Case Presentation
Chief Symptom
A sixty six year-old Hispanic Caucasian lady with a 14 year history of diffuse scleroderma and a history of bilateral breast cancer presented for evaluation of a vasculitic rash and hemoptysis with positive Proteinase-3 (PR-3) serology raising concern for possible granulomatosis with polyangiitis (GPA) or ANCA-associated vasculitis (AAV).
History of Present Illness
The patient developed new onset of a petechial rash on her lower extremities along with punched out ulcerations on the feet and dorsal hands (Figure 1A) two months prior to the current presentation. Four weeks prior to presentation, she developed cough with hemoptysis. There was no fever, but she did report weight loss. She had not chest pain but was short of breath with minimal exertion. Chest radiograph revealed diffuse interstitial lung disease with superimposed multifocal peripheral airspace disease and pleural based cavitating lesions (Figure 2A). She was started on antibiotic and non-contrast CT thorax was performed.
Figure 1.
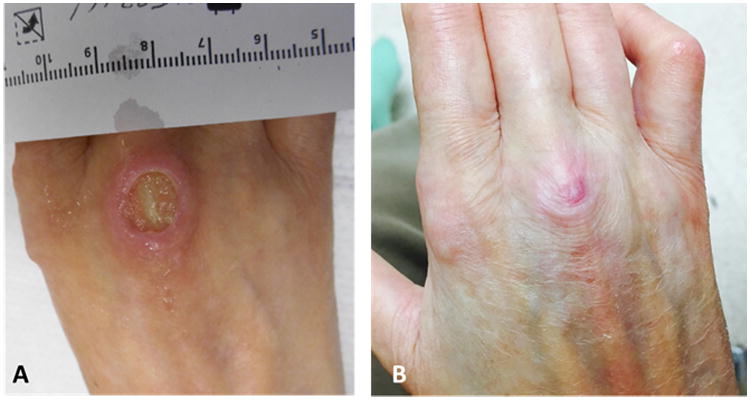
A: Punched out vasculitic skin ulcerations overlying the 3rd MCP at presentation. B: Healed lesion overlying the 3rd MCP 4 months after induction therapy with steroids and rituximab.
Figure 2.
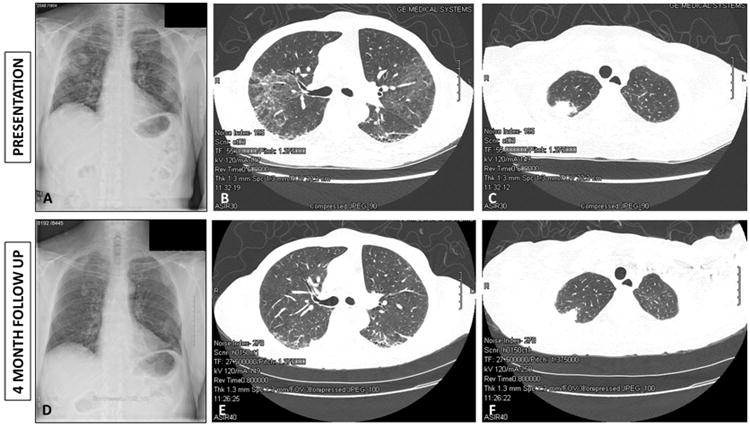
A: Chest radiograph at time of presentation with hemoptysis showing apical lesions with central cavitation. B: Thoracic computed tomography at time of presentation demonstrating fibrotic changes with honey combing and traction bronchiectasis. C: Thoracic computed tomography demonstrating right apical lung lesion with central cavitation. D: Follow-up Chest radiograph four months after commencing induction therapy with steroids and rituximab demonstrating interval improvement in the lung lesions. E: Follow-up thoracic computed tomography demonstrating interval improvement in the interstitial lung disease. F: Follow-up thoracic computed tomography demonstrating partial resolution of the apical cavitating lesions four months after institution of induction therapy with steroids and rituximab.
Past Medical History
The patient had a 14 year history of diffuse scleroderma. She had originally presented with raynauds, and sclerodactyly with puffy hands. Over the first few years her disease manifestations predominantly consisted of skin involvement with contractures at the metacarpophalangeal joints (MCPs) and proximal interphalangeal joints (PIPs), severe gastrointestinal dysmotility and interstitial lung disease. She was initially treated with only hydroxychloroquine monotherapy. Immunosuppressive therapies that could have predisposed her to malignancy were not used during the first 5 years of her disease.
In regards her pulmonary function, her most recent pulmonary function tests (PFT) 1 month prior to the current admission revealed FVC 61% predicted, FEV1 64% predicted, with DLCO 49% predicted. This was an improvement from 2 years prior when she had FVC 55% predicted, FEV1 58% predicted and DLCO 42% predicted. Six minute walk test was performed 1 month prior to this presentation and the patient walked 450 meters (89% predicted) without desaturation. The last echo prior to the current admission showed normal ejection fraction with no valve lesions, normal left ventricular size and function, normal right ventricular size and function and right ventricular systolic pressure (RVSP) of 31mmHg.
The patient was diagnosed with bilateral breast cancer 9 years prior to the current presentation (5 years after the original diagnosis of scleroderma). Histology revealed stage IIB invasive and in situ mammary carcinoma with lobular and ductal features. She underwent bilateral mastectomy followed by chemotherapy. The histology from both mastectomy specimens showed estrogen receptor positive (100%), progesterone receptor positive (80%) and the tumor was Her2 negative (HercepTest™ score 0 bilaterally). Bilateral axillary dissection revealed 2 out of 17 nodes positive on the left and 1 out of 10 nodes positive on the right. She was treated with bilateral mastectomy and chemotherapy. Initially she received Adriamycin and Cyclophosphamide, however the Adriamycin had to be discontinued due to severe mucositis. Following completion of the cyclophosphamide she received 2 doses of Paclitaxel and 2 doses of Docetaxel. She subsequently received 5 years of continuous therapy with the aromatase inhibitor Anastrazole. Her interstitial lung disease pre-dated the breast cancer and chemotherapy treatment and was thus felt to be unrelated to chemotherapy. She elected not to undergo breast reconstruction. Two years prior to the current presentation she developed bilateral scleritis, sinusitis and hearing loss which was attributed to relapsing polychondritis. She was started on prednisone and mycophenolate mofetil. Several months later note was made of a positive ANCA; however, because she was clinically stable no action was taken.
Family History and Social History
The patient was adopted. She lived alone. She was a former smoker of 2 packs per day for 15 years, but quit 28 years prior to the current presentation. She was medically retired due to scleroderma. She had previously worked in educational research.
Medications
At the time of presentation the patient's outpatient medications included: Losartan 25mg orally once daily; Omeprazole 40mg orally twice daily; Bromfenac ophthalmic 0.07% 1 drop left eye twice daily; Brimonidine/timolol ophthalmic 0.2-0.5% 1 drop both eyes twice daily; Cyclobenzaprine 5mg at bedtime; Ergocalciferol 50,000 units orally once per month; Mycophenolate mofetil 2g orally in the morning and 1g in the afternoon (total daily dose 3g); Prednisone 12mg once daily; ibuprofen 200mg daily; Tobramycin/dexamethasone ophthalmic 0.3-0.1% ophthalmic ointment twice daily to left eyelid; mupirocin to hand ulcers; Doxycycline hyclate 100mg orally twice daily.
Allergies
The patient had no known drug allergies. She was allergic to shrimp.
Physical Examination
On physical examination the patient was afebrile, blood pressure was 154/80, pulse 70 beats per minute with regular rate and rhythm, oxygen saturation was 100% on room air. Height was 66 inches with a weight of 138 pounds giving a body mass index of 22.27 Kg/m2.
She was in no acute distress at rest. She had scleroderma facies with skin thickening, tapered nose and microstomia with reduced salivary pooling. She had no malar or discoid rashes, but did have dryness of the eyes and conjunctival injection on the right. She had no scalp lesions and temporal arteries were pulsatile and non-tender. Cardiovascular examination revealed normal heart sounds with no murmurs, rubs or gallops and a normal P2. Chest examination revealed coarse crackles to the mid-zones bilaterally with bronchial breath sounds at the left apex. The abdomen was soft and non-tender, with no hepatosplenomegaly and no masses. Bowel sounds were active. Breast examination was notable for bilateral mastectomy. She had tethered retracted skin on the anterior chest wall with no prior reconstruction. No masses were appreciated. Musculoskeletal examination revealed bilateral sclerodactyly with contractures of the MCPs and PIPs bilaterally consistent with longstanding scleroderma. She had a large punched out ulceration over the right 3rd MCP measuring 1×0.7×0.2cm with a punched out border and desiccated tendon in the base (Figure 1A). There were additional ulcers over the left 3rd PIP measuring 0.4×0.4×0.1cm, right 5th PIP 0.3×0.2×0.1cm, left lateral toe 0.5×0.4×0.1cm, right medial toe 0.4×0.2×0cm, right medial second toe 0.4×0.3×0.1cm and right lateral 4th toe measuring 0.8×0.3×0.1cm. She had a petechial rash over her lower extremities consistent with a leukocytoclastic vasculitis. Her modified Rodnan skin score (MRSS) was 14. Joint examination revealed bilateral MCP joint contractures but no active synovitis. Her swollen joint count was 0 and tender joint count was 0.
Laboratory evaluation
Laboratory testing at diagnosis with scleroderma, at breast cancer diagnosis and at the time of the current presentation is listed in Table 1. At the time of this current presentation, the patient had a normal white cell count and normocytic anemia. Her metabolic panel and liver function tests were within normal limits. Erythrocyte sedimentation rate (ESR) was normal at 23mm/hr but C-reactive protein (CRP) was elevated at 14 mg/L. Urinalysis was unremarkable with no active sediment, no white cells and no protein.
Table 1. Laboratory values at the time of scleroderma onset, at breast cancer diagnosis, at episcleritis onset, and at the time of the current presentation.
| Normal Range | Diffuse systemic sclerosis onset | Breast Cancer Diagnosis | Episcleritis onset | Current presentation | 4 months after rituximab induction therapy | |
|---|---|---|---|---|---|---|
| Time prior to current presentation (years) | -14 | -10 | -2 | 0 | +0.3 | |
| White Blood Cell Count (×103/uL) | 3.4-10.8 | 4.5 | 4.4 | 8.3 | 7.18 | 10.2 |
| Hemoglobin (g/dL) | 11.1-15.9 | 12.8 | 12.7 | 12.1 | 10.1 | 11.4 |
| Hematocrit (%) | 34.0-46.6 | 37.9 | 36.0 | 37.0 | 33.1 | 35.4 |
| Mean cell volume (femtoliters) | 80-100 | 89.2 | 90 | 91.9 | 83 | |
| Platelets (×103/uL) | 155-379 | 246 | 210 | 293 | 356 | 290 |
| Sodium (mMol/L) | 134-144 | 139 | 141 | 139 | 138 | 140 |
| Potassium (mMol/L) | 3.5-5.2 | 4.1 | 4.2 | 4.3 | 4.0 | 4.1 |
| BUN (mg/dL) | 8-27 | 15 | 7 | 13 | 11 | 22 |
| Creatinine (mg/dL) | 0.57-1.00 | 0.6 | 0.6 | 0.55 | 0.50 | .54 |
| Glucose (mg/dL) | 65-99 | 105 | 97 | 82 | 129 | 103 |
| AST (iU/L) | 0-40 | 25 | 29 | 28 | 23 | 18 |
| ALT (iU/L) | 0-32 | 19 | 21 | 27 | 22 | 10 |
| Erythrocyte sedimentation rate (mm/hr) | 0-40 | 25 | 29 | 28 | 23 | 9 |
| C-Reactive protein (mg/L) | 0-4.9 | 4 | <1 | 2.7 | 14 | 2.5 |
| ANA | Negative | 1:160 homogeneous | 1:160 homogeneous | |||
| Scl-70 | Negative | Negative | Negative | Negative | Negative | |
| Centromere | Negative | Negative | Negative | |||
| RNA polymerase III (units) | <20 | Not done | 33.4 | |||
| THTO | Negative | Not Done | Negative | |||
| U3RNP | Negative | Not Done | Negative | |||
| U1RNP | Negative | Not Done | Negative | |||
| Anti-Smith | Negative | Not Done | Negative | |||
| Anti-dsDNA | Negative | Not Done | Negative | |||
| p-ANCA | Negative | Not Done | <1:20 | <1:20 | ||
| c-ANCA | Negative | Not Done | 1:80 | 1:640 | ||
| Anti-proteinase-3 (U/mL) | 0-3.5 | Not Done | 47.5 | 39.9 | ||
| Anti-myeloperoxidase (MPO) | <9.0 | Not Done | <9.0 | <9.0 | ||
| Rheumatoid Factor (IU/mL) | 0-13.5 | Not Done | 17.3 | |||
| Cyclic Citrullinated peptide (units) | 0-19 | Not Done | 3 | |||
| Quantiferon gold | Negative | Not Done | Negative | |||
| Ca27-29 (U/mL) | 0-37 | 40 | 39.8 | 46.8 |
Serologic work up at time of presentation with episcleritis and at the time of the current presentation with hemoptysis revealed positive cANCA with titer rising from 1:80 initially to 1:640 by the time of the hemoptysis presentation. Proteinase-3 antibody was 39.9 U/mL. Quantiferon gold was negative. Baseline scleroderma antibody profile had revealed a positive ANA 1:160 homogeneous standing but negative Scl-70 and centromere antibodies. At that time additional scleroderma specific autoantibodies were not commercially available. At the time of the current presentation scleroderma specific antibodies were sent and revealed negative THTO, negative U3RNP but positive RNA polymerase III antibody at 33.4 units (negative <20 units).
Imaging
CT thorax revealed multiple lung nodules and masses which were peripheral and pleural based and noted to be new since the previous scan 2 years prior. There were also notable bilateral lower lobe predominant honeycombing and bibasilar reticular changes with traction bronchiectasis consistent with interstitial fibrosis (Figure 2B). Ground glass opacities were noted in the bilateral upper lobes with multiple calcified granulomata but no significant hilar or mediastinal lymphadenopathy. The largest cavitating lesions were 3×2cm in the left apex and 2.2×2cm in the right upper lobe; both lesions had cavitary changes (Figure 2C).
Case Summary
This was a sixty six year-old lady with longstanding diffuse scleroderma and bilateral stage IIB breast cancer diagnosed 5 years after scleroderma onset who now presents with a vasculitic rash, punched out ulcerations, and cavitating pulmonary lesions on a background of scleroderma-associated interstitial lung disease.
Differential Diagnosis
The differential diagnosis of cavitating pulmonary nodules is listed in Table 2 and includes benign and malignant etiologies (1). The most common benign pathologies which could result in cavitating pulmonary nodules, particularly since this patient was immunosuppressed, include infections such as cavitating pneumonias, mycobacterial infections including tuberculosis and invasive fungal infections such as aspergillus. While this patient carried a diagnosis of relapsing polychondritis, she also had positive PR-3 serology. As outlined below, relapsing polychondritis does not typically cause cavitating lung lesions. However, granulomatosis with polyangiitis and other ANCA associated vasculitidies are known to cause cavitary lung lesions and were on the differential in this case. Finally, both primary and secondary malignant lung lesions may cavitate and were on the differential in this case due to the known association between malignancy and scleroderma, in addition to the patient's history of smoking. We will review each of these differentials as they pertain to this case.
Table 2. Differential diagnosis of cavitary pulmonary nodules(1).
| Benign cavitating pulmonary lesions | Malignant cavitating pulmonary lesions | ||
|---|---|---|---|
| Infectious | Bacterial necrotizing pneumonia: S. pneumoniae, H. influenza, K. pneumoniae, S. aureus Lung abscesses: Prevotella, Fusobacterium, and S. milleri group Uncommon infections: Actinomyces, Burkholderia pseduomallei, and Rhodococcus equi. Mycobacterial: M. tuberculosis, M. avium, M. intracellulare, M. malmoense, M. xenopi. Fungal: Aspergillus sp., Zygomycetes, Histoplasma capsulatum, Blastomyces dermatidis, Coccidioides immitis, Coccidioides posadasii, Paracoccidioides brasiliensis, Cryptococcosis neoformans, Penicillum marneffei, Pneumocystis jiroveci |
Primary | Squamous Cell Carcinoma Lymphoma (particularly in HIV) Kaposi's Sarcoma Lymphomatoid granulomatosis |
| Autoimmune | Granulomatosis with polyangiitis Sarcoidosis Ankylosing Spondylitis Rheumatoid nodules Primary amyloidosis |
Secondary | Metastatic tumors of squamous cell origin are more likely to cavitate than tumors of other origins |
| Pulmonary | Pulmonary Embolism Bronchiolitis Obliterans Organizing Pneumonia (up to 6% may have cavitary lesions (55)) Pulmonary Langerhans' cell Histiocytosis |
||
Necrotizing pneumonia
Several infections may cause pulmonary cavitation either by development of necrotizing pneumonia or lung abscess, or by seeding to the lung via hematogenous spread as is seen with septic emboli. While pulmonary cavitation is not commonly seen with community-acquired pneumonia, occasional cases of cavitary pneumonia due to Streptococcus pneumoniae, Pneumococcal pneumoniae and Hemophilus influenza have been reported, particularly in the immunocompromised host (2). Lung abscesses may also occur in patients prone to chronic aspiration and are typically caused by anerobes normally present in the gingival crevice. Additionally, Staphylococcus aureus (3) and Klebsiella pneumonia (4) are both common causes of necrotizing pneumonia in immunocompromised individuals. Finally, Nocardia spp. can cause lung abscesses in immunocompromised individuals, especially those receiving prolonged courses of glucocorticoids (5).
Chronic aspiration secondary to this patient's longstanding diffuse scleroderma with gastrointestinal dysmotility, and her longstanding immunosuppression with glucocorticoids and mycophenolate mofetil, were both risk-factors for cavitary pneumonia in this case. However, the patient did not have systemic features suggestive of necrotizing pneumonia at presentation. She had a normal white blood cell count, was afebrile and had no rigors or chills leading us to investigate other etiologies.
Atypical infections associated with cavitating lung lesions
Several atypical infections can also result in cavitary lung lesions. Mycobacterium tuberculosis is the most common mycobacterial disease resulting in pulmonary cavitation with 50% of cases having cavitation on radiographs (6). Similarly, atypical mycobacterial infections can be associated with radiographic evidence of cavitation including myocobacterium avium complex (7) and mycobacterium kansasii (8). Fungal pulmonary infections may also result in cavitary lung lesions. Cavitation may be seen in aspergillus infections in three situations; aspergilloma formation in patients with pre-existing cavities, chronic necrotizing aspergillosis in patients with a history of chronic lung disease, and invasive aspergillosis in immunocompromised patients (9). Other opportunistic fungal infections including Zygomycetes, Histoplasmosis, Coccidioides, Blastomycosis, Crypotococcosis and Pneumocystis jivroveci infection are all also associated with cavity formation (1). This patient's ongoing immunosuppression predisposed her to such atypical infections and was the predominant driver to obtain a definitive tissue diagnosis.
Malignancy
The most common primary lung malignancy known to form cavitary lung lesions is squamous cell carcinoma accounting for approximately 80% of malignant cavitating lesions (10). This patient was at risk for a primary lung malignancy owing to her smoking history. However, she also had a history of bilateral breast carcinoma raising the question as to whether the pulmonary lesions could be metastatic. Cavitation is relatively rare in metastatic lung lesions with only 4% of metastatic lesions resulting in cavitation (1). Furthermore, metastatic tumors of squamous cell origin are more likely to cavitate than tumors of other primary origin(1), again making it imperative that a tissue diagnosis be obtained in this case.
Rheumatoid nodules
Pulmonary rheumatoid nodules are a rare manifestation of rheumatoid arthritis (RA) occurring in between 0.4-32% of cases (11). Most commonly, pulmonary rheumatoid nodules develop in male smokers with seropositive RA, and development of the pulmonary nodules can sometimes predate joint involvement (12). However, this patient did not have clinical evidence of RA. She had a weakly positive rheumatoid factor, however her articular contractures were secondary to sclerodactyly and joint involvement from scleroderma. She did not have erosive disease and did not have clinical evidence of synovitis so it was felt that the lung nodules were unlikely to be rheumatoid nodules.
Relapsing polychondritis
Relapsing polychondritis (RPC) is a chronic, recurrent, immune-mediated, inflammatory disease of cartilage commonly presenting with inflammation of the cartilage of the ear, eye, nose, joints or respiratory tract (13). Clinical manifestations can be quite variable, and due to the indolent onset of the disease, the early manifestations are difficult to recognize. Although RPC can affect all ages and races, it is most common in Caucasians aged 40-60 years old. The disease is equally common in men and women. The incidence of RPC is estimated at 3.5 per million population per year (13).
Although RPC can present with a non-erosive oligoarticular asymmetric arthritis, it would not explain the sclerodactyly and joint contractures seen in this case which were clearly related to systemic sclerosis. While RPC can occur with co-existent systemic autoimmune disease in 1/3 of cases (13), it has not been specifically reported in association with systemic sclerosis. Up to 14-25% of cases of RPC are complicated by vasculitis which may involve the large, medium or small vessels(14, 15). Systemic vasculitis in RPC carries a high mortality, with a longitudinal study from the Mayo Clinic demonstrating only 45% 5 year survival in patients with RPC and systemic vasculitis (13, 16, 17).
RPC can cause pulmonary involvement but typically this is inflammation and stenosis of the large airways resulting in tracheobronchialmalacia. RPC could not explain the interstitial lung disease seen in this case, nor could it explain the cavitating lung lesions. This led us to explore alternative differential diagnoses as an explanation.
ANCA-Associated Vasculitis
On review of the records, it became apparent that serology for anti-neutrophil cytoplasmic antibodies initially became positive in this case 1 year prior to the presentation with hemoptysis raising the diagnostic question of whether ANCA-associated vasculitis could play a role in the lung manifestations in this case.
Granulomatosis with polyangiitis (GPA, formerly known as Wegener's Granulomatosis) is an ANCA-associated vasculitis that is well-recognized to cause cavitary lung lesions due to granulomatous inflammation with cavitation, nodules and pulmonary infiltrates seen in up to 70% of patients (18, 19). While many cases will present with both lung and renal involvement, referred to as pulmonary-renal syndrome, approximately 25% of cases of GPA have clinical manifestations limited to the lungs and upper airways, a syndrome termed limited GPA(20). In a French series of patients presenting with alveolar hemorrhage and positive ANCA serology, 61.25% of cases were ultimately found to be due to GPA, 26.25% were due to microscopic polyangiitis (MPA) and 10% due to eosinophilic granulomatous polyangiitis (EGPA, formerly known as Churg-Strauss Syndrome)(21). Of patients with pulmonary involvement in GPA, 69% have lung nodules which are bilateral in 74% of cases and cavitating in 49%(22).
The histologic findings in GPA are well described and pathognomic for the diagnosis. Angiocentric transmural inflammation is seen extending through the vessel wall and interrupting the internal elastic lamina. Granulomatous inflammation may be seen in the surrounding tissues with mixed infiltrate including neutrophils, lymphocytes and multinucleated giant cells with palisading histiocytes oriented perpendicular to the necrotic center of the granuloma. However, in patients already on immunosuppressive therapy, the histologic findings can be blunted (23). In patients with hemoptysis, diffuse alveolar hemorrhage secondary to pulmonary capillaritis is also often seen. This complication may occur in between 5-45% of patients with ANCA-associated vasculitis and may be the presenting manifestation. In such cases, early pathologic diagnosis is crucial to exclude infectious etiologies and facilitate initiation of immunosuppressive therapy. Initial immunosuppressive therapy in GPA typically consists of induction with glucocorticoids combined with either cyclophosphamide or rituximab. Several studies have demonstrated comparable efficacy of cyclophosphamide and rituximab as induction agents (24).
ANCA-associated vasculitis as a paraneoplastic syndrome
Cutaneous vasculitis has been reported as a presenting symptom of underlying malignancy (25-27) and this patient's age and prior history of breast cancer increased concern for paraneoplastic vasculitis in this case. However, in a large cutaneous vasculitis series from Northern Spain only one of 16 cases of paraneoplastic vasculitis was associated with breast cancer(25). None of these cases was associated with ANCA positivity. ANCA-associated vasculitis has been reported in association with hematologic malignancies, but this patient had relatively normal hematologic parameters, no B-symptoms and no lymphadenopathy or hepatosplenomegaly. Systematic literature review investigating the association of ANCA positivity with the various chemotherapeutic agents that this patient had received did not identify any prior cases of ANCA-associated vasculitis caused by Adriamycin, Cyclophosphamide, Paclitaxel, Docetaxel or aromatase inhibitors. Taken together these factors led us to investigate alternative explanations for the ANCA-associated vasculitis in this case.
Interstitial lung disease associated with ANCA-associated vasculitis
While the interstitial lung disease in this case was originally attributed to the scleroderma, various types of interstitial lung disease (ILD) have been described in association with ANCA-associated vasculitis. In a recent series describing radiographic findings, the radiographic pattern was consistent with usual interstitial pneumonia (UIP) in 43% of cases, combined pulmonary fibrosis and emphysema in 20%, atypical UIP in 14%, and non-specific interstitial pneumonia (NSIP) in 10%. In 45% of cases the ILD precedes the vasculitis presentation (28).
Cutaneous ulceration in scleroderma
Scleroderma is a rare autoimmune disease characterized by inflammation, progressive fibrosis of the skin and various internal organs and vasculopathic changes. The disease is divided into clinical subsets (diffuse, limited, sine and overlap) based on the extent of skin involvement and other clinical features. Autoantibodies are present in 95% of patients with scleroderma and autoantibody profiles are helpful predictors of clinically distinct subsets of scleroderma (29, 30), assisting with assessment of likely complications and prognosis. This patient had clinical manifestations of diffuse scleroderma with diffuse skin involvement, interstitial lung disease, and gastrointestinal involvement.
Cutaneous ulceration is a well-recognized complication of many autoimmune diseases (31, 32) and is seen in both limited and diffuse scleroderma (33) with a prevalence of 4%. It is thought that delayed wound healing in scleroderma may be a manifestation of vasculopathy, and some studies have suggested an association with antiphospholipid antibodies (33-36). However, in this case, antiphospholipid testing was negative, and the patient's ulcerations did not have the typical morphology of scleroderma ulcers. The punched out appearance and associated petechial rash along with the positive PR-3 antibody raised concern for possible scleroderma-associated vasculitis.
Scleroderma-associated ANCA-positivity and ANCA-associated vasculitis
While rare, ANCA-associated vasculitis is a recognized phenomenon in association with scleroderma (37) and it is most commonly identified in patients presenting with normotensive renal-failure, often in the setting of pulmonary-renal syndrome (37, 38). ANCA-associated vasculitis has also been reported in association with skin manifestations including digital necrosis (39). Some authors have also described serologic identification of ANCA immunofluorescence in 6.9% of scleroderma patients without specific vasculitic manifestations (40-42). This has led some to question whether ANCA positivity in patients with other autoimmune diseases may simply be antibody cross-reactivity. However, an inception cohort study designed to answer this question investigated the prevalence of ANCA by inmunofluoresence and ELISA in patients with rheumatoid arthritis, lupus, scleroderma, myositis, Sjogrens syndrome and antiphospholipid syndrome and found that, while among patients with connective tissue disease there was a high prevalence of antibodies to neutrophils detected by immunofluorescence, there was a low prevalence of anti-proteinase 3 (PR3) or anti-myeloperoxidase (MPO) detected by antigen specific ELISA. P-ANCA positivity on immunofluorescence was often seen as a cross reactivity from ANA antibodies. However, no other autoantibodies consistently resulted in cANCA positivity(43). Furthermore, many of the reports of ANCA positivity in conjunction with systemic vasculitis in scleroderma were associated with antibodies to MPO ANCA (44-48) rather than the PR-3 ANCA seen in this case. This data supports our clinical concern that cANCA, and specifically PR-3 positivity as seen in this case, is unlikely to be attributable to cross reactivity to other autoantibodies.
Association between scleroderma and malignancy
The temporal association between scleroderma onset and malignancy has now been well described (49). Recent data suggests that in some cases of scleroderma the autoimmune process may be triggered by autoantigen mutation in the inciting malignancy (50, 51). An association has been described between anti-RNA polymerase III (RNAP III) antibodies, malignancy and scleroderma (50, 52), and several studies have demonstrated that scleroderma patients with RNAP III antibodies have a higher prevalence of malignancy and are more likely to be diagnosed with malignancy concurrently with, or within five years of, scleroderma onset (49, 53, 54). Patients with somatic mutations in the gene encoding RNA polymerase III (POLR3A) have evidence of mutation specific T-cell immune responses with generation of cross-reactive RNA polymerase III autoantibodies suggesting that there may be a pathologic mechanism for this association. However, the data on whether prevalence of malignancy is higher in RNAP III positive patients remains controversial. Some studies show a higher prevalence of cancer in RNAP III compared to other antibody subgroups(50, 51) but other studies did not corroborate this(52, 53) despite all studies confirming that there is a higher risk of co-temporal presentation of malignancy in scleroderma patients who are RNAP III positive. Furthermore, approximately 85% of patients with scleroderma and RNAP III antibodies do not have an identifiable malignancy. While some have postulated that this may be a result of effective immune clearance of malignant cells, the relationship between malignancy and RNAP III antibodies remains an active area of investigation (49, 50).
In this patients case, RNAP III antibodies were not available for commercial testing at the time of her original diagnosis but at the time of presentation with hemoptysis they were tested and were positive.
Diagnostic Procedure
At the time of presentation our main differential diagnosis was whether this patient had granulomatosis with polyagniitis as a manifestation of scleroderma-associated ANCA positive vasculitis, and whether the lung nodules were infectious in etiology, or secondary to malignant spread of her breast cancer. In order to make a definitive diagnosis, the patient underwent left thoracoscopy with wedge resection of the left lower lobe. Gram stain, acid fast bacilli, fungal stains and cultures were all negative.
The predominant histopathologic findings are summarized in Figure 3. The background lung parenchyma exhibits fibrosis with cyst formation and bronchial metaplasia (Figure 3A) consistent with chronic scleroderma-associated lung disease. There are areas of geographic “blue” necrosis rimmed by palisading histiocytes consistent with necrotizing granulomatous inflammation (Figure 3B). Foci of vasculitis are highlighted by an elastic stain (Figure 3C). Rare vessels are seen showing disruption of the vessel wall by inflammatory cells and histiocytes. Subjacent to the pleura is a collection of monomorphic epithelial cells (confirmed by MAK-6 cytokeratin immunohistochemical stain). The cells are monomorphic with bland cytology, and exhibit a single file and single cell pattern of infiltration in some areas. Intracytoplasmic vacuoles and occasional mitotic figures are seen in some cells (Figure 3D). Additional immunohistochemical stains performed revealed that these atypical cells were diffusely positive for MAK-6, GATA-3, estrogen receptor (ER, Figure 3E) and focally positive for mammoglobulin (Figure 3F). Immunostains for progesterone receptor, E-Cadherin and GCDFP-15 and HER-2 were negative (not shown). Tissue stains were also negative for pathogens. Taken together the overall morphology and immunophenotype were consistent with breast adenocarcinoma, lobular type, metastatic to the lung with coexistent but geographically distinct ANCA-associated vasculitis on a background of fibrotic lung disease secondary to diffuse scleroderma.
Figure 3.
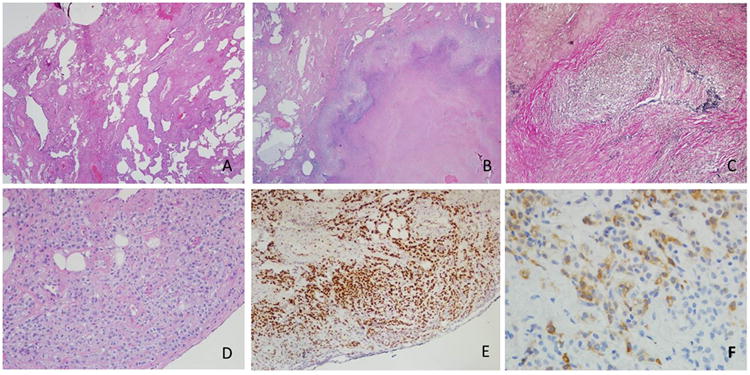
A: Section of lung parenchyma from video-assisted thoracoscopic lung biopsy demonstrating bronchial metaplasia, cyst formation and widening of alveola by fibrosis (H&E, 2×). B: Area of geographic necrosis rimmed by palisading histiocytes; metaplastic bone is seen (H&E, 2×) C: Destruction of vessel wall and disruption of internal elastic lamina by inflammatory cells and histiocytes (Elastic Von Gieson stain, 10×). D: Subpleural collection of atypical epithelial cells demonstrating discohesion, single cell infiltration and prominent intracytoplasmic vacuoles (H&E, 10×). E: Diffuse nuclear positivity in the atypical cells (Estrogen receptor (ER) immunohistochemical stain, 10×) F: Cytoplasmic positivity in the atypical cells supporting breast origin (Mammoglobin immunohistochemical stain, 20×).
Patients Clinical Course
The patient was treated with intravenous glucocorticoids (solumedrol 1000mg every 24 hours ×3 doses) followed by oral prednisone 60mg daily. For her breast cancer, because her recurrent tumor remained hormone sensitive, and the prevailing standard of care is to utilize single agent monotherapy at the onset of metastatic disease she was commenced on oral anastrazole 1mg daily. After discussion at a multidisciplinary tumor board meeting this was felt to be sufficient for management of the breast cancer and additional chemotherapy was not felt to be warranted at this time but remains an option if her disease progresses in the future. Additionally, she received induction therapy for ANCA associated vasculitis (GPA) with intravenous Rituximab 1000mg for two doses 14 days apart. Over the subsequent 4 months the patient's respiratory symptoms improved and there was substantial improvement in the pulmonary findings on the CT scan (Figure 2 D-F). Her skin ulcerations completely healed (Figure 1B) and she continues to do well clinically.
Final Diagnosis
The final diagnosis in this case was RNAP III positive diffuse scleroderma with associated breast cancer presenting with lung metastases associated with ANCA-associated vasculitis consistent with limited GPA. It is unclear whether the ANCA-associated vasculitis in this case was secondary to scleroderma or triggered by metastatic malignancy; however, the patient has done very well with combined therapy using the aromatase inhibitor anastrazole as hormonal therapy for breast cancer along with steroids and rituximab as therapy for the ANCA-associated vasculitis. This case emphasizes the now well-recognized co-temporal relationship between scleroderma and malignancy and the emerging pathophysiologic understanding of the relationship between autoantigen mutations in malignant tissues and autoimmune triggering.
Acknowledgments
This work was supported by award R01NR013888 from the National Institute of Nursing Research and by award number UL1 TR000075 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosures: Falin B. Patel: None
Kara S. Couch: Supported by R01NR013888 from the National Institute of Nursing Research
Sean McNish: Supported by R01NR013888 from the National Institute of Nursing Research
Jonathan D. Miller: None
Robert Siegel: None
Samantha Easley: None
Victoria K. Shanmugam: Supported by R01NR013888 from the National Institute of Nursing Research
Footnotes
Author Contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Shanmugam had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Falin B. Patel: 1, 2, 3
Kara S. Couch: 1, 2, 3
Sean McNish: 1, 2, 3
Jonathan D. Miller: 1, 2, 3
Robert Siegel: 1, 2, 3
Samantha Easley: 1, 2, 3
Victoria K. Shanmugam: 1, 2, 3
References
- 1.Gadkowski LB, Stout JE. Cavitary Pulmonary Disease. Clinical Microbiology Reviews. 2008;21(2):305–33. doi: 10.1128/CMR.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada F, Ando Y, Matsushita S, Ishii R, Nakayama T, Morikawa K, Ono A, Maeda T, Mori H. Thin-section CT findings of patients with acute Streptococcus pneumoniae pneumonia with and without concurrent infection. The British Journal of Radiology. 2012;85(1016):e357–e64. doi: 10.1259/bjr/18544730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. The Lancet. 2002;359(9308):753–9. doi: 10.1016/S0140-6736(02)07877-7. doi: http://dx.doi.org/10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 4.Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC. Association between rmpA and magA Genes and Clinical Syndromes Caused by Klebsiella pneumoniae in Taiwan. Clinical Infectious Diseases. 2006;42(10):1351–8. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 5.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clinical Microbiology Reviews. 1994;7(2):213–64. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadlock FP, Park SK, Awe RJ, Rivera M. Unusual radiographic findings in adult pulmonary tuberculosis. American Journal of Roentgenology. 1980;134(5):1015–8. doi: 10.2214/ajr.134.5.1015. [DOI] [PubMed] [Google Scholar]
- 7.Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995;19(3):353–60. doi: 10.1097/00004728-199505000-00003. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 8.Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Hurst GA. Radiographic manifestations of pulmonary Mycobacterium kansasii infections. American Journal of Roentgenology. 1978;131(6):985–93. doi: 10.2214/ajr.131.6.985. [DOI] [PubMed] [Google Scholar]
- 9.Soubani AO, Chandrasekar PH. THe clinical spectrum of pulmonary aspergillosis*. Chest. 2002;121(6):1988–99. doi: 10.1378/chest.121.6.1988. [DOI] [PubMed] [Google Scholar]
- 10.Chiu FT. Caviation in lung cancers. Aust N Z J Med. 1975;5(6):523–30. doi: 10.1111/j.1445-5994.1975.tb03856.x. Epub 1975/12/01. [DOI] [PubMed] [Google Scholar]
- 11.Yousem SA, Colby TV, Carrington CB. Lung biopsy in rheumatoid arthritis. Am Rev Respir Dis. 1985;131(5):770–7. doi: 10.1164/arrd.1985.131.5.770. Epub 1985/05/01. [DOI] [PubMed] [Google Scholar]
- 12.Jolles H, Moseley PL, Peterson MW. NOdular pulmonary opacities in patients with rheumatoid arthritis. a diagnostic dilemma. Chest. 1989;96(5):1022–5. doi: 10.1378/chest.96.5.1022. [DOI] [PubMed] [Google Scholar]
- 13.Kent PD, Michet Clement J, Jr, Luthra HS. Relapsing polychondritis. Current Opinion in Rheumatology. 2004;16(1):56–61. doi: 10.1097/00002281-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Cipriano P, Alonso D, Baltaxe H, Gay WJ, Smith J. Multiple aortic aneurysms in relapsing polychondritis. Am J Cardiol. 1976;37(7):1097. doi: 10.1016/0002-9149(76)90432-x. [DOI] [PubMed] [Google Scholar]
- 15.Barzegar C, Vrtovsnik F, Devars J, Mignon F, Pradalier A. Vasculitis with mesangial IgA deposits complicating relapsing polychondritis. Clin Exp Rheumatol. 2002;20(1):89. [PubMed] [Google Scholar]
- 16.Michet JCJ, McKenna CH, Luthra HS, O'Fallon WM. Relapsing PolychondritisSurvival and Predictive Role of Early Disease Manifestations. Annals of Internal Medicine. 1986;104(1):74–8. doi: 10.7326/0003-4819-104-1-74. [DOI] [PubMed] [Google Scholar]
- 17.Michet C. Vasculitis and relapsing polychondritis. Rheum Dis Clin North Am. 1990;16(2):441–4. [PubMed] [Google Scholar]
- 18.Martinez F, Chung JH, Digumarthy SR, Kanne JP, Abbott GF, Shepard JAO, Mark EJ, Sharma A. Common and Uncommon Manifestations of Wegener Granulomatosis at Chest CT: Radiologic-Pathologic Correlation. RadioGraphics. 2011;32(1):51–69. doi: 10.1148/rg.321115060. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlman JE, Hruban RH, Fishman EK. Wegener granulomatosis: CT features of parenchymal lung disease. J Comput Assist Tomogr. 1991;15(6):948–52. Epub 1991/11/01. [PubMed] [Google Scholar]
- 20.Stone JH. Limited versus severe Wegener's granulomatosis: Baseline data on patients in the Wegener's granulomatosis etanercept trial. Arthritis & Rheumatism. 2003;48(8):2299–309. doi: 10.1002/art.11075. [DOI] [PubMed] [Google Scholar]
- 21.Kostianovsky A, Hauser T, Pagnoux C, Cohen P, Daugas E, Mouthon L, Miossec P, Cordier J, Guillevin L (FVSG) FVSG. Alveolar haemorrhage in ANCA-associated vasculitides: 80 patients' features and prognostic factors. Clin Exp Rheumatol. 2012;30(1 Suppl 70):S77–82. Epub 2012 May 11. [PubMed] [Google Scholar]
- 22.Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. PUlmonary wegener's granulomatosis. a clinical and imaging study of 77 cases. Chest. 1990;97(4):906–12. doi: 10.1378/chest.97.4.906. [DOI] [PubMed] [Google Scholar]
- 23.Mark EJ, Flieder DB, Matsubara O. Treated Wegener's granulomatosis: Distinctive pathological findings in the lungs of 20 patients and what they tell us about the natural history of the disease. Human Pathology. 1997;28(4):450–8. doi: 10.1016/s0046-8177(97)90034-4. doi: http://dx.doi.org/10.1016/S0046-8177(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 24.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CGM, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. New England Journal of Medicine. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loricera J, Calvo-Río V, Ortiz-Sanjuán F, González-López MA, Fernández-Llaca H, Rueda-Gotor J, Gonzalez-Vela MC, Alvarez L, Mata C, González-Lamuño D, Martínez-Taboada VM, González-Gay MA, Blanco R. The Spectrum of Paraneoplastic Cutaneous Vasculitis in a Defined Population: Incidence and Clinical Features. Medicine. 2013;92(6):331–43. doi: 10.1097/md.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solans-Laque R, Bosch-Gil JA, Perez-Bocanegra C, Selva-O'Callaghan A, Simeon-Aznar CP, Vilardell-Tarres M. Paraneoplastic vasculitis in patients with solid tumors: report of 15 cases. J Rheumatol. 2008;35(2):294–304. Epub 2007/12/19. [PubMed] [Google Scholar]
- 27.Hutson TE, Hoffman GS. Temporal concurrence of vasculitis and cancer: a report of 12 cases. Arthritis Care Res. 2000;13(6):417–23. doi: 10.1002/1529-0131(200012)13:6<417::aid-art13>3.0.co;2-t. Epub 2003/11/26. [DOI] [PubMed] [Google Scholar]
- 28.Comarmond C, Crestani B, Tazi A, Hervier B, Adam-Marchand S, Nunes H, Cohen-Aubart F, Wislez M, Cadranel J, Housset B, Lloret-Linares C, Sève P, Pagnoux C, Abad S, Camuset J, Bienvenu B, Duruisseaux M, Hachulla E, Arlet JB, Hamidou M, Mahr A, Resche-Rigon M, Brun AL, Grenier P, Cacoub P, Saadoun D. Pulmonary Fibrosis in Antineutrophil Cytoplasmic Antibodies (ANCA)-Associated Vasculitis: A Series of 49 Patients and Review of the Literature. Medicine. 2014;93(24):340–9. doi: 10.1097/md.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steen VD. Autoantibodies in Systemic Sclerosis. Semin Arthritis Rheu. 2005;35(1):35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Steen VD. The Many Faces of Scleroderma. Rheumatic Disease Clinics of North America. 2008;34(1):1–15. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Shanmugam VK, Schilling A, Germinario A, Mete M, Kim P, Steinberg J, Attinger CE. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre. International Wound Journal. 2011:1–9. doi: 10.1111/j.1742-481X.2011.00899.x. Epub Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanmugam VK, Steen VD, Attinger CE, Mavromatis B, Kessler C, Cupps TR. Lower extremity ulcers in connective tissue diseases: a case series describing clinical and laboratory features. Arthritis & Rheumatism. 2006;54(12):4105. [Google Scholar]
- 33.Shanmugam V, Price P, Attinger C, Steen V. Lower Extremity Ulcers in Systemic Sclerosis: Features and Response to Therapy. Int J Rheumatol. 2010 doi: 10.1155/2010/747946. Epub 2010 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alivernini S, De Santis M, Tolusso B, Mannocci A, Bosello SL, Peluso G, Pinnelli M, D'Antona G, LaTorre G, Ferraccioli G. Skin ulcers in systemic sclerosis: Determinants of presence and predictive factors of healing. J Am Acad Dermatol. 2009;60:426–35. doi: 10.1016/j.jaad.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RHWM, De Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) Journal of Thrombosis and Haemostasis. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 36.Merkel PA, Chang Y, Pierangeli SS, Convery K, Nigel Harris E, Polisson RP. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. The American Journal of Medicine. 1996;101(6):576–83. doi: 10.1016/s0002-9343(96)00335-x. [DOI] [PubMed] [Google Scholar]
- 37.Rho Y, Choi S, Lee Y, Ji J, Song G. Scleroderma associated with ANCA-associated vasculitis. Rheumatology International. 2006;26(5):369–75. doi: 10.1007/s00296-005-0011-5. [DOI] [PubMed] [Google Scholar]
- 38.Kamen DL, Wigley FM, Brown AN. Antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis in scleroderma--a different kind of renal crisis. The Journal of Rheumatology. 2006;33(9):1886–8. [PubMed] [Google Scholar]
- 39.Wong M, Ranganath VK, Clements PJ. Antineutrophil Cytoplasmic Antibody-positive Digital Necrosis in a Patient with Limited Systemic Sclerosis. The Journal of Rheumatology. 2010;37(1):214–5. doi: 10.3899/jrheum.090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manolova I, Dancheva M. Antineutrophil cytoplasmic antibodies in patients with systemic sclerosis. The Journal of Rheumatology. 2003;30(9):2078–9. [PubMed] [Google Scholar]
- 41.Ruffatti A, Sinico RA, Radice A, Ossi E, Cozzi F, Tonello M, Grypiotis P, Todesco S. Autoantibodies to proteinase 3 and myeloperoxidase in systemic sclerosis. The Journal of Rheumatology. 2002;29(5):918–23. [PubMed] [Google Scholar]
- 42.Khanna D, Aggarwal A, Bhakuni DS, Dayal R, Misra R. Bactericidal/permeability-increasing protein and cathepsin G are the major antigenic targets of antineutrophil cytoplasmic autoantibodies in systemic sclerosis. The Journal of Rheumatology. 2003;30(6):1248–52. [PubMed] [Google Scholar]
- 43.Merkel PA, Polisson RP, Chang Y, Skates SJ, Niles JL. Prevalence of Antineutrophil Cytoplasmic Antibodies in a Large Inception Cohort of Patients with Connective Tissue Disease. Annals of Internal Medicine. 1997;126(11):866–73. doi: 10.7326/0003-4819-126-11-199706010-00003. [DOI] [PubMed] [Google Scholar]
- 44.Carvajal I, Bernis C, Sanz P, Garcia A, Garcia-Vadillo A, Traver JA. Antineutrophil cytoplasmic autoantibodies (ANCA) and systemic sclerosis. Nephrology Dialysis Transplantation. 1997;12(3):576–7. doi: 10.1093/ndt/12.3.576. [DOI] [PubMed] [Google Scholar]
- 45.Derrett-Smith EC, Nihtyanova SI, Harvey J, Salama AD, Denton CP. Revisiting ANCA-associated vasculitis in systemic sclerosis: clinical, serological and immunogenetic factors. Rheumatology. 2013;52(10):1824–31. doi: 10.1093/rheumatology/ket213. [DOI] [PubMed] [Google Scholar]
- 46.Quéméneur T, Mouthon L, Cacoub P, Meyer O, Michon-Pasturel U, Vanhille P, Hatron PY, Guillevin L, Hachulla E. Systemic Vasculitis During the Course of Systemic Sclerosis: Report of 12 Cases and Review of the Literature. Medicine. 2013;92(1):1–9. doi: 10.1097/MD.0b013e31827781fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casari S, Haeney M, Farrand S, Herrick A. Antineutrophil cytoplasmic antibodies a “Red Flag” in patients with systemic sclerosis. The Journal of Rheumatology. 2002;29(12):2666–7. [PubMed] [Google Scholar]
- 48.Endo H, Hosono T, Kondo H. Antineutrophil cytoplasmic autoantibodies in 6 patients with renal failure and systemic sclerosis. J Rheumatol. 1994;21(5):864–70. [PubMed] [Google Scholar]
- 49.Shah AA, Casciola-Rosen L, Rosen A. Review: Cancer-Induced Autoimmunity in the Rheumatic Diseases. Arthritis & Rheumatology. 2015;67(2):317–26. doi: 10.1002/art.38928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah AA, Hummers LK, Casciola-Rosen L, Visvanathan K, Rosen A, Wigley FM. Examination of Autoantibody Status and Clinical Features Associated With Cancer Risk and Cancer-Associated Scleroderma. Arthritis & Rheumatology. 2015;67(4):1053–61. doi: 10.1002/art.39022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, Boin F, Fava A, Thoburn C, Kinde I, Jiao Y, Papadopoulos N, Kinzler KW, Vogelstein B, Rosen A. Association of the Autoimmune Disease Scleroderma with an Immunologic Response to Cancer. Science. 2014;343(6167):152–7. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moinzadeh P, Fonseca C, Hellmich M, Shah AA, Chighizola C, Denton CP, Ong VH. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Research & Therapy. 2014;16(1):R53–R. doi: 10.1186/ar4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Airo' P, Ceribelli A, Cavazzana I, Taraborelli M, Zingarelli S, Franceschini F. Malignancies in Italian Patients with Systemic Sclerosis Positive for Anti-RNA Polymerase III Antibodies. The Journal of Rheumatology. 2011;38(7):1329–34. doi: 10.3899/jrheum.101144. [DOI] [PubMed] [Google Scholar]
- 54.Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, Roddy J, Nash P, Sturgess A, Proudman S, Stevens W. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Research & Therapy. 2011;13(6):R211–R. doi: 10.1186/ar3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KS, Kullnig P, Hartman TE, Müller NL. Cryptogenic organizing pneumonia: CT findings in 43 patients. American Journal of Roentgenology. 1994;162(3):543–6. doi: 10.2214/ajr.162.3.8109493. [DOI] [PubMed] [Google Scholar]


