before imaging in oxygenated artificial cerebrospinal fluid (aCSF) composed of 130 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 10 mM glucose, 24 mM NaHCO3, 3 mM MgSO4, and 1 mM CaCl2 as previously described (Gyoneva et al., 2014b). This solution is adequate to preserve excitatory and inhibitory synaptic transmission, plasticity, and ultrastructure of neurons and spines. Furthermore, microglia in the slices show a ramified morphology, constantly sample the parenchyma and respond to tissue damage in a fashion similar to that observed with in vivo imaging, suggesting that slices faithfully capture tissue physiology.
Slice imaging and image analysis
For imaging, the slices were transferred to the stage of an inverted Olympus IX51 microscope equipped with a disk spinning confocal unit and constantly perfused with oxygenated aCSF (composition as above, except for 1.5 mM each MgSO4 and CaCl2) at 1 mL/min flow rate for the duration of the experiment (Gyoneva et al., 2014b). Optical sections were taken every minute at 1 µm steps through the slices (~35 µm total) to capture microglial morphology. The GFP signal in microglia was obtained by excitation at 490 nm and detected with a FITC filter set. The signal from plaque-associated Methoxy-X04 was excited at 350 nm and detected with a DAPI filter set. Two types of recordings were obtained: baseline recordings over 20 min to examine the dynamics of microglia in physiological conditions, and injury recordings over 20 min to determine the response of microglia to tissue damage. Damage was generated by lowering a 100 µm rod at constant velocity (100 µm/s) for 180 µm into the slice with a motorized micromanipulator, as described (Gyoneva et al., 2014b); the rod was inserted into the tissue in less than 2 s and left in place. In slices containing the substantia nigra pars compacta, this typically injures ~10 cells (Gyoneva et al., 2014b). We chose this modest injury over more severe injuries (like laser ablation) because it should more closely model the slow, occasional death of a few neurons in an Alzheimer’s patient, as opposed to injury paradigms that rapidly kill many cells. The location of plaques in each recording was noted by taking a single stack in the DAPI channel before beginning a time sequence in the GFP channel.
For some experiments, the slices were perfused with the adenosine A2A receptor selective antagonist preladenant, which was synthesized as previously described (Gyoneva et al., 2014a). Preladenant has over 1000-fold selectivity for A2A receptors compared to the other adenosine receptors in in vitro expression systems and has been studied in models of PD where it reduces motor impairments and depression-like behavior and affects microglial motility in brain slices (Hodgson et al., 2009; Hodgson et al., 2010; Gyoneva et al., 2014b; Neustadt et al., 2007).
For quantification of the response, the optical sections at each time point were converted from a 3D dataset to a 2D image by maximum intensity projection on the xy-plane. The 2D time sequences were then analyzed with Imaris 7.4 software (Bitplane AG), as previously described (Gyoneva et al., 2014b), to calculate several parameters that characterize microglial motility: displacement, total length of movement, average velocity, and fraction of tracks with displacement over 5 µm. Here the term “displacement” refers to the net difference between the final and starting position of an object, taking the direction of the movement into account (positive direction towards injury). Similarly, “velocity” refers to both the direction of the movement and the magnitude of the rate of movement. “Total distance” is a directionless measurement that designates the total distance an object travels before it reaches its final position. If an object moves back and forth many times between two positions, it will have a small displacement, but large total distance travelled.
Immunohistochemistry and quantification
The expression of adenosine A2A receptor was determined with fluorescent immunohistochemistry. 5xFAD:mg-GFP mice or non-Tg littermates were injected with Methoxy-X04 as above. On the next day, the brains were isolated and drop-fixed in 4% paraformaldehyde overnight. After cryoprotection in 30% sucrose, the brains were sectioned on a cryostat at 40 µm thickness. Staining was performed for 5–6 animals in each group (Tg and non-Tg) with 3–5 sections per animal. After a 1 hr block with 10% normal goat serum (NGS) and 0.15% Triton X-100 in PBS, primary anti-A2A receptor antibody (1:500 or 1:1000 dilution, depending on lot; Millipore 05-717) was applied in the blocking solution overnight at 4°C. Following a wash, the sections were incubated with secondary goat anti-mouse IgG antibody conjugated to AlexaFluor-594 (AF594, Jackson ImmunoResearch). The sections were cover-slipped and imaged on a confocal microscope (1 µm step). In addition to the AF594 channel, GFP (microglia) and DAPI (Aβ plaques) fluorescence was also captured. For each channel, the same imaging settings were used for all sections. Sections containing the striatum were used as positive control for A2A receptor expression because of its constitutive expression there. Quantification was performed on 3–5 sections from each animal, and then the mean was calculated for the experimental group. The total number of microglia per unit area was calculated as the number of cell bodies of GFP-positive cells within a field of view and adjusted for the dimensions of the image. The number of A2A receptor-positive cells and A2A receptor/GFP-double positive cells per unit area were calculated with the Cell Counter tool in FIJI (National Institutes of Health).
Statistical analysis
Data were analyzed with SigmaStat v10 and PrismGraphPad v6. All statistical tests performed, sample sizes and significance values are indicated in the corresponding text and figure legends. Each individual time-lapse recording was considered an independent observation as it contains a slightly different area of the hippocampus and rested for a different amount of time before imaging. Moreover, the average response of the slices from each animal was comparable to those from other animals (data not shown). The figures represent group averages and standard error of the mean (SEM). All datasets being compared showed equal variance (Brown-Forsythe or Bartlett’s test). In general, microglia in 5xFAD:mg-GFP mice, both around and away from plaques, were compared to non-Tg littermate controls with analysis of variance (ANOVA) and Dunnett’s post hoc test. Effects of preladenant on plaque-associated microglia and microglia distributed throughout the whole slice from wild type and 5xFAD mice were examined with two-way ANOVA. Results were considered to be significantly different if p < 0.05.
Results
Imaging microglial motion in acute brain slices from the 5xFAD mouse model of AD
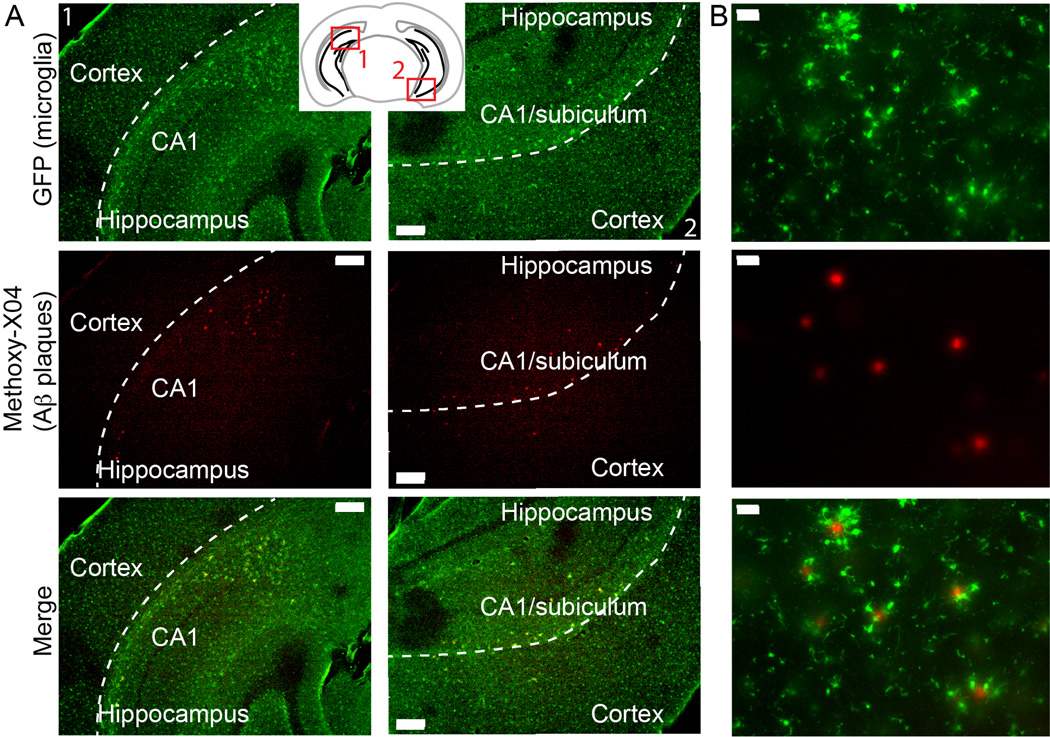
We prepared acute 200 µm-thick brain slices from 5xFAD:mg-GFP and non-transgenic (non-Tg) littermate controls, both of which contain Cx3cr1-driven GFP expression in microglia (see Methods for description of strains). To visualize plaques in tissues, we injected the mice with the congophilic dye Methoxy- X04 (Klunk et al., 2002) one day before slice preparation (Hefendehl et al., 2011). In our system, we were able to detect multiple plaques in the hippocampus and cortex as early as 2 month of age (Figure 1A), an early stage of plaque deposition, with many plaques concentrated in the CA1 region and possibly the subiculum of the hippocampus. Examining the sections at higher magnification confirmed that all plaques were surrounded by GFP-positive microglia (Figure 1B). As seen before, plaque-associated microglia possessed thickened processes and an amoeboid shape (Baron et al., 2014; Sheng et al., 1997), consistent with activated phenotype compared to microglia that were not contacting plaques.
Figure 1. Detection of amyloid β plaques in 5xFAD:mg-GFP mice.
Mice were injected i.p. with 10 mg/kg of the amyloid-binding dye Methoxy-X04 24 hr before brain isolation. Microglia were visualized by their GFP signal, and Methoxy-X04-positive plaques were detected by the intrinsic fluorescence of the dye. A. Plaques are present in the hippocampus of 2-month old mice, mostly in the CA1 region. Inset shows locations of the representative images. Scale bar: 200 µm. B. Higher magnification images show that the Methoxy-X04-positive plaques are surrounded by GFP-positive microglia. Scale bar: 30 µm.
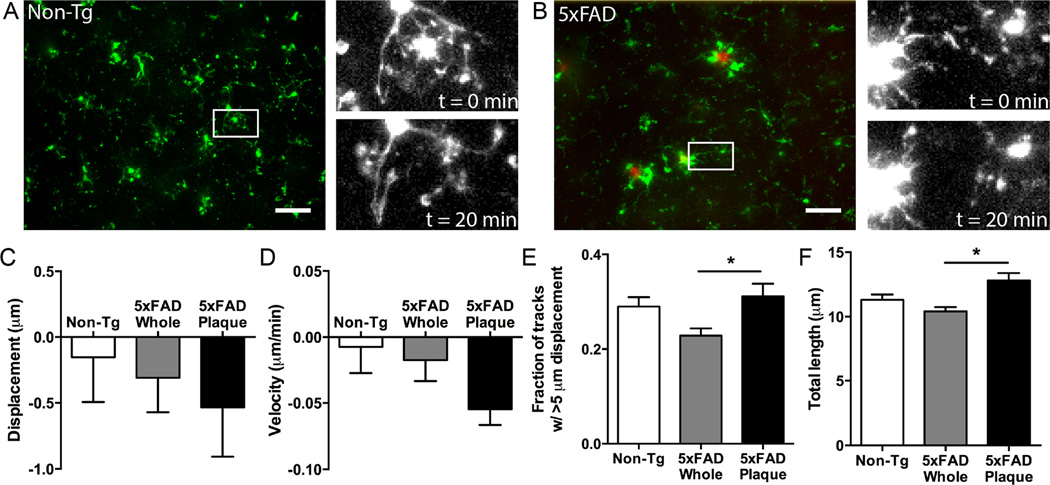
To study microglial motion in real time, we performed time-lapse confocal imaging. Initially, we recorded microglial motion in the tissues of non-Tg or 5xFAD:mg- GFP mice in the absence of any experimental disturbances to capture the baseline dynamics of microglia. Similar to microglia in non-Tg animals, microglia in 5xFAD:mg-GFP mice constantly move their processes during baseline conditions (Figure 2A, B). Measuring the process displacement, the velocity of movement, the total distance traveled, and the number of tracks that had displacement longer than 5 µm showed no difference in movement of microglial processes in 5xFAD:mg-GFP and non-transgenic mice under baseline conditions (Figure 2C-F).
Figure 2. Microglial motion in slices from 5xFAD:mg-GFP mice in the absence of injury.
5xFAD:mg-GFP or non-AD littermate controls (non-Tg) were injected with 10 mg/kg Methoxy-X04 and brains were isolated for slice preparation 24 hr later. Acute brain slices were subjected to time-lapse confocal imaging. Microglia were identified from GFP fluorescence and amyloid plaques were identified from Methoxy-X04 fluorescence. A, B. Representative images showing microglial distribution in acute slices from non-Tg (A) and 5xFAD (B) mice used for time-lapse recordings. Green: microglia; red: Methoxy-X04-positive plaques. In both genotypes, microglial processes move over time. Scale bar: 25 µm. Insets show select cells at the beginning (t = 0 min) and at the end (t = 20 min) of baseline recordings. C-F. Microglial motion was analyzed by automatically tracking objects (microglial processes) larger than 2 µm with Imaris software to quantify process displacement (C), velocity (D), fraction of objects moving more than 5 µm (E), and total length of movement (F). To study plaque-associated microglia (5xFAD Plaque), a region of interest containing only a plaque and the microglia immediately surrounding it was compared to the motion of all microglia in the recording. While plaque-associated microglia do not show altered displacement (C), or velocity (D), they display significantly increased fraction of tracks moving over 5 µm (E), and total length of movement (F) when compared to microglia in the whole image. N = 10 slices for all groups. Statistics: one-way ANOVA and Dunnett’s post hoc test compared to 5xFAD Whole; *, p < 0.05.
We also analyzed the motility of cells that were physically contacting plaques, which by eye appeared to show different motility, and compared them to the average movement of all microglial processes in the same slice; the processes of plaque-associated microglia represented ~10% of all processes in a recording. While the displacement and velocity of tracks were unaffected by association with plaques (Figure 2C, D), we found that more processes of microglia surrounding plaques had displacements longer than 5 µm and longer total distances traveled (Figure 2E, F). Therefore, microglia around plaques in 5xFAD:mg-GFP mice appear to have an increased baseline motility compared to microglia that are not associated with plaques, possibly moving their processes back-and-forth faster (resulting in longer total length), but not extending over longer distances (same displacement).
Microglia in 5xFAD:mg-GFP mice show reduced response to tissue damage
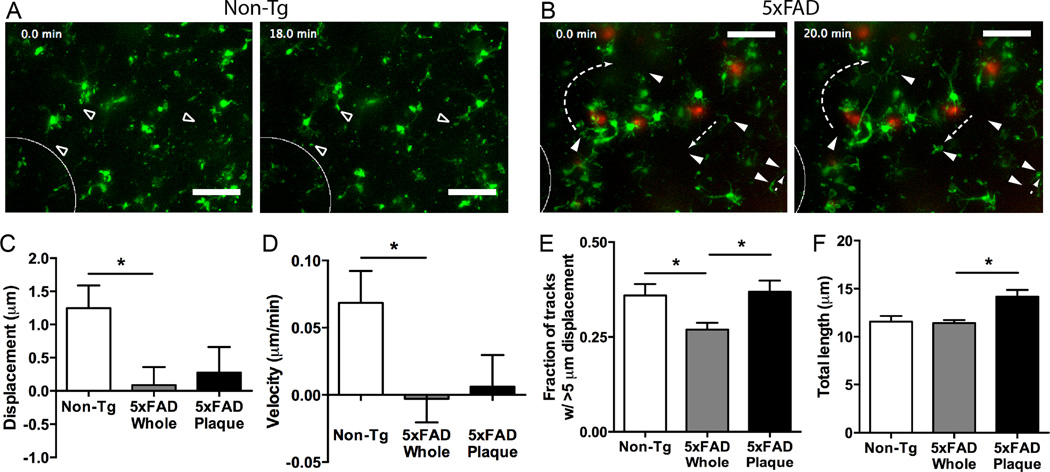
In the APP/PS1 model of AD, microglial processes respond to laser ablation at a slower rate than non-transgenic mice both in slice preparations and in vivo (Krabbe et al., 2013). To assess whether the 5xFAD:mg-GFP mice respond to more modest damage, we used a mechanical damage paradigm in which we quickly lowered a thin rod into the tissue at constant velocity while recording the subsequent microglial response. Microglia in healthy non-transgenic animals responded to the damage by extending their processes to the site of damage (Movie 1, Figure 3A). This is represented by a significantly increased displacement, increased velocity in the direction of the injury, and an increase in processes that move more than 5 µm (Figure 3C-E). In contrast, microglia in 5xFAD:mg-GFP mice failed to extend processes in the direction of the injury and had significantly reduced displacement and velocity (Movie 2, Figure 3B-D). These results extend the findings in the APP/PS1 model (Krabbe et al., 2013) to the 5xFAD model and from laser-induced damage to modest mechanical damage. Despite the differences in directional movement towards the injury, there was no change in the total length of movement after injury between non-transgenic and 5xFAD:mg-GFP mice (Figure 3F). The reduced net displacement and the unchanged total length of movement for plaque-associated microglial processes suggest that processes were hypermotile – moving back and forth despite little net displacement.
Figure 3. Microglial response to injury in 5xFAD and non-Tg mice.
Brain slices from 5xFAD:mg-GFP and non-Tg mice were prepared and imaged as described above. To assess microglial response to injury, a thin rod was lowered into the slice to generate tissue damage (white ellipse shows site of rod insertion). A, B. Representative slices showing response to injury. Microglia from non-Tg mice extend processes to the site of injury (open arrowheads) (A), but microglia from 5xFAD mice do not (B). Microglia are shown in green and Methoxy-X04-positive plaques are shown in red. Occasionally, microglia in 5xFAD mice (but not WT mice) showed unexpected movements and cell body displacement (filled arrowheads). Dashed arrows show unexpected process or cell body movement for a subset of cells. Scale bar: 25 µm. C-F. Microglial motion was analyzed by automatically tracking objects (microglial processes) larger than 2 µm with Imaris software to quantify process displacement (C), velocity (D), fraction of objects moving more than 5 µm (E), and total length of movement (F). To study plaque-associated microglia (5xFAD Plaque), a region of interest containing only a plaque and the microglia immediately surrounding it was compared to the motion of all microglia in the recording. Overall, microglia in 5xFAD mice show a significantly reduced displacement and velocity in the direction of the injury and fraction of tracks with long displacement. Plaque-associated microglia do not show different displacement and velocity in the direction of injury. However, they display significantly increased number of processes moving more than 5 µm (E) and total length of movement (F), suggesting random movement that is not directed to the injury. N = 16 slices for Non-Tg and 18 for 5xFAD (Whole and Plaque) mice. Statistics: one-way ANOVA and Dunnett’s post hoc test compared to 5xFAD Whole; *, p < 0.05.
Occasionally, we also noticed GFP-positive cells that displayed uncharacteristic motility (Movie 2; Figure 3B), which may be another feature of the altered motility of microglia in AD. There are several cells that crawl over significant distances through the tissue (two cells in right half of panels in Figure 3B). Other cells extend their processes over very long distances to unidentified targets (cell in left half of panels in Figure 3B). These types of motility have not been described for microglia in short live recordings before.
Because of the altered baseline motility of plaque-associated microglia, we examined whether these microglia had a differential response to tissue damage. The displacement and velocity in the direction of injury of processes on plaque-associated microglia were not significantly altered from the average velocity of all microglia in a 5xFAD slice (Figure 3C, D). However, microglial processes near plaques displayed a significantly increased fraction of tracks with length over 5 µm and an increase in the total length traveled when compared to all cells in the slice (Figure 3E, F). This may have accounted for the increased variability of displacement and velocity in Fig 3C, D (note SEM for 5xFAD plaque bars). Thus, plaque-associated microglia continued to show hypermotile activity, but the movement was not directed to the injury. Taken together, our data so far indicate that microglia in the 5xFAD:mg-GFP mouse model of AD show altered motility both under baseline conditions and in response to tissue damage.
Modulation of microglial motility by adenosine A2A receptors in tissues from 5xFAD:mg-GFP mice
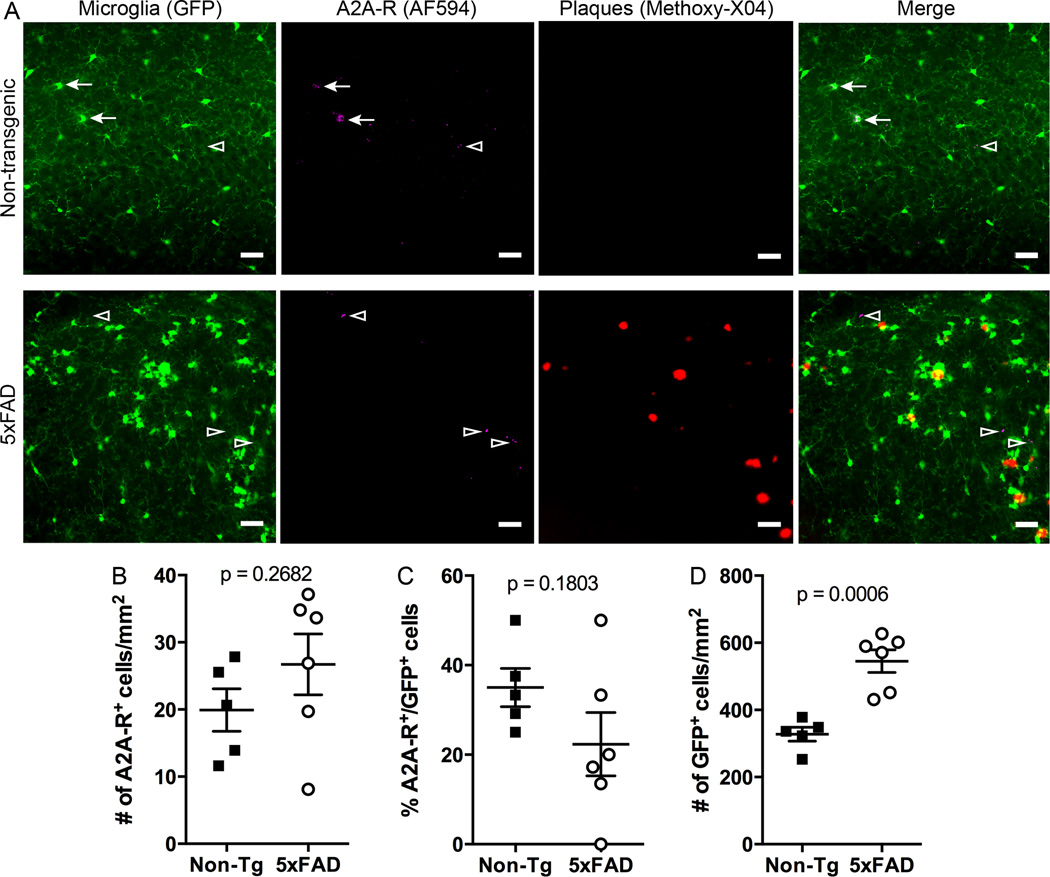
Given the precedent for a role of A2A receptors in regulating microglial dynamics (Gyoneva et al., 2014a; Gyoneva et al., 2014b), we examined whether A2A receptors may be responsible for the delayed response to mechanical damage seen in 5xFAD:mg-GFP mice. We first determined whether the environment around plaques alters A2A receptor expression. To accomplish this, we examined A2A receptor levels in 5xFAD:mg-GFP and non-Tg mice by fluorescent immunohistochemistry. A2A receptor immunoreactivity was detected both in microglia (GFP-positive cells) and in unidentified cell types (GFP-negative cells) in non-Tg and 5xFAD:mg-GFP mice (Figure 4A). To determine if the number of A2A receptor-positive microglia is different in non-Tg and 5xFAD mice, we counted GFP+/A2A-R+ cells. Although the total number of A2A-positive cells appeared to increase in 5xFAD mice (Figure 4B), the number of microglia expressing A2A receptors appeared slightly lower (Figure 4C); neither of these differences was statistically significant. The number of GFP-positive cells was significantly higher in the 5xFAD mice (Figure 4D), likely because of the high number of microglia around plaques.
Figure 4. Adenosine A2A receptor expression in 5xFAD mice.
5xFAD:mg-GFP and non-Tg mice were injected with Methoxy-X04 and brains were isolated and fixed 24 hr later. A. Brain sections (40 µm) were stained for A2A receptor and imaged on a confocal microscope to obtain GFP (microglia, shown in green), AlexaFluor-594 (A2A receptors, shown in magenta) and Methoxy-X04 (plaques, shown in red) signals. Merged images show A2A receptor-positive microglia in the hippocampus of both non-Tg and 5xFAD mice. Scale bar: 30 µm. Arrows point to GFP-positive cells that appear to colocalize with A2A receptors. Arrowheads point to A2A receptor-positive cells that do not colocalize with GFP-positive microglia. B-D. Quantification of A2A receptor-positive cells. The total number of A2A receptor-positive cells in the hippocampus is not significantly different between 5xFAD and non-Tg mice (B). The number of A2A receptor-expressing microglia is not significantly different between non-Tg and 5xFAD mice (C), despite the overall higher number of microglia in 5xFAD mice (D). Statistics: Student’s t test.
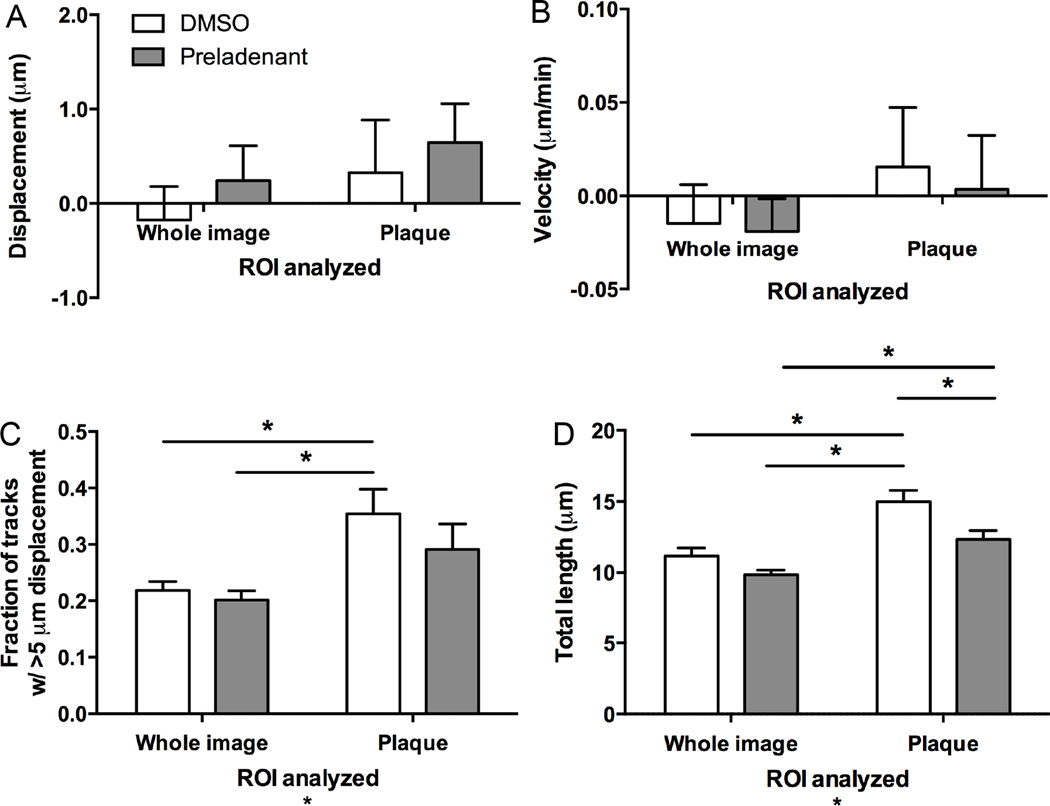
Although A2A receptor expression was not selectively altered in microglia in 5xFAD mice, their presence in the hippocampus in regions containing plaques, together with the potential ability of the non-selective adenosine receptor antagonist caffeine to affect Aβ pathology and memory, prompted us to examine how A2A receptors might modulate microglial motility. In order to assess the effects of A2A receptor function, we added the highly selective A2A receptor antagonist preladenant (5 µM) to the slice perfusion solution 10 min before and during injury induction and for the duration of imaging thereafter. When whole slices were examined, preladenant did not significantly alter either the displacement of microglia in the direction of the injury (Figure 5A), the velocity of the response to injury (Figure 5B), the fraction of processes with track length over 5 µm (Figure 5C), or the total distance travelled (Figure 5D). However, when considering only plaque-associated microglia, there was a significant decrease in the total length traveled by microglial processes after preladenant treatment compared to vehicle control, but not the number of processes moving over 5µm (Figure 5C, D). Thus, preladenant specifically reduced the motility of microglia around plaques, although these microglia were still hypermotile compared to the rest of the slice (Figure 5C, D).
Figure 5. Effect of A2A receptor antagonism on microglial motility in 5xFAD mice.
Brain slices from 5xFAD:mg-GFP mice were prepared and imaged as described above to assess response to injury. The slices were constantly perfused with 5 µM of the A2A receptor antagonist preladenant or DMSO vehicle. Microglial motion was analyzed by automatically tracking objects (microglial processes) larger than 2 µm with Imaris software to quantify process displacement (A), velocity (B), fraction of objects moving more than 5 µm (C), and total length of movement (D). Plaque-associated microglia (analysis of a region of interest containing only a plaque) show increased number of tracks with more than 5 µm displacement (C) and total distance travelled (D). A2A receptor blockade with preladenant did not significantly affect microglial motility when the whole slice is analyzed, but reduced the total distance traveled by processes of plaque-associated microglia (D). N = 9 slices for all conditions. Statistics: two-way ANOVA and Tukey’s post hoc test; *, p < 0.05.
Discussion
In the present study, we examined microglial motility at baseline and following antagonism of adenosine A2A receptors in acute brain slices of an animal model of Alzheimer’s disease. We crossed 5xFAD mice, which carry five total familial mutations in APP and PS1 (Oakley et al., 2006), to Cx3cr1GFP/GFP mice that exhibit microglial GFP expression (Jung et al., 2000) and monitored microglial motility with confocal microscopy (Figure 1). Our data allow us to draw three main conclusions. First, microglia that are associated with amyloid plaques show a dysregulated, hyperactive motility compared to non-plaque microglia. Second, neither plaque-associated, nor plaque-free microglia respond appropriately to mechanically induced tissue damage in slices from 5xFAD mice. Third, the adenosine A2A receptor antagonist preladenant failed to enhance the microglial response to damage, although it reduced the hypermotility of plaque-associated microglia. Thus, plaque-associated microglia may represent a unique microglial phenotype with hypermotile processes and altered response to tissue damage (as in other inflammatory conditions). There appears to be no contribution of A2A receptors in response to injury, but these receptors are able to influence motility of microglia near plaques.
Altered microglial motility patterns in 5xFAD:mg-GFP mice
Microglial surveying of the brain parenchyma is thought to clear debris and is considered essential for the maintenance of brain homeostasis (Nimmerjahn et al., 2005). Here we observed that microglia surrounding plaques show altered motility patterns compared to the rest of microglia (Figure 2A, B). While the processes that directly contacted plaques appeared stationary, processes pointing away from the plaques moved with higher velocity and over longer total distances (Figure 2C-F). Baron et al. (2014) recently described the morphology of microglia in the APPSwe,Ind and APP/PS1 AD models, concluding that plaque-associated microglia have an activated morphology that contrasted the normal-appearing morphology of non-plaque microglia. Here we show that microglia that contact plaques are not simply morphologically different, but also differ functionally in regard to velocity of process motility under both baseline and injury conditions.
The hypermotile behavior by processes of activated microglia seen here is reminiscent of the increased motility of processes in lipopolysaccharide (LPS)-activated microglia in vitro and in vivo described before (Gyoneva et al., 2014a; Orr et al., 2009). Thus, increased process motility may be a general sign of microglial activation and an indication of a pathological condition. However, the functional consequences of this remain to be examined.
Microglial response to tissue damage in 5xFAD:mg-GFP mice
We recently established an acute brain slice preparation to study the response of microglia to mechanical damage within parenchyma that involved a limited number (~10) of cells (Gyoneva et al., 2014b). This preparation allows time lapse imaging of microglia embedded in brain tissue from structures that are distant to the pial surface and thus inaccessible to in vivo imaging. Acute brain slice preparations have been used extensively in neurophysiological studies, and show neuronal function and microglia appear to be in a resting state by most measures. We previously showed that microglia in the substantia nigra of slices prepared from LPS- or MPTP-treated mice exhibit a delayed response to tissue damage (Gyoneva et al., 2014b).
We now extend our findings to the 5xFAD model of Alzheimer’s disease, showing that microglia in the hippocampus of slices from 5xFAD:mg-GFP mice did not extend their processes to the site of damage (Figure 3). Despite their increased baseline motility, plaque-associated microglia did not respond to the damage either (Figure 3). Importantly, the lack of response to tissue damage was also described both in slices and in vivo with multiphoton microscopy in another AD mouse model (Krabbe et al., 2013). Thus, it suggests that the phenomenon is modelindependent and occurs both to significant laser-induced tissue damage and in the milder rod-induced damage used herein. In addition, the delay in microglial process extension in response to tissue damage under inflammatory conditions may be a widespread condition, as it also occurs in various models of microglial activation ranging from systemic inflammation to the MPTP-model of Parkinson’s disease (Gyoneva et al., 2014a; Gyoneva et al., 2014b). Yet, care should be taken to generalize this to other conditions as microglia can also show an accelerated response to damage, such as in kainate-induced status epilepticus model in mice (Avignone et al. 2008). Therefore, microglial motility should be examined in each individual context.
Adenosine A2A receptors in 5xFAD mice
Because of our previous findings that adenosine A2A receptor antagonism restores the ability of microglia to respond to tissue damage (Gyoneva et al., 2014a; Gyoneva et al., 2014b), we examined whether this receptor system would mediate a similar effect in 5xFAD:mg-GFP mice. However, the inclusion of the selective A2A receptor antagonist preladenant in the perfusion solution during injury recordings did not accelerate microglial response to injury in 5xFAD:mg-GFP mice (Figure 5), which is consistent with the lack of change in A2A receptor immunoreactivity in microglia in 5xFAD:mg-GFP mice compared to non-Tg controls (Figure 6C-E). This suggests that the microglial activation that is seen in models of AD and PD likely represent two different microglial phenotypes characterized by differential cell surface receptor expression, which is not surprising considering the disparate disease etiologies.
Even though preladenant did not significantly alter the approach of microglial processes to the site of damage, the addition of the antagonist affected certain aspects of the motility of plaque-associated microglia (Figure 5). Specifically, the total distance traveled was significantly decreased, suggesting that preladenant may decrease the hyperactivity of plaque-associated microglia. Moreover, it is possible that acute A2A receptor antagonism affects other microglial functions that were not examined here, such as phagocytosis of Aβ plaques or cytokine release, and it remains to be determined whether preladenant exerted its effects directly by modulating motility pathways or by overall suppression of microglial functions. Additionally, the ability of preladenant to acutely affect plaque-associated microglial motility warrants future studies that examine the effects of chronic preladenant treatment on microglial functions in AD models.
It is worth noting that we detected A2A expression in multiple cell types in the hippocampus, both in 5xFAD and non-Tg mice. This is consistent with A2A receptor expression in the cortex and hippocampus of humans with AD as assessed in post-mortem samples (Albasanz et al., 2008; Angulo et al., 2003). Much of this A2A receptor expression in humans was initially reported to be in microglia (Angulo et al., 2003), however more recent work also suggests astrocytic expression (Orr et al., 2015). In our study, some of the A2A receptor-expressing cells appeared to be GFP-positive microglia, including plaque-associated microglia. However there was no difference in A2A receptor protein expression in hippocampus between 5xFAD and non-Tg mice despite the ability of Aβ peptides to upregulate A2A receptor mRNA (Orr et al., 2009). While the total number of A2A receptor-expressing cells appeared higher in 5xFAD mice (Figure 4B), there was no difference in A2A receptor-expressing microglia between 5xFAD and non-Tg animals (Figure 4C). This is not due to decreased microglial density in 5xFAD mice because these mice had more microglia per unit area than their wild type littermates (Figure 4D). Hence, it is possible that in our studies the presence of AD pathology is associated with an apparent increase in A2A receptors in non-microglial cell types (neurons or astrocytes), which is consistent with astrocytic expression of the receptor seen by Orr et al. (2015). These non-microglial A2A receptors might then modulate microglial motility via unknown mechanisms to reduce hyperactivity of plaque-associated microglia. Furthermore, the presence of A2A receptors in the hippocampus (Figure 4A) could potentially explain some of the effects of A2A receptor modulation on cognition and memory (Arendash et al., 2009; Canas et al., 2009; Cao et al., 2009; Eskelinen and Kivipelto, 2010; Santos et al., 2010).
Our data on adenosine A2A receptor expression and modulation of microglial function in models of AD (here) and PD (Gyoneva et al., 2014b) provide an example of a differentially regulated signaling system with varied properties in different conditions associated with neuroinflammation and neurodegeneration. While microglia in the MPTP model of PD upregulate A2A receptors and respond to A2A receptor antagonists (Gyoneva et al., 2014b), microglia in 5xFAD mice do not alter A2A receptor expression. Moreover, A2A receptors in 5xFAD mice are slightly responsive to A2A receptor antagonists (reduced motility of plaque-associated microglia; Figure 5), suggesting some tonic activation is present in the tissue. The variability of microglial phenotypes in AD and PD might also explain the divergent effects of A2A receptor antagonists on microglial motility: increased response to injury in a model of PD and decreased motility of plaque-associated microglia in a model of AD.
In conclusion, we studied the motility of microglia in the 5xFAD model of Alzheimer’s disease. We show that microglia that are physically associated with plaques show hypermotile behavior for the processes pointing away from plaques and confirm that microglia in tissues with AD-like pathology show a delayed response to tissue damage. While the adenosine A2A receptor antagonist preladenant was able to ameliorate the hyperactivity of plaque-associated microglia, it did not restore the response to tissue damage. The exact roles of adenosine A2A receptors in modulating microglial functions in the hippocampus and the identification of additional receptor systems that regulate microglial motility in the context of AD warrant further investigation.
Supplementary Material
Acute brain slices were prepared from mice with microglia-specific GFP expression. Injury was induced by lowering a thin rod into the slice; the location of the injury is indicated with a white ellipse. Time-lapse confocal imaging captures microglial response to the injury over 20 min. Arrow point to cells that extended their processes to the site of injury.
Acute brain slices were prepared from mice with microglia-specific GFP expression which express 5 familial mutations seen in AD (5xFAD). Injury was induced by lowering a thin rod into the slice; the location of the injury is indicated with a white ellipse. Time-lapse confocal imaging captures microglial response to the injury over 20 min. Amyloid plaques are shown in red and microglia in green. Arrowheads point to cells that show unexpected motility of their processes or cell body displacement.
Highlights.
Plaques-associated microglia show a hyperactive motility in slices from 5xFAD mice
Microglia in 5xFAD mice do not respond appropriately to tissue damage
An adenosine A2A receptor antagonist did not enhance microglial response to damage
A2A receptor antagonism reduced the hypermotility of plaque-associated microglia
Acknowledgments
Funding was provided by NINDS NRSA (F31NS076215, S.G.), NIH Pharmacological Sciences institutional training grant (T32GM008602, S.G.), and a pilot grant from the Emory University Alzheimer's Disease Research Center (NIHP50 AG025688, S.F.T.). We thank Dr. Ethel Garnier-Amblard for synthesizing preladenant.
Abbreviations
- Aβ
amyloid β peptide
- aCSF
artificial cerebrospinal fluid
- AD
Alzheimer’s disease
- AF594
AlexaFluor-594
- APP
amyloid precursor protein
- CCL
CC-type chemokine ligand
- CX3CR
CX3C-type chemokine receptor
- FAD
familial Alzheimer’s disease
- IL
interleukin
- LPS
lipopolysaccharide
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NGS
normal goat serum
- PD
Parkinson’s disease
- PS1
presenilin 1
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
S.G., S.A.S., S.F.T. and D.W. planned the experiments. S.G., S.A.S. and J.Z. performed the research. S.G. and S.A.S. analyzed the data. S.G., S.A.S., D.W. and S.F.T. discussed the results. All authors wrote the manuscript.
Disclosure:
The authors have no conflict of interest to declare.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Piet E, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L-F, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasanz JL, Perez S, Barrachina M, Ferrer I, Martin M. Up-regulation of Adenosine Receptors in the Frontal Cortex in Alzheimer's Disease. Brain Pathol. 2008;18:211–219. doi: 10.1111/j.1750-3639.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo E, Casado V, Mallol J, Canela EI, Vinals F, Ferrer I, Liuis C, Franco R. A1 Adenosine Receptors Accumulate in Neurodegenerative Structures in Alzheimer's Disease and Mediate Both Amyloid Precursor Protein Processing and Tau Phosphorylation and Translocation. Brain Pathol. 2003;13:440–451. doi: 10.1111/j.1750-3639.2003.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tan J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer's Disease Mice. J Alzheimer Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- Avignone E, Ulmann L, Levacasseur F, Rassendren F, Audinat E. Status Epilepticus Induces a Particular Microglial Activation State Characterized by Enhanced Purinergic Signaling. J Neurosci. 2008;28:9133–9144. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Babcock AA, Nemirovsky A, Finsen B, Monsonego A. Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer's disease. Aging Cell. 2014;13:584–595. doi: 10.1111/acel.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canas PM, Porciuncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA. Adenosine A2A Receptor Blockade Prevents Synaptotoxicity and Memory Dysfunction Caused by β-Amyloid Peptides via p38 Mitogen-Activated Protein Kinase Pathway. J Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A, Mamcarz M, Zhang C, Mori T, Arendash GW, Holtzman DM, Potter H. Caffeine Suppresses Amyloid-β Levels in Plasma and Brain of Alzheimer's Disease Transgenic Mice. J Alzheimer Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Eskelinen M, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer's disease. J Alzheimer Dis. 2010;20:S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial Response to Amyloid Plaques in APPsw Transgenic Mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Davalos D, Biswas D, Swanger SA, Garnier-Amblard E, Loth F, Akassoglou K, Traynelis SF. Systemic Inflammation Regulates Microglial Responses to Tissue Damage In Vivo. Glia. 2014a;62:1345–1360. doi: 10.1002/glia.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Shapiro L, Lazo C, Garnier-Amblard E, Smith Y, Miller GW, Traynelis SF. Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson's disease. Neurobiol Dis. 2014b;67:191–202. doi: 10.1016/j.nbd.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan W-B, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Hefendehl J, Wegenast-Braun BM, Liebig C, Eicke D, Milford D, Calhoun ME, Kohsaka S, Eichner M, Jucker M. Long-term in vivo imaging of β-amyloid appearance and growth in a mouse model of cerebral β-amyloidosis. J Neurosci. 2011;31:624–629. doi: 10.1523/JNEUROSCI.5147-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer's disease. J Neural Transm. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S, Cohen-Williams ME, Higgins GA, Impagnatiello F, Nicolussi E, Parra LE, Foster C, Zhai Y, Neustadt BR, Stamford AW, Parker EM, Areggiani A, Hunter J. Characterization of the Potent and Highly Selective A2A Receptor Antagonists Preladenant and SCH 412348 [7-[2-[4-2,4-Difluorophenyl]-1-piperazinyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in Rodent Models of Movement Disorders and Depression. J Pharmacol Exp Therapeutics. 2009;330:294–303. doi: 10.1124/jpet.108.149617. [DOI] [PubMed] [Google Scholar]
- Itagaki S, Akiyama H, Saito H, McGeer PL. Ultrastructural localization of complement membrane attack complex (MAC)-like immunoreactivity in brains of patients with Alzheimer's disease. Brain Res. 1994;645:78–84. doi: 10.1016/0006-8993(94)91640-3. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of Fractalkine Receptor CX3CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Wood WG, Sun GY, Erb L, Petris M, Weisman GA. Nucleotides released from Aβ1-42-treated microglial cells increase cell migration and Aβ1-42 uptake through P2Y2 receptor activation. J Neurochem. 2012;121:228–238. doi: 10.1111/j.1471-4159.2012.07700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Bacskai BJ, Mathis CA, Kajdazz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging Aβ Plaques in Living Transgenic Mice with Multiphoton Microscopy and Methoxy-X04, a Systemically Administered Congo Red Derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holzman D. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Berngardt U, Miller KR, Prokop S, Kettenman H, Heppner FL. Functional Impairment of Microglia Coincides with Beta-Amyloid Deposition in Mice with Alzheimer-Like Pathology. PloS One. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holzman D, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neustadt H, Liu J, Hao WJ, Greenlee AW, Stamford C, Foster L, Arik J, Lachowicz H, Zhang R, Bertorelli S, Fredduzzi G, Varty M, Cohen-Williams K. Ng Potent and selective adenosine A2A receptor antagonists: 1,2,4-Triazolo[1,5-c]pyrimidines. Bioorg Med Chem Lett. 2007;19(3):967–971. doi: 10.1016/j.bmcl.2008.11.075. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuronal Loss in Transgenic Mice with Five Familial Alzheimer's Disease Mutations: Potential Factors in Amyloid Plaque Formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li X-J, Gross RE, Traynelis SF. Adenosine A2A receptor mediates microglial process retraction. Nat Neurosci. 2009;12:872–878. doi: 10.1038/nn.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Hsiao EC, Wang MX, Ho K, Kim DH, Wang X, Guo W, Kang J, Yu G-Q, Adame A, Devidze N, Dubal DB, Masliah E, Conklin BR, Mucke L. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci. 2015;18:423–434. doi: 10.1038/nn.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter L, Scott S, Barron E, Chui H. HMC Class II-Positive Microglia in Human Brains: Association with Alzheimer Lesions. J Neurosci Res. 1992;33:549–558. doi: 10.1002/jnr.490330407. [DOI] [PubMed] [Google Scholar]
- Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimer Dis. 2010;20:S187–S204. doi: 10.3233/JAD-2010-091387. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WST. Neuritic plaque evolution in Alzheimer's disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94:1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer's Disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Acute brain slices were prepared from mice with microglia-specific GFP expression. Injury was induced by lowering a thin rod into the slice; the location of the injury is indicated with a white ellipse. Time-lapse confocal imaging captures microglial response to the injury over 20 min. Arrow point to cells that extended their processes to the site of injury.
Acute brain slices were prepared from mice with microglia-specific GFP expression which express 5 familial mutations seen in AD (5xFAD). Injury was induced by lowering a thin rod into the slice; the location of the injury is indicated with a white ellipse. Time-lapse confocal imaging captures microglial response to the injury over 20 min. Amyloid plaques are shown in red and microglia in green. Arrowheads point to cells that show unexpected motility of their processes or cell body displacement.