Abstract
Patient-centered health risk assessments (HRAs) that screen for unhealthy behaviors, prioritize concerns, and provide feedback may improve counseling, goal setting, and health. To evaluate the effectiveness of routinely administering a patient-centered HRA, My Own Health Report, for diet, exercise, smoking, alcohol, drug use, stress, depression, anxiety, and sleep, 18 primary care practices were randomized to ask patients to complete My Own Health Report (MOHR) before an office visit (intervention) or continue usual care (control). Intervention practice patients were more likely than control practice patients to be asked about each of eight risks (range of differences 5.3–15.8 %, p < 0.001), set goals for six risks (range of differences 3.8–16.6 %, p < 0.01), and improve five risks (range of differences 5.4–13.6 %, p < 0.01). Compared to controls, intervention patients felt clinicians cared more for them and showed more interest in their concerns. Patient-centered health risk assessments improve screening and goal setting.
Trial Registration
Clinicaltrials.gov identifier: NCT01825746
Keywords: Health risk assessment, Primary care, Patient reported measures, Health behaviors, Mental health, Pragmatic trial
Introduction
Unhealthy behaviors and mental health issues cause significant morbidity and mortality [1–5]. Despite the ubiquity of these conditions, they often go unrecognized and untreated. Primary care, because of its focus on providing comprehensive longitudinal care [6–8], is well positioned to help identify patients at risk and either provide health behavior and mental health counseling or refer patients for more intensive support and counseling in the community or from specialists. In fact, guideline bodies recommend that primary care clinicians routinely screen and provide or refer patients for counseling for a number of health behavior and mental health topics [9]. There are, however, a host of structural, financial, and policy barriers that make this difficult. To successfully perform these tasks, primary care needs interventions and supports that are feasible to implement [10].
Policy makers and researchers have suggested that one solution may be the routine use of patient-centered health risk assessments (HRAs) that systematically screen for such risks, allow patients to prioritize concerns, provide patients immediate feedback about positive screens and potential next steps, support goal setting, and alert clinicians to patients’ concerns [11, 12]. In fact, the Centers for Medicare and Medicaid Services (CMS) has mandated patient-centered HRAs for inclusion as part of Annual Wellness Visits [11, 13]. Essential to this process is goal setting and follow-up, which is a key component of changing individual-level behaviors [14]. Theory indicates that setting or prioritizing manageable, specific goals could improve health behaviors and lead to improved outcomes [15]. Individuals who prioritize a specific goal and are involved in decision-making are more likely to engage in health behavior change, but results are mixed on the specific characteristics of goal setting activities that are needed [14, 16, 17].
While HRAs have been demonstrated to improve outcomes in the workplace and community settings [18, 19], there is inadequate evidence to demonstrate that these efforts will achieve similar benefits in primary care [20]. Yet, addressing these issues in primary care makes sense. Patients frequently cite clinician advice as a key motivating factor for making a health behavior change [7, 21]. Behaviors and mental health directly influence a patient’s ability to manage chronic conditions, adherence with medications, intention to get recommended preventive care, health care utilization, and, ultimately, outcomes. Unfortunately, clinicians are often overwhelmed by multiple competing demands during office visits—often appropriately so [22, 23]—and frequently do not ask about behaviors and mental health issues, let alone provide counseling, goal setting, assistance, and follow-up [24, 25]. Compounding these issues, effective health behavior and mental health counseling is time-consuming, often requiring 20 or more hours of counseling over six or more months of time [26, 27].
Although much has been written about HRAs, there are few rigorous studies that evaluate the feasibility and effectiveness of HRAs in real-world settings. This manuscript reports on whether implementing a patient-centered HRA, called My Own Health Report (MOHR), improves screening and goal setting—the first steps of identifying and initiating counseling—for eight behaviors and mental health concerns in a diverse range of primary care practices.
Methods
In this cluster-randomized non-blinded trial, 18 primary care practices were randomized to routinely offer MOHR to all patients presenting for wellness and/or chronic care or to provide usual care. While the study methods, implementation findings, and frequency of unhealthy behaviors and mental health risks have been previously reported, the study design is detailed below [5, 28–30]. The study was approved by the Virginia Commonwealth University (VCU) (#HM12746), University of California, Los Angeles (#12-0017900), and five other participating institutional review boards.
Nine matched pairs of practices affiliated with eight nationally distributed practice-based research networks (PBRNs) or Cancer Prevention and Control Research Networks (CPCRNs) were purposefully selected to represent the full spectrum of care including practice type, ownership, location, electronic health record (EHR) infrastructure, and patient population. Practices were located in California, North Carolina, Texas, Vermont, and Virginia—ten were rural, four suburban, and six urban. All were small to medium in size, with one to six clinicians and an annual practice patient panel of 1500 to 10,000 adults. No practice systematically offered a HRA prior to participating in the study. Eight practices had some behavioral or mental health counselors on site, although usually for only several half days per week. Practices were matched in pairs by location, size, ownership, patient demographics, and EHR infrastructure. To conceal allocation, practices were numbered in paired blocks; researchers were blinded to practice name, and researchers randomly allocated each practice to intervention and control condition by coin toss.
MOHR was available in an electronic or paper format and addressed diet, exercise, smoking, alcohol, drug use, stress, depression and anxiety, and sleep [31]. Topics were selected based on US Preventive Services Task Force recommendations [9]. MOHR started with brief, practical, valid, and evidence-based screening questions [32, 33]. Responses were scored and categorized as being of “no concern,” “some concern,” or “high concern.” Patients were asked if they were ready to change and/or discuss topics of some or high concern with their clinician. MOHR then provided patients a summary containing feedback that identified any health behavior or mental health risks paired with the healthy goal, initial steps to make improvements, and a worksheet to start creating specific, measurable, achievable, realistic, and timely (SMART) goals [34, 35]. Clinicians received a summary of any positive screens prior to the visit.
Intervention practices were asked to adopt, implement, and field MOHR one time to a minimum of 300 patients between March and December 2013. As a pragmatic trial, practices were asked to decide which patients would be invited to complete MOHR, when and where MOHR would be completed, whether they would use the electronic or paper version, and who would counsel patients in response to summaries—replicating what would naturally occur in practice. Clinicians were invited to attend two 1-h training webinars prior to MOHR being offered to their patients. The webinars reviewed basic health behavior and mental health recommendations, discussed health behavior counseling techniques (the five As, SMART goals, and motivational interviewing concepts), demonstrated what patients would see with MOHR, and shared example patient summaries that clinicians would receive. Clinicians and practices were not specifically asked to do anything different beyond the optional webinar training and fielding MOHR. How they responded to conditions identified by MOHR was left to their discretion.
The primary outcome was whether patients seen at intervention practices were more likely to set a change goal for each of the eight behaviors or mental health concerns than patients seen at control practices, which can be viewed as the first critical step to delivering recommended counseling. Secondary outcomes are actions recommended by the guidelines that include whether intervention patients were more likely to be screened, referred, or report that they had made improvements compared to control patients. Additional outcomes included patient trust in their health care team and perceived clinician communication style derived from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Clinician and Group survey—a validated instrument commonly used for patients to report on and evaluate their experience with health care [36]. Outcomes were measured by survey mailed to patients starting 2 weeks after the office visit using a modified Dillman technique [37]. Non-responders were called, and the survey was administered by phone. As the study goal was to assess the intervention impact on the entire practice’s patient population, all patients presenting for a wellness and chronic care visit from intervention and control practices were mailed the outcomes survey, whether or not they participated in the MOHR health risk assessment.
Survey outcomes from all respondents—regardless of MOHR participation—were analyzed using a generalized linear mixed model with a fixed effect for intervention status (intervention or control) and a random effect to account for matching between practices. The model was also used to account for patient characteristics including patient age, race, ethnicity, and time with the clinic. SAS version 9.4 (Cary, NC, USA) was used for all analyses.
Results
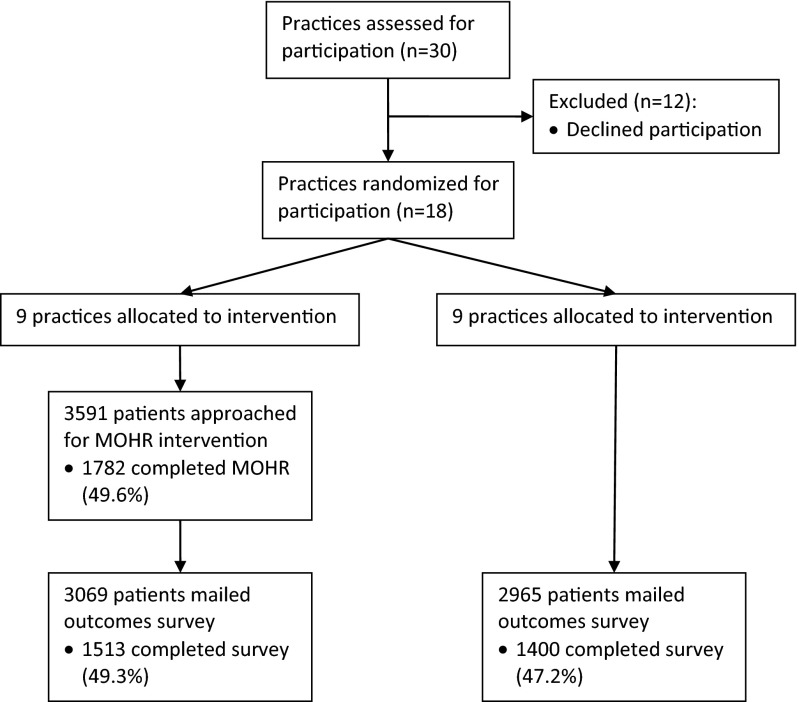
At intervention practices, 49.6 % (1782 of 3591) of approached patients completed MOHR prior to their office visit. Fully, 49.3 % (1513 of 3069) of patients at intervention practices and 47.2 % (1400 of 2965) of patients at control practices completed the outcomes survey (Fig. 1). Intervention and control patients were similar with the exception of small differences in patients’ age and length of time seen in the practice, but the magnitude in differences was minimal (Table 1). Based on responses to the MOHR assessment, a majority of patients from intervention practices reported risks for diet (44.7–84.5 %), exercise (70.8 %), sleep (63.9 %), stress (59.6 %), and weight (79.6 %). While less common, a sizable minority of patients had alcohol (23.8 %), tobacco (23.8 %), illegal drug use (3.2 %), anxiety (15.5 %), and depression risks (8.9 %). These results have been previously detailed [5].
Fig. 1.
CONSORT MOHR study flow diagram. CONSORT study flow diagram for this practice-level randomized controlled trial. All practices randomized to the intervention were able to field the MOHR assessment. Practices offered MOHR to patients presenting for a wellness and/or chronic care visit. Using an intention to treat approach, all patients presenting for a wellness and/or chronic care visit were offered the outcomes survey—whether or not they completed the intervention. Once 150 patients completed the survey, survey administration was discontinued, even if the practice continued to MOHR assessment
Table 1.
Demographics of intervention and control patients completing the outcome survey
| Intervention (n = 1513) | Control (n = 1400) | p Value | |
|---|---|---|---|
| Gender (female) | 67.3 % | 68.1 % | 0.653 |
| Age | 0.002 | ||
| 18–25 years | 4.7 % | 5.7 % | |
| 26–39 years | 14.8 % | 17.4 % | |
| 40–49 years | 19.2 % | 20.8 % | |
| 50–64 years | 41.4 % | 39.4 % | |
| 65–79 years | 18.1 % | 15.2 % | |
| 80+ years | 1.8 % | 1.5 % | |
| Race/ethnicity | 0.07 | ||
| Latino | 27.2 % | 29.2 % | |
| Black | 10.0 % | 11.4 % | |
| White | 61.8 % | 58.3 % | |
| Asian | 0.4 % | 0.5 % | |
| Native American | 0.5 % | 0.6 % | |
| Other | 0.1 % | 0.1 % | |
| Highest education | 0.675 | ||
| Less than high school | 18.1 % | 18.6 % | |
| High school | 32.5 % | 32.3 % | |
| Some college | 23.8 % | 24.1 % | |
| College or more | 25.6 % | 25.0 % | |
| How long have you been at this clinic | 0.038 | ||
| <6 months | 14.2 % | 17.9 % | |
| 6 months–1 year | 6.4 % | 5.1 % | |
| 1–3 years | 16.1 % | 14.2 % | |
| 3–5 years | 14.3 % | 13.2 % | |
| >5 years | 49.3 % | 49.7 % | |
| In general would you say you health is… | 0.530 | ||
| Excellent | 7.2 % | 8.7 % | |
| Very good | 23.4 % | 23.8 % | |
| Good | 37.9 % | 35.8 % | |
| Fair | 25.5 % | 25.2 % | |
| Poor | 6.0 % | 6.6 % | |
| How confident are you at filling out your medical forms by yourself? (% extremely) | 49.3 % | 47.4 % | 0.839 |
| Have you used the internet for health information? (% yes) | 62.2 % | 64.2 % | 0.387 |
Compared to patients from control practices, more patients from intervention practices reported greater screening rates for each of the eight behaviors and mental health risks (range of differences 5.3–15.8 %, p < 0.001) (Tables 2 and 3). Significantly more patients from intervention versus control practices reported setting specific goals to improve their diet (48.2 vs. 31.5 %, p < 0.001), exercise more (46.5 vs. 33.5 %, p < 0.001), reduce risky drinking (15.1 vs. 10.8 %, p < 0.01), reduce stress (27.3 vs. 19.2 %, p < 0.001), reduce depression/anxiety (28.9 vs. 20.7 %, p < 0.001), and improve sleep (27.7 vs. 22.1 %, p < 0.01). Less frequent behaviors (e.g., smoking and drug use) did not reach statistical significance but trended toward improvements. There were no differences between practices in referral rates to ancillary behavior and mental health counseling staff, including internal referrals. Overall, more intervention than control practice patients reported making substantial diet, exercise, stress, anxiety/depression, and sleep improvements (range of differences 5.4–13.6 %, p < 0.01).
Table 2.
Comparison of patient reported screening, goal setting, referrals, and improvement for intervention versus control practice patients
| Were you asked about the following? | Did anyone work with you to set specific goals to make changes? | Did anyone recommend you seek assistance from another provider or program? | Have you made any positive changes since your visit? | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Eating/diet | 73.9 % | 59.2 %* | 48.2 % | 31.5 %* | 19.7 % | 15.6 % | 63.4 % | 49.8 %* |
| Physical activity/exercise | 79.4 % | 69.9 %* | 46.3 % | 33.5 %* | 17.4 % | 15.6 % | 54.4 % | 45.5 %* |
| Tobacco/smoking | 77.4 % | 72.1 %** | 17.2 % | 14.8 % | 6.9 % | 7.0 % | 13.2 % | 12.9 % |
| Alcohol use | 74.6 % | 67.7 %* | 15.1 % | 10.8 %** | 4.7 % | 4.4 % | 10.8 % | 9.7 % |
| Drug use | 68.9 % | 59.9 %* | 12.3 % | 10.3 % | 4.3 % | 3.7 % | 10.3 % | 9.4 % |
| Stress level | 63.9 % | 50.5 %* | 27.3 % | 19.2 %* | 10.2 % | 8.2 % | 29.0 % | 22.2 %* |
| Anxiety/depression | 65.2 % | 49.4 %* | 28.9 % | 20.7 %* | 11.2 % | 9.4 % | 26.4 % | 20.6 %* |
| Sleep | 66.0 % | 52.9 %* | 27.7 % | 22.1 %** | 11.7 % | 11.1 % | 30.1 % | 24.7 %* |
Values adjusted for patient age, race/ethnicity, and time with the practice
*p value comparing intervention to control <0.001
**p value comparing intervention to control <0.01
Table 3.
Comparison of patient reported screening, goal setting, referrals, and improvement for intervention versus control practice patients
| Intervention | Control | p Value | |
|---|---|---|---|
| They really cared about me as a person: | |||
| Yes, definitely | 81.3 % | 75.1 % | <0.001 |
| Yes, somewhat | 16.0 % | 20.5 % | |
| No | 2.7 % | 4.4 % | |
| I could trust them with my medical care: | |||
| Yes, definitely | 84.5 % | 79.5 % | 0.004 |
| Yes, somewhat | 12.9 % | 16.5 % | |
| No | 2.6 % | 4.0 % | |
| They encouraged me to ask questions: | |||
| Always | 56.7 % | 47.2 % | <0.001 |
| Usually | 20.2 % | 22.0 % | |
| Sometimes | 15.9 % | 19.5 % | |
| Never | 7.2 % | 11.3 % | |
| They show interest in my questions and concerns: | |||
| Always | 71.5 % | 63.5 % | <0.001 |
| Usually | 15.2 % | 19.1 % | |
| Sometimes | 9.8 % | 13.1 % | |
| Never | 3.6 % | 4.3 % | |
| They explain things in a way that is easy to understand: | |||
| Always | 73.9 % | 68.9 % | 0.031 |
| Usually | 15.1 % | 17.9 % | |
| Sometimes | 7.9 % | 9.1 % | |
| Never | 3.0 % | 4.1 % | |
Questions from the Consumer Assessment of Healthcare Provider and Systems Clinical & Group survey (CAHPS-CG) [36]
With respect to the care delivery process assessed by the CAHPS survey, a greater percent of patients from intervention practices than control practices reported that their clinician definitely cared about them as a person (81.8 vs. 75.3 %, p < 0.001); they could definitively trust their clinician with their medical care (85.0 vs. 79.5 %, p < 0.001); they were encouraged to ask questions (57.1 vs. 47.6 %, p < 0.001); and they were explained information in a way that was easy for them to understand (74.4 vs. 69.0 %, p < 0.001).
Discussion
By systematically fielding a patient-centered HRA, primary care practices can improve their care delivery process for addressing health behaviors and mental health concerns. This process improved adherence to screening recommendations for risky health behaviors and mental health concerns [9], resulting in more patients setting improvement goals with their clinician. Patients also reported making health behavior and mental health improvements and having greater satisfaction with their care. Ideally, these effects can occur both through patients learning about their health needs, becoming activated, and making the necessary changes; as well as through clinicians being proactively alerted of patient’s needs and reaching out to help patients.
These findings are particularly significant given the patient population studied. While practices were approached for participation to represent the full spectrum of primary care, the final sample of study practices included a high proportion of disadvantaged patients and minorities. These vulnerable patient populations have a greater prevalence of risks and greater difficulties with making improvements [38]. Of additional significance, this study evaluated the impact of the intervention on an entire primary care practice’s population, not just a subpopulation of patients participating in the study (i.e., completing the MOHR assessment). Our findings suggest that if practices use similar patient-centered HRAs routinely during care, they could improve the health of their overall patient panel.
A key element of MOHR is that it was a patient-centered “HRA-plus.” An HRA-plus has previously been defined as moving beyond merely asking about risk for disease and disability and including feedback, prioritizing, shared goal setting, referrals, progress monitoring, and regular follow-up [11]. Without these additional elements, MOHR may not have resulted in the reported improvements. Of note, MOHR did not automate goal setting, referrals, progress monitoring, or follow-up but rather relied on the clinician and patient during an office visit to do these next steps based on their interests and clinical judgment. Advances are still needed to patient-centered HRAs, such as MOHR, and more evidence is needed to understand which elements of the HRA-plus model are effective and how best to deliver them.
Most notably, MOHR did not result in any significant increase in referrals. This is particularly interesting given that seven practices had integrated health behavior or mental health support. Not only were there no differences between intervention and control practices, but there were no differences in referrals between practices with and without health behavior and mental health counselors. One explanation is that in exit interviews, clinicians reported not referring more patients because onsite counselors were already overburdened prior to participating in this study. Additionally, practices reported counseling patients about risky behaviors and mental health concerns for an additional 14 min per patient [30]. While this is a substantial time investment for practices, patients will require greater support over an extended period of time to sustain improvements [9]. Future iterations of patient-centered HRAs, like MOHR, could serve to facilitate the integration of primary and community care providers to better leverage existing resources and better support patient needs [39]. Effective partnerships with health behavior and mental health counselors will be especially important.
This study has four key limitations. First, the study was not designed to directly measure behavior and mental health changes. The primary outcome was goal setting, an intermediary step to making improvements. Encouragingly, patients from intervention practices perceived that they made more improvements than patients from control practices, but whether they actually changed behaviors or had improved mental health is unknown. Second, outcome surveys were not linked to MOHR assessments. As a result, all patients were asked about screening, goal setting, and improvements for every behavior and mental health domain, whether healthy or unhealthy for the patient. This may explain why there were no observed improvements in goal setting or behaviors for the lower prevalence conditions of smoking and drug abuse. This design was purposely used given this study’s objective of assessing whether an HRA improves screening and goal setting for an entire primary care practice. Third, only about half of patients completed the post visit survey which is the basis for our study’s outcomes. While this is lower than the ideal ∼60 % response rate for a modified Dillman technique, it is not unexpected given our attempt to survey such a large proportion of the intervention and control practices’ patients—everyone with a chronic care and or wellness visit. Finally, it is unclear whether these findings can be generalized outside of a research setting. While the study design was highly pragmatic to increase external validity [28, 40], fielding MOHR required substantial resources and time from practices (an additional 28 min per patient) [30]. However, practice redesign efforts such as patient-centered medical homes, accountable care organizations, primary care and public health integrations, and primary care and mental health integrations are advancing team-based care models that can support all of the needed patient-centered HRA steps. Additionally, new economic models focused on the quality of care, and the health of patients as opposed to fee for service-based payment models will provide resource support and incent patient-centered HRA activities [41, 42]. Current practice mandates to perform a patient-centered HRA as part of a Medicare Annual Wellness Visit highlight the changes that are already occurring in practice [11, 13].
The inclusion of patient-centered HRAs into routine care holds great promise to effectively initiate the process of helping patients improve behaviors and mental health. Given the high prevalence of risks, this activity could result in substantial public health improvements. Whether the intended benefits can be realized on a national scale remains to be seen. Paramount to success will be effective implementation and follow-up counseling and support.
Acknowledgments
Funding for the MOHR project was provided by the National Cancer Institute, Agency for Healthcare Research and Quality, Office of Behavioral and Social Sciences Research, and National Center for Advancing Translational Sciences (CTSA Grant Number ULTR00058). The opinions expressed in this manuscript are those of the authors and do not necessarily reflect those of the funders.
We would like to thank the research teams for their valuable efforts including Melissa Hayes, Steve Mitchell, Mark Greenawald, Mark Kelly, Rodger Kessler, Jill Arklind, Joseph Carroll, Christine Nelson, John Heintzman, Maria Fernandez, Kayla Fair, Julie Ribardo, Dylan Roby, Jennifer Leeman, and Alexis Moore.
Most of all, we would like to thank our practices for their partnership, insights, and hard work: Vienna Primary and Preventive Medicine, Little Falls Family Practice, Charles City Regional Health Services, Chester Family MedCare, Carilion Family Medicine—Roanoke Salem, Carilion Family Medicine—Southeast, Berlin Family Health, Milton Family Practice, Humbolt Open Door Clinic, McKinleyville Community Health Center, St. Johns’ Well Child and Family Center, Spring Branch Community Health Center—Pitner Clinic, Spring Branch Community Health Center—Hillendahl Clinic, HealthPoint Community Health Centers—Navasota, HealthPoint Community Health Centers—Hearne, Murfeesboro Clinic, and Snow Hill Clinic
Compliance with ethical standards
The authors have adhered to ethical principles throughout this research study including writing and submitting this manuscript. Specifically, we adhered to the Committee on Publication Ethics (COPE) guidelines, seven institutional IRB protocols, and appropriate informed consent processes for research participants.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Implications
Practice
Administering a patient-centered health risk assessment in primary care will identify a significant number of unhealthy behaviors and mental health needs allowing patients and clinicians to better set goals that will improve health.
Policy
Better integration of primary care with mental health and health behavior counselors is needed to address the modifiable risks that will be identified as health risk assessments are more commonly used in primary care.
Research
A greater understanding about how to routinely support the use of health risk assessments in primary care as well as how to deliver intensive counseling to patients ready to make changes is needed.
References
- 1.Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Bauer UE, Briss PA, Goodman RA, et al. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 3.Woolf SH, Aron LY, National Academies (U.S.). Panel on Understanding Cross-National Health Differences Among High-Income Countries, Institute of Medicine (U.S.). Board on Population Health and Public Health Practice. U.S. Health in International Perspective : Shorter Lives, Poorer Health. [PubMed]
- 4.U.S. Department of Health and Human Services. Healthy People 2020. 2012; http://www.healthypeople.gov/2020/default.aspx. Accessed Jan. 2013.
- 5.Phillips SM, Glasgow RE, Bello G, et al. Frequency and prioritization of patient health risks from a structured health risk assessment. Ann Fam Med. 2014;12(6):505–513. doi: 10.1370/afm.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGruy FV, Etz RS. Attending to the whole person in the patient-centered medical home: the case for incorporating mental healthcare, substance abuse care, and health behavior change. Fam Syst Health: J Collaborative Fam Healthcare. 2010;28(4):298–307. doi: 10.1037/a0022049. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med. 2004;27(2 Suppl):61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 8.IOM (Institute of Medicine). Primary Care and Public Health . Exploring Integration to Improve Population Health. Washington: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 9.U.S. Preventive Services Task Force. Preventive Services. 2014; http://www.uspreventiveservicestaskforce.org/. Accessed Jan. 2015
- 10.Krist AH, Baumann LJ, Holtrop JS, et al. Evaluating feasible and referable behavioral counseling interventions. Am J Prev Med. 2015;49(3 Suppl 2):S138–149. doi: 10.1016/j.amepre.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Goetzel RZ, Staley P, Ogden L, Stange P, Fox J, Spangler J, Tabrizi M, Beckowski M, Kowlessar N, Glasgow RE, Taylor MV. A framework for patient-centered health risk assessments - providing health promotion and disease prevention services to Medicare beneficiaries. Atlanta, GA: US Department of Health and Human Services, Centeres for Disease Control and Prevention. 2011; http://www.cdc.gov/policy/opth/hra/. Accessed Feb. 2014.
- 12.Goodyear-Smith F, Warren J, Bojic M, et al. eCHAT for lifestyle and mental health screening in primary care. Ann Fam Med. 2013;11(5):460–466. doi: 10.1370/afm.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The patient protection and affordable care act. Public Law 111–1148. 2nd Session ed 2010.
- 14.Pearson ES. Goal setting as a health behavior change strategy in overweight and obese adults: a systematic literature review examining intervention components. Patient Educ Couns. 2012;87(1):32–42. doi: 10.1016/j.pec.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Strecher VJ, Seijts GH, Kok GJ, et al. Goal setting as a strategy for health behavior change. Health Educ Q. 1995;22(2):190–200. doi: 10.1177/109019819502200207. [DOI] [PubMed] [Google Scholar]
- 16.Cullen KW, Baranowski T, Smith SP. Using goal setting as a strategy for dietary behavior change. J Am Diet Assoc. 2001;101(5):562–566. doi: 10.1016/S0002-8223(01)00140-7. [DOI] [PubMed] [Google Scholar]
- 17.Shilts MK, Horowitz M, Townsend MS. Goal setting as a strategy for dietary and physical activity behavior change: a review of the literature. Am J Health Promot. 2004;19(2):81–93. doi: 10.4278/0890-1171-19.2.81. [DOI] [PubMed] [Google Scholar]
- 18.Shekelle PG, Tucker JS, Maglione M, et al. Health risk appraisals and medicare. Santa Monica: RAND Corporation; 2003. [Google Scholar]
- 19.Guide to Community Preventive Services. 2015; http://www.thecommunityguide.org/. Accessed Feb. 2015.
- 20.Halpin HA, McMenamin SB, Schmittdiel J, et al. The routine use of health risk appraisals: results from a national study of physician organizations. Am J Health Promot. 2005;20(1):34–38. doi: 10.4278/0890-1171-20.1.34. [DOI] [PubMed] [Google Scholar]
- 21.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stange KC, Flocke SA, Goodwin MA, et al. Direct observation of rates of preventive service delivery in community family practice. Prev Med. 2000;31(2 Pt 1):167–176. doi: 10.1006/pmed.2000.0700. [DOI] [PubMed] [Google Scholar]
- 23.Stange KC, Zyzanski SJ, Jaen CR, et al. Illuminating the ‘black box’. A description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46(5):377–389. [PubMed] [Google Scholar]
- 24.Elwy AR, Horton NJ, Saitz R. Physicians’ attitudes toward unhealthy alcohol use and self-efficacy for screening and counseling as predictors of their counseling and primary care patients’ drinking outcomes. Subst Abuse Treat Prev Policy. 2013;8:17. doi: 10.1186/1747-597X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson KE, Hersh AL, Nkoy FL, et al. Primary care physician smoking screening and counseling for patients with chronic disease. Prev Med. 2014;71C:77–82. doi: 10.1016/j.ypmed.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Preventive Services Task Force. Screening for and management of obesity in adults. 2012; http://www.uspreventiveservicestaskforce.org/uspstf/uspsobes.htm. Accessed Jan. 2013.
- 27.U.S. Preventive Services Task Force. Screening for obesity in children and adolescents. 2010; http://www.uspreventiveservicestaskforce.org/uspstf/uspschobes.htm. Accessed Mar, 2013.
- 28.Krist AH, Glenn BA, Glasgow RE, et al. Designing a valid randomized pragmatic primary care implementation trial: the my own health report (MOHR) project. Implement Sci: IS. 2013;8:73. doi: 10.1186/1748-5908-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez HP, Glenn BA, Olmos TT, et al. Real-world implementation and outcomes of health behavior and mental health assessment. J Am Board Fam Med. 2014;27(3):356–366. doi: 10.3122/jabfm.2014.03.130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krist AH, Phillips SM, Sabo RT, et al. Adoption, reach, implementation, and maintenance of a behavioral and mental health assessment in primary care. Ann Fam Med. 2014;12(6):525–533. doi: 10.1370/afm.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.My Own Heath Report. www.MyOwnHealthReport.org. Accessed Jan. 2014.
- 32.Estabrooks PA, Boyle M, Emmons KM, et al. Harmonized patient-reported data elements in the electronic health record: supporting meaningful use by primary care action on health behaviors and key psychosocial factors. J Am Med Inform Assoc. 2012;19(4):575–582. doi: 10.1136/amiajnl-2011-000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glasgow RE, Kaplan RM, Ockene JK, et al. Patient-reported measures of psychosocial issues and health behavior should be added to electronic health records. Health Aff (Millwood) 2012;31(3):497–504. doi: 10.1377/hlthaff.2010.1295. [DOI] [PubMed] [Google Scholar]
- 34.Croteau J, Ryan D. Acheiving your SMART health goals. BeWell@Stanford. 2013; http://bewell.stanford.edu/smart-goals. Accessed Jan. 2013.
- 35.O’Neil j. SMART Goals, SMART Schools. Educational Leadership. 2000:46–50.
- 36.Agency for Healthcare Research and Quality. CAHPS Clinician & Group Surveys. http://cahps.ahrq.gov/clinician_group/. Accessed Nov. 2014.
- 37.Dillman DA. Mail and internet surveys: the tailored design method. 2. John Wiley Company: Hoboken; 1999. [Google Scholar]
- 38.Lu N, Samuels ME, Wilson R. Socioeconomic differences in health: how much do health behaviors and health insurance coverage account for? J Health Care Poor Underserved. 2004;15(4):618–630. doi: 10.1353/hpu.2004.0053. [DOI] [PubMed] [Google Scholar]
- 39.Krist AH, Shenson D, Woolf SH, et al. Clinical and community delivery systems for preventive care: an integration framework. Am J Prev Med. 2013;45(4):508–516. doi: 10.1016/j.amepre.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasgow RE, Kessler RS, Ory MG, et al. Conducting rapid, relevant research: lessons learned from the My Own Health Report project. Am J Prev Med. 2014;47(2):212–219. doi: 10.1016/j.amepre.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joint Principles of the Patient-Centered Medical Home. 2009; http://www.pcpcc.net/. Accessed Sept. 2014.
- 42.McClellan M, McKethan AN, Lewis JL, et al. A national strategy to put accountable care into practice. Health Aff (Millwood) 2010;29(5):982–990. doi: 10.1377/hlthaff.2010.0194. [DOI] [PubMed] [Google Scholar]