Abstract
Regain of lost weight is a universal problem for behavioral treatments. An increased understanding of theory-based psychosocial predictors of decay in behavioral correlates of weight loss might improve treatments. Data were derived from a previous weight loss investigation of 110 women with obesity. A subsample from the experimental treatment who lost ≥3 % body weight and regained at least one third of that over 24 months (N = 36) was assessed. During months 6 through 24, there were unfavorable changes in behavioral (fruit/vegetable and sweet intake; physical activity) and psychosocial variables. Mood change predicted change in fruit/vegetable and sweet intake, with emotional eating change mediating the latter relationship. Change in self-regulation predicted changes in sweet and fruit/vegetable intake and physical activity, with self-efficacy mediating the self-regulation–fruit/vegetable intake and self-regulation–physical activity relationships. Findings suggest that after treatment-induced weight loss, addressing indicated theory-based psychosocial variables might mitigate decay in behavioral predictors of healthier weight.
KEYWORDS: Weight loss, Maintenance, Treatment, Self-regulation, Self-efficacy, Physical activity
Although behavioral (non-pharmacological/non-surgical) treatments have consistently been associated with a 5–8 % loss in initial body weight, even more consistent has been a gradual climb toward baseline weight after approximately 6 months [1–3]. Although as little as 3 % weight loss can have health benefits [4, 5], from one third to two third regain of weight is expected over 2 years [2, 6]. Often, regain will continue beyond a participant’s initial weight [1]. Repeating a pattern of weight loss and regain might increase individuals’ health risks [7]. Researchers suggest that weight loss maintenance differs considerably from weight loss and thus should be distinctly studied and addressed [8]. In a recent trial incorporating a year-long, state-of-the-science behavioral weight management protocol directed primarily at reductions in energy intake [9], an initial weight loss of 9 % demonstrated a 91 % regain of baseline weight over the subsequent 3 years [10]. Because of the failure of this seemingly comprehensive protocol that was highly focused upon maintaining weight loss from its outset, along with the long history of behavioral treatments’ association with weight regain [1–3], its developers questioned the merits of any further pursuits of such weight management interventions [10]. Although other researchers disagreed [11–13], it seemed clear that new and different approaches were needed.
As a reaction to this and (a) a consensus that exercise is a primary predictor of maintained weight loss [6, 14, 15], (b) suggestions that the development of self-regulation prior to pursuing reductions in energy intake is advisable [8], (c) proposed behavioral models of the relationship between exercise and weight loss [11, 16, 17], and (d) indications that physical activity affects weight loss more through its impact on psychosocial predictors of controlled eating than energy expenditure [11], a novel experimental intervention was recently tested with women with obesity [18]. This treatment administered a component of supported exercise to improve psychosocial factors such as self-regulatory skills, self-efficacy, and mood 2 months prior to the administration of group nutrition-change sessions. The nutrition sessions then focused upon generalizing and reinforcing those gains for long-term maintenance of controlled eating and weight loss [18].
For example, for improving both physical activity and eating, there was a concentration on the use of self-regulation for overcoming common behavioral barriers and building self-efficacy to persevere. Also, the impact that exercise-induced mood change has on emotional eating [19] was leveraged. This impact is a factor previously shown to be associated with fruit and vegetable intake and consumption of sweets in women [20]. It was thought that this focus on building resilient self-regulatory skills, and accounting for psychosocial factors such as self-efficacy, mood, and emotional eating, would mitigate expected decays in the behaviors that are predictive of maintained weight loss [18, 21].
Outcomes of this experimental treatment were considerably stronger than a comparison condition of theory-based education and most other behavioral weight loss treatments. At the end of its second year, 89 % of participants’ initial mean weight loss of 5.8 kg was maintained [18]. Also, this experimental treatment was relatively inexpensive to administer. Theoretical tenets of social cognitive [22] and self-efficacy [23] theory were supported. However, as has been the case with most weight management studies [8], behavioral changes and the resulting mean weight regain of only 0.6 kg over 2 years varied greatly between participants (SD = 4.6 kg).
Although there are a few exceptions, which also suggest the importance of building self-efficacy (e.g., perceptions of ability and competence) and addressing eating in response to emotional cues [24, 25], there is little actionable research on theory-based psychosocial factors that might influence success at maintaining weight management behaviors. Analyses of dynamic patterns of changes in psychosocial correlates of eating and physical activity trajectories in participants who were unsuccessful at maintaining their weight loss might be especially instructive beginning just after the time when most weight loss is expected (e.g., after the initial 6 months of treatment [1–3]). Thus, the aim of this preliminary, small-sample study was to develop a better understanding of changes within malleable psychosocial factors as they relate to their association with changes in fruits/vegetable consumption, intake of sweets, and physical activity outputs after the completion of a 6-month period of expected weight loss. Based on theory [16, 22, 23] and previous research over primarily brief time frames [11, 25], changes in self-regulation, self-efficacy, mood, and emotional eating were incorporated as predictors of behavioral changes within this research because of their respective roles in (a) overcoming inevitable life-related barriers, (b) perceiving success that motivates persistence, (c) establishing a positive mental climate that is advantageous for persistence, and (d) overcoming emotion-based triggers for lapses into past unproductive eating behaviors.
The ultimate goal of the research was to advance behavioral treatments to better-facilitate sustained weight loss in all participants. Data from a subsample of participants of the aforementioned experimental treatment [18], who were initially successful at weight loss by losing at least the 3 % of their initial weight required for a reduction in health risks [4, 5] but regained a minimum of one third of that weight over 2 years (which is indicative of a failure to sustain long-term effects [2, 6]), were assessed. After considering previously suggested relationships between changes in psychosocial and behavioral correlates of weight [8, 10, 11, 16, 17, 20, 25], and incorporating suggestions for the use of mediation analyses to inform the architecture of interventions [26], the following hypotheses were provided:
Measures of eating, physical activity, and their psychosocial predictors will proceed in the unfavorable direction from month 6 to month 12, month 6 to month 24, and month 12 to month 24.
Over the above temporal periods, there will be a significant inverse relationship between changes in negative mood and fruit/vegetable intake that will be mediated by emotional eating change. That relationship will be positive when change in the consumption of sweets is the measure of eating.
Over the aforementioned temporal periods, there will be a significant positive relationship between changes self-regulation and fruit/vegetable intake that will be mediated by self-efficacy change. That relationship will be negative when change in the consumption of sweets is the measure of eating.
Over the aforementioned temporal periods, there will be a significant positive relationship between changes in self-regulation and physical activity that will be mediated by change in exercise self-efficacy.
METHODS
Participants
Participants were a subsample of a recent study of 110 women with obesity (body mass index (BMI) = 30–40 kg/m2) [18]. That sample type and size was established a priori to match a study being contrasted within that report [10]. For inclusion in the present research, at least 3 % of initial body weight must have been lost over the first 6 months after treatment initiation, and at least one third (≥33.3 %) of the lost weight must have been regained over 24 months while participating in the experimental group. Exclusion criteria were under 21 years of age, pregnancy, current use of medications for weight loss or a psychological/psychiatric condition, participation in a medical or commercial weight loss program, and exercise of ≥20 min/week.
The mean age of this subsample (N = 36) was 45.9 years (SD = 9.2), with a mean BMI of 34.5 kg/m2 (SD = 3.2). There were 72 % White, 19 % African-American, and 9 % of other races/ethnicities. This approximates the racial make-up of the USA, as a whole. Nearly all self-reported a middle family-income range. Mean weight loss at month 6 was 7.3 % of original body weight (SD = 3.1). Mean weight loss at month 24 was 4.1 % (SD = 5.2). Institutional review board approval and written informed consent were obtained from each participant. The research protocols followed requirements of the Helsinki Declaration.
Measures
Because of the large number of self-report survey responses required, abbreviated instruments that were appropriately validated were used to minimize participant burden and maximize quality of responses where possible [27].
Fruits/vegetables and sweets
As suggested as applicable for the present research context [28], single items were used to measure typical daily intake of servings of fruits (e.g., apple, orange [1 small or 118 mL canned], 100 % fruit juice [118 mL]), vegetables (e.g., broccoli, carrots, green beans [118 mL], raw spinach [236 mL]), and sweets (e.g., candy [one small piece or 27 mL], cake [one small piece or 59 mL]) corresponding to both the U.S. Department of Agriculture’s Food Plate and its earlier Food Guide Pyramid [29]. Scores from fruits and vegetables were summed (i.e., fruits/vegetables). Correlations with comprehensive food frequency questionnaires and the full-length Block Food Frequency Questionnaire [30] were reported to be strong at .70–.85 [30, 31]. Test-retest reliabilities over 3 weeks were .77–.83 for women [11].
Physical activity
The Godin-Shephard Leisure-Time Physical Activity Questionnaire [32] was used to measure weekly physical activity. Its measurement unit is metabolic equivalent of tasks (METs) per week, where 1 MET ~ 3.5 mL of O2/kg/minute [33]. Participants’ self-reported numbers of weekly sessions of strenuous (~9 METs; e.g., running), moderate (~5 METs; e.g., fast walking), and light (~3 METs; e.g., easy walking) physical activities for “more than 15 min” were summed. Test-retest reliability over 2 weeks was reported to be .74 [34]. Construct validity was indicated through strong correlations reported with both accelerometer and VO2max measurements [35, 36].
Negative mood
The Total Mood Disturbance scale of the Profile of Mood States Short Form [37] was used to measure negative mood. Its 30 items assess feelings related to depression, tension/anxiety, vigor, fatigue, anger, and confusion (e.g., “sad,” “tense,” “energetic”) occurring during the past week. Responses range from 0 (not at all) to 4 (extremely) and are summed (after reversing the scores on vigor). A lower score indicates less negative mood. Internal consistencies (all reported as Cronbach’s α) were reported to be .84–.95 across factors [37] and were .79–.89 for the present sample [18]. Reported 3-week test-retest reliabilities averaged .69 [37].
Emotional eating
A 15-item version of the Emotional Eating Scale [38] was used to measure emotional eating. Items assess the extent a feeling related to anxiety, depression, and anger/frustration leads to an urge to eat (e.g., “nervous,” “irritated”). Item responses range from 0 (no desire to eat) to 4 (an overwhelming urge to eat) and were summed. A higher score indicated more emotional eating. Internal consistencies were reported to be α = .72–.78, and test-retest reliability over 2 weeks was .79 [38]. For the present sample, α = .70–.79 [18].
Self-regulation for controlled eating and self-regulation for exercise
Consistent with previous research [11], a validated scale [39] that was adapted for the present context separately measured self-regulation for exercise and self-regulation for controlled eating through 10 items each. Responses range from 1 (never) to 5 (often) and assess the degrees specific self-regulatory skills/methods are presently incorporated to manage barriers to controlled eating (e.g., “I say positive things to myself about eating well”) and completing exercise/physical activity (e.g., “I set physical activity goals”). Scores within each scale are summed, with a higher score indicating greater use of self-regulation to manage either eating or exercise/physical activity. Internal consistency of the original instrument was reported to be .75 [39] and was .83 and .80, respectively, for the present versions and sample [18]. Test-retest reliabilities over 2 weeks were .74 and .78, respectively [17].
Self-efficacy for controlling eating
The 20-item Weight Efficacy Lifestyle Scale [40] was used to measure self-efficacy for controlling eating when negative emotions, food availability, physical discomfort, positive activities, and social pressure might be challenging (e.g., “I can resist eating even when I have to say ‘no’ to others”). Item responses range from 0 (not confident) to 9 (very confident) and are summed. A higher score indicates greater self-efficacy. Internal consistencies were reported to be α = .70–.90 [40] and were .74–.81 for the present sample [18].
Exercise self-efficacy
The 5-item Exercise Self-Efficacy Scale [41] was used to measure self-efficacy for completing exercise/physical activity or “… how confident you are that you can persist with exercising (continue with exercising) under the listed conditions” (e.g., “I feel I don’t have the time,” “I have more enjoyable things to do”). Responses range from 1 (not at all confident) to 11 (very confident) and are summed. A higher score indicates greater self-efficacy. Internal consistencies were reported to be α = .76–.82, and test-retest reliability over 2 weeks was .90 [42]. For the present sample, α = .80 [18].
Procedure
The treatment setting was small community-based wellness/fitness centers in the eastern USA. Processes and procedures are more fully explained elsewhere [18]; however, an overview will be provided here. The weight loss treatment consisted of two components. The exercise support component incorporated the previously validated Coach Approach protocol [43] which was administered via six individual meetings (treatment initiation–month 7; Fig. 1). The nutrition behavior change component was administered through small group sessions (months 2–14; Fig. 1). Both treatment components were based on social cognitive [22] and self-efficacy [23] theories where individuals are viewed as being capable of self-organization, managing their environments, and being self-reflective of their abilities. Wellness leaders with at least one national certification (e.g., American Council on Exercise) received training in, and administered, all treatment sessions.
Fig. 1.
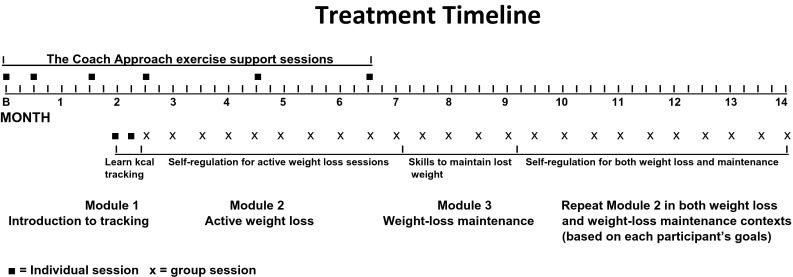
Weight loss treatment timeline
Within the exercise support component, physical activity plans were based on each participant’s ability and preferences. However, the officially recommended minimum volume for health (i.e., 150 min/week of moderate-intensity physical activity [44]) was also described. Most session time was spent on the development of distinct self-regulatory skills including goal setting/progress monitoring, stimulus control, cognitive restructuring, relapse prevention, behavioral contracting, and how to best address behavioral cues and triggers. Physical activity-induced mood change was assessed both in response to a single bout of physical activity and over 1- to 2-month intervals. The individual one-on-one sessions were conducted in a private office supported by the use of a dedicated computer program [43].
The experimental nutrition component began after 2 months of focusing on exercise only. As diagrammed in Fig. 1, module 1 of the weight loss treatment component consisted of two, one-on-one sessions that introduced all participants to food and kcal tracking. Energy-intake limits were assigned based on each individual’s present weight (e.g., 1500 kcal/day limit for a body weight of 79–99 kg). Module 2 of the treatment consisted of 10 group nutrition sessions of 60 min each. They were each spaced by 2 weeks. Consistent with previous research [11], its goal of active weight loss focused upon generalizing and adapting self-regulatory skills developed and practiced in the context of supporting regular physical activity, to regulating eating behaviors. For example, dissociation from exercise-induced discomfort was extended to dissociating from cues to overeating. Module 3 of the nutrition component consisted of four group sessions of 45 min each that again focused on the use of self-regulatory skills but were adapted to a required goal of maintaining, rather than seeking continuation of, lost weight. A final nutrition module provided content from module 2 tailored for either obtaining additional weight loss, or maintenance of lost weight, based on each participant’s goals.
Throughout, the treatment processes emphasized the need to (a) maintain regular physical activity, (b) increase consumption of fruits and vegetables and minimize sweets, (c) incorporate self-regulatory skills to overcome behavioral barriers, and (d) develop self-efficacy through acknowledgement of enhanced capabilities to persevere. Because the emphases on behavioral change processes required considerable time within sessions, participants were referred to a United States Department of Agriculture website [29] for further evidence-based nutrition information. Study staff completed fidelity checks on approximately 15 % of treatment sessions. They indicated the need for only minor, easily implemented, corrective actions. Surveys were completed in a private area.
Data analyses
The intention-to-treat format that was used incorporated the expectation-maximization algorithm [45] for imputation of the 12 % of missing scores within the present data set. Based on consistent directionality of score changes and relationships in earlier related research [11], statistical significance was set at α = .05 (one-tailed). Statistical analyses were conducted using SPSS Version 22 (IBM, Armonk, NY).
Repeated measures ANOVAs assessed changes in scores at months 6, 12, and 24. This was followed-up with t tests to evaluate all possible pairwise contrasts. Consistent with suggestions for this research context, change scores were unadjusted for baseline values [46].
Mediation analysis was then used incorporating 20,000 bootstrapped resamples [47]. The direct relationship between a predictor and outcome measure was first calculated (path c). Then, a potential mediator of that relationship was added to the model. Based on previous suggestions [48], a significant relationship between the predictor and outcome variable was not required for proceeding with mediation analyses. Statistical significance of mediation is identified when its corresponding 95 % confidence interval does not include zero. The relationships of mediator to predictor (path a), mediator to outcome (path b), and predictor to outcome while accounting for the effect of the mediator (path c′) were also calculated. Temporal periods for changes (month 6–month 12, month 6–month 24, and month 12–month 24) were consistent for variables within each equation. However, to assess directionality vs. bi-directionality in relationships, a procedure for assessment of reciprocal relationships [45] was used. Within this procedure, a reciprocal, mutually reinforcing, relationship between an outcome and mediator variable is identified if, after reversing their positions in a complementary mediation model, both equations demonstrate significant mediation [49].
RESULTS
Score changes
Each of the behavioral predictors of weight measured in this study—fruit/vegetable intake, consumption of sweets, and physical activity output—significantly changed in the undesirable direction starting 12 months after treatment initiation (Table 1). The proposed psychosocial correlates of these behavioral changes, however, demonstrated a more linear unfavorable trajectory starting at month 6. Only self-efficacy for controlling eating did not show a significant degradation over the course of this study (Table 1).
Table 1.
Descriptive statistics and change scores from month 6 to month 24 (N = 36)
| Measure | Month 6 | Month 12 | Month 24 | Month 6–12 change | Month 6–24 change | Month 12–24 change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Fruit/vegetable intake (servings/day) | 6.40 | 2.41 | 6.31 | 2.33 | 5.43 | 1.99 | −.97 | 1.21 | −.97 | 2.33** | −.88 | 2.23* |
| Consumption of sweets (servings/day) | 1.06 | .83 | 1.11 | .78 | 1.44 | .97 | .06 | .64 | .38 | 1.25* | .32 | 1.01* |
| Physical activity/week (METs/week) | 36.14 | 14.95 | 35.79 | 14.77 | 22.96 | 15.45 | −.34 | 8.13 | −13.18 | 16.83*** | −12.84 | 16.44*** |
| Negative mood | 2.44 | 10.23 | 6.94 | 12.29 | 9.56 | 15.37 | 4.50 | 7.84*** | 7.12 | 13.69** | 2.61 | 13.66 |
| Emotional eating | 17.47 | 9.61 | 18.67 | 11.59 | 20.43 | 11.14 | 1.19 | 5.37 | 2.96 | 10.05* | 1.76 | 10.07 |
| Self-regulation for controlled eating | 33.14 | 4.14 | 31.81 | 4.43 | 28.97 | 6.34 | −1.33 | 3.94* | −4.17 | 6.31*** | −2.83 | 5.90** |
| Self-efficacy for controlling eating | 130.57 | 25.25 | 128.26 | 25.37 | 124.11 | 30.34 | −2.31 | 14.93 | −6.46 | 32.17 | −4.15 | 27.37 |
| Self-regulation for exercise | 33.67 | 4.08 | 32.58 | 4.24 | 29.58 | 6.21 | −1.08 | 3.45* | −4.09 | 4.69*** | −3.00 | 5.41** |
| Exercise self-efficacy | 36.44 | 9.43 | 34.69 | 10.98 | 30.33 | 11.04 | −1.75 | 7.31 | −6.11 | 10.86*** | −4.36 | 9.06** |
Significance of change within the labeled temporal interval is based on paired one-tailed t tests (dfs = 35)
METs metabolic equivalent of tasks (a measure of energy costs)
*p < .05, **p < .01, ***p < .001
Psychosocial predictors of behavioral changes
Results for all mediation analyses are shown in Table 2. Statistically significant relationships between changes in mood and fruit/vegetable intake were not significantly mediated by changes in emotional eating (models 1a, 4a, and 7a). However, during months 6–24, the significant prediction of changes in the intake of sweets by mood change was significantly mediated by changes in emotional eating (model 4b). Change in self-efficacy for controlling eating significantly mediated the significant relationship between changes in self-regulation for controlled eating and fruit/vegetable intake only when month 24 data were included (models 5a and 8a). However, self-efficacy for controlled eating change was not a significant mediator of the prediction of changes in the consumption of sweets by self-regulation for controlled eating changes. In all temporal intervals tested, change in exercise self-efficacy significantly mediated a statistically significant relationship between changes in self-regulation for exercise and physical activity (models 3, 6, and 9).
Table 2.
Results from mediation and reciprocal effects analyses (N = 36)
| Predictor | Mediator | Outcome | Model R 2 | Path a | Path b | Path c | Path c′ | Indirect effect |
|---|---|---|---|---|---|---|---|---|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | ||||
| p | p | p | p | p | [95% CI] | |||
| Changes from month 6 to month 12 | ||||||||
| Model 1a | ||||||||
| ΔNegative mood | ΔEmotional eating | ΔFruit/vegetable | .17 | −.15 (.06) | .07 (.04) | −.02 (.01) | −.01 (.01) | −.01 (.01) |
| .047 | .006 | .038 | .042 | .183 | −.03, .00 | |||
| Model 1b | ||||||||
| ΔNegative mood | ΔEmotional eating | ΔSweets | .00 | −.15 (.06) | .01 (.02) | .00 (.01) | .00 (.01) | .00 (.01) |
| .968 | .006 | .419 | .439 | .412 | −.01, .01 | |||
| Model 2a | ||||||||
| ΔSelf-regulation-eating | ΔSelf-efficacy-eating | ΔFruit/vegetable | .20 | −.06 (.71) | −.02 (.01) | −.11 (.05) | −.12 (.05) | .00 (.03) |
| .023 | .466 | .032 | .021 | .016 | −.04, .04 | |||
| Model 2b | ||||||||
| ΔSelf-regulation-eating | ΔSelf-efficacy-eating | ΔSweets | .09 | −.06 (.71) | .00 (.01) | −.05 (.03) | −.05 (.03) | .00 (.01) |
| .223 | .466 | .448 | .041 | .043 | −.01, .01 | |||
| Model 3 | ||||||||
| ΔSelf-regulation-exercise | ΔExercise self-efficacy | ΔPhysical activity | .23 | .78 (.26) | .35 (.22) | .93 (.35) | .65 (.38) | .27 (.18) |
| .007 | .003 | .061 | .006 | .050 | .04, .67 | |||
| Model 3 (reciprocal analysis) | ||||||||
| ΔSelf-regulation-exercise | ΔPhysical activity | ΔExercise self-efficacy | .23 | .26 (.09) | .65 (.38) | .52 (.20) | .35 (.22) | .17 (.11) |
| .014 | .003 | .050 | .008 | .061 | .02, .41 | |||
| Changes from month 6 to month 24 | ||||||||
| Model 4a | ||||||||
| ΔNegative mood | ΔEmotional eating | ΔFruit/vegetable | .12 | .24 (.12) | −.07 (.04) | −.05 (.03) | −.03 (.03) | −.02 (.02) |
| .050 | .025 | .040 | .044 | .135 | −.06, .01 | |||
| Model 4b | ||||||||
| ΔNegative mood | ΔEmotional eating | ΔSweets | .30 | .24 (.12) | .06 (.02) | .03 (.01) | .01 (.01) | .01 (.01) |
| .003 | .025 | .002 | .032 | .161 | .01, .03 | |||
| Model 4b (reciprocal analysis) | ||||||||
| ΔNegative mood | ΔSweets | ΔEmotional eating | .31 | .03 (.01) | 3.80 (1.22) | .24 (.12) | .13 (.11) | .11 (.08) |
| .002 | .032 | .002 | .025 | .122 | .03, .30 | |||
| Model 5a | ||||||||
| ΔSelf-regulation-eating | ΔSelf-efficacy-eating | ΔFruit/vegetable | .24 | 2.63 (.75) | .02 (.01) | .15 (.06) | .09 (.07) | .06 (.04) |
| .010 | .001 | .040 | .006 | .084 | .01, .15 | |||
| Model 5a (reciprocal analysis) | ||||||||
| ΔSelf-regulation-eating | ΔFruit/vegetable | ΔSelf-efficacy-eating | .33 | .15 (.06) | 3.88 (2.15) | 2.63 (.75) | 2.03 (.80) | .59 (.41) |
| .001 | .006 | .040 | .001 | .084 | .01, 1.35 | |||
| Model 5b | ||||||||
| ΔSelf-regulation-eating | ΔSelf-efficacy-eating | ΔSweets | .32 | 2.63 (.75) | −.004 (.01) | −.11 (.03) | −.10 (.03) | −.01 (.01) |
| .001 | .001 | .295 | <.001 | .002 | −.05, .01 | |||
| Model 6 | ||||||||
| ΔSelf-regulation-exercise | ΔExercise self-efficacy | ΔPhysical activity | .52 | 1.66 (.28) | .64 (.27) | 2.36 (.46) | 1.30 (.62) | 1.06 (.52) |
| <.001 | <.001 | .012 | <.001 | .022 | .30, 1.99 | |||
| Model 6 (reciprocal analysis) | ||||||||
| ΔSelf-regulation-exercise | ΔPhysical activity | ΔExercise self-efficacy | .58 | 2.36 (.46) | .23 (.10) | 1.66 (.28) | 1.11 (.35) | .54 (.24) |
| <.001 | <.001 | .012 | <.001 | .001 | .19, .99 | |||
| Changes from month 12 to month 24 | ||||||||
| Model 7a | ||||||||
| ΔNegative mood | ΔEmotional eating | ΔFruit/vegetable | .52 | −.12 (.06) | −.04 (.03) | .06 (.01) | .05 (.01) | .01 (.01) |
| <.001 | .022 | .065 | <.001 | <.001 | .00, .02 | |||
| Model 7b | ||||||||
| ΔNegative mood | ΔEmotional eating | ΔSweets | .18 | −.12 (.06) | .03 (.02) | −.01 (.01) | −.01 (.01) | .00 (.00) |
| .039 | .022 | .057 | .022 | .077 | −.01, .00 | |||
| Model 8a | ||||||||
| ΔSelf-regulation-eating | ΔSelf-efficacy-eating | ΔFruit/vegetable | .50 | 1.98 (.72) | .06 (.01) | .08 (.06) | −.04 (.05) | .12 (.06) |
| <.001 | .005 | <.001 | .110 | .216 | .05, .24 | |||
| Model 8a (reciprocal analysis) | ||||||||
| ΔSelf-regulation-eating | ΔFruit/vegetable | ΔSelf-efficacy-eating | .57 | .08 (.06) | 7.80 (1.43) | 1.98 (.72) | 1.36 (.54) | .62 (.54) |
| <.001 | .110 | <.001 | .005 | .008 | −.19, 1.58 | |||
| Model 8b | ||||||||
| ΔSelf-regulation-eating | ΔSelf-efficacy-eating | ΔSweets | .27 | 1.98 (.72) | −.01 (.01) | −.09 (.03) | −.08 (.03) | −.01 (.01) |
| .005 | .005 | .189 | <.001 | .005 | −.03, .01 | |||
| Model 9 | ||||||||
| ΔSelf-regulation-exercise | ΔExercise self-efficacy | ΔPhysical activity | .49 | 1.13 (.21) | .92 (.31) | 1.81 (.42) | .78 (.51) | 1.04 (.51) |
| <.001 | <.001 | .003 | <.001 | .070 | .32, 2.00 | |||
| Model 9 (reciprocal analysis) | ||||||||
| ΔSelf-regulation-exercise | ΔPhysical activity | ΔExercise self-efficacy | .57 | 1.81 (.42) | .23 (.08) | 1.13 (.21) | .71 (.24) | .42 (.22) |
| <.001 | <.001 | .003 | <.001 | .003 | .12, .87 | |||
Analyses were based on a bootstrapping method for mediation incorporating 20,000 resamples (Preacher and Hayes 2008). A reciprocal analysis was conducted when mediation within the initial model was significant
Path a predictor → mediator, Path b mediator → outcome, Path c = predictor → outcome, Path c′ = predictor → outcome, after controlling for the mediator, 95% CI 95% confidence interval, one-tailed test
In each of the above models, increased negative mood significantly predicted more emotional eating (ps < .03), and for intervals where month 24 data were included, increased use of self-regulation significantly predicted greater self-efficacy (see path a [predictor → mediator] in Table 2; models 5a, 5b, 6, 8a, 8b, and 9). Also, for each interval tested, there was a reciprocal, bi-directional relationship between changes in exercise self-efficacy and physical activity (Table 2; see reciprocal analyses in models 3, 6, and 9).
Post hoc analysis
Over the duration of the study, participants’ change in fruit/vegetable intake was significantly (inversely) associated with change in their consumption of sweets, r = −.44, p = .003.
DISCUSSION
The aim of this preliminary study was to re-analyze a subset of existing data from an earlier investigation [18] to determine psychosocial predictors of decays in healthy eating and physical activity after behavioral treatment-induced weight loss occurred. An additional goal was to translate these findings to inform follow-up treatment processes to better sustain lost weight in individuals vulnerable to regain. A subgroup of experimental treatment participants who lost weight and then began a trajectory of substantial regain from months 6 to 24 were assessed. As hypothesized, findings indicated significant unfavorable changes over time in self-regulatory skill usage, self-efficacy, mood, and emotional eating associated with less healthy eating and reduced physical activity soon after the 6-month period of weight loss. Results suggested a need to bolster some participants’ use of self-regulatory skills to maintain healthy behaviors, possibly for years after initial weight loss is attained. The predicted effects of self-regulation on the weight management behaviors of increased fruit/vegetable intake and physical activity outputs were found to be mediated by changes in self-efficacy. Therefore, periodic communications to some participants that not only serve to reinforce previously incorporated self-regulatory skills, but highlight how using these skills can help to overcome challenges and barriers to maintaining their healthy eating and physical activity behaviors, appear to be indicated. Possibly the use of technology will also aid in the reinforcement of self-regulatory skill usage, especially when adherence is challenged. In that manner, lagging feelings of competence (self-efficacy) could be increased through a realization that an enhanced use of self-regulation yields well-earned pay-offs for increasing both healthy behaviors and maintaining, or even furthering, loss in weight. The identified reciprocal relationships between gains in self-efficacy and improved behavioral changes could also be reinforced through such processes.
A significant association between increased negative mood and increased intake if sweets was, as expected, mediated by emotional eating over the length of the study. Emotional and binge eating is often related to excess consumption of sweets and fats [20, 50]. Because a minimum of only two to three moderate bouts of exercise per week has been shown to be associated with significant improvements in both mood [11] and emotional eating [19], treatment methods that were previously incorporated to maximize adherence to exercise (e.g., cognitive restructuring, relapse prevention) [42] might be reviewed with a participant to increase her physical activity to a point where mood is improved. Behavioral methods that were previously used to respond to emotion-based cues to overeating should also be re-addressed within follow-ups for participants in need.
Although the need for long-term follow-up is consistent with previous suggestions [2], evidence-based guidance for the content of those processes has been lacking. In two studies that did suggest targets for promoting long-term weight loss maintenance, the need for increased self-efficacy, self-regulation strategies, control over emotion-cued overeating, and physical activity was in agreement with the present findings [24, 25]. Continued research will be needed to determine optimal modalities, frequencies, and durations of such follow-up methods, especially because many participants might be averse to engaging in lengthy or intensive treatment sessions over the long term. Although some research indicated the possible helpfulness of phone- and/or Internet-based follow-up messages [51, 52], the associated research is in its early stages and the efficacy of those modalities remains unclear [6]. It is also possible that follow-up protocols should be tailored by individual factors (e.g., amount of weight lost and regained, psychological make-up).
In addition to limitations already stated in the research report where these data were derived such as the use of primarily White, middle-age females with class 1 and 2 obesity with possible expectation biases reflected in survey responses [18], the present preliminary study was also of a particularly small subsample. This modest-size sample was largely due to restrictive participant inclusion criteria that were associated with weight change parameters consistent with the aims of this study. Also, men might respond differently than women on changes in the psychosocial variables tested, especially emotional eating [53]. Therefore, replications with larger sample sizes and more objective measures (e.g., accelerometers) are required to increase confidence in findings, and replications across demographic variables and degrees of overweight/obesity are required to assess their generalizability. The present investigation did, however, serve to foster directed inquiry into actionable steps for the mitigation of weight regain through analyses of theory-based psychosocial predictors of eating and physical activity behaviors—primary behavioral correlates of weight change [11].
In summary, research should continue into the implications of psychosocial predictors of decays in healthy eating and physical activity so that the typical pattern of weight loss and regain [1–3] might be improved. Additionally, outcomes of the experimental treatment used within this research [18] might be maximized. Because of the efficiency, scalability, and relative success suggested within the initial application of that protocol [18], improvements in its long-term effects might have a considerable impact on community-based weight management efforts. As suggested by Baranowski, the most positive practical effects in weight management might be derived from the effective translation of behavioral theory to practice [54].
ACKNOWLEDGMENTS
This research was funded, in part, by a grant from Thrivent Foundation to the YMCA of Metro Atlanta.
Compliance with Ethical Standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Conflict of Interest
The author has no conflict of interest.
Informed Consent
Informed consent was obtained from all participants included in the study.
Footnotes
Implications
Practice: Practitioners should expect a decay in healthy eating and physical activity after weight loss in many individuals and plan follow-ups to improve deteriorations in psychological predictors of those behavioral changes such as mood, emotional eating, self-regulation, and self-efficacy.
Researchers: Future research is warranted to further evaluate methods in which decays in post-behavioral intervention eating and physical activity behaviors, that are expected for many individuals, might be improved in order to better-enable sustained losses in weight.
Policymakers: Resources should be focused on scalable evidence-based methods designed to sustain behaviors that are consistent with maintaining loss of weight after behavioral intervention.
REFERENCES
- 1.Mann T, Tomiyama J, Westling E, Lew AM, Samuels B, Chatman J. Medicare’s search for effective obesity treatments: diets are not the answer. American Psychologist. 2007;62:220–233. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- 2.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychology. 2000;19(Suppl):5–16. doi: 10.1037/0278-6133.19.Suppl1.5. [DOI] [PubMed] [Google Scholar]
- 3.Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review of meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute. (1998). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health Publication No. 98-4083.
- 5.Jensen MD, Ryan DH, Donato KA, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22(Suppl 2):S1–S409. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 6.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 7.Kroke A, Liese AD, Schulz M, et al. Recent weight changes and weight cycling as predictors of subsequent two year weight change in a middle-aged cohort. International Journal of Obesity. 2002;26:403–406. doi: 10.1038/sj.ijo.0801920. [DOI] [PubMed] [Google Scholar]
- 8.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper Z, Fairburn CG, Hawker DM. Cognitive-behavioral treatment of obesity: a clinician’s guide. New York: Guilford; 2003. [Google Scholar]
- 10.Cooper Z, Doll HA, Hawker DM, et al. Testing a new cognitive behavioural treatment for obesity: a randomized controlled trial with three-year follow-up. Behaviour Research and Therapy. 2010;48:706–713. doi: 10.1016/j.brat.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annesi JJ. Supported exercise improves controlled eating and weight through its effects on psychosocial factors: extending a systematic research program toward treatment development. Permanente Journal. 2012;16(1):7–18. doi: 10.7812/11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brownell KD. The humbling experience of treating obesity: should we persist or desist? Behaviour Research and Therapy. 2010;48:717–719. doi: 10.1016/j.brat.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Jeffery RW, Levy RL. Overgeneralization from limited data. A commentary on Cooper et al., 2010. Behaviour Research and Therapy. 2010;48:714–716. doi: 10.1016/j.brat.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogelholm M, Kukkomen-Harjula K. Does physical activity prevent weight gain—a systematic review. Obesity Reviews. 2000;1:95–111. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obesity Research. 1998;6(Suppl 2):S51–S209. [PubMed] [Google Scholar]
- 16.Baker CW, Brownell KD. Physical activity and maintenance of weight loss: physiological and psychological mechanisms. In: Bouchard C, editor. Physical activity and obesity. Champaign: Human Kinetics; 2000. pp. 311–328. [Google Scholar]
- 17.Annesi JJ, Marti CN. Path analysis of exercise treatment-induced changes in psychological factors leading to weight loss. Psychology and Health. 2011;26:1081–1098. doi: 10.1080/08870446.2010.534167. [DOI] [PubMed] [Google Scholar]
- 18.Annesi, J. J., Johnson, P. H., Tennant, G. A., Porter, K. J., & McEwen, K. L. (2016). Weight loss and the prevention of weight regain: evaluation of a treatment model of exercise self-regulation generalizing to controlled eating. Permanente Journal, 20(3). Available at http://www.thepermanentejournal.org/issues/2016/summer/6034-weight-loss.html. Accessibility verified February 16, 2016. [DOI] [PMC free article] [PubMed]
- 19.Annesi, J. J., & Vaughn, L. L. (2011). Relationship of exercise volume with change in depression and its association with self-efficacy to control emotional eating in severely obese women. Advances in Preventive Medicine, Article ID 514271. [DOI] [PMC free article] [PubMed]
- 20.Elfag K, Tholin S, Rasmussen F. Consumption of fruits, vegetables, sweets, and soft drinks are associated with psychological dimensions of eating behaviour in parents and their 12-year-old children. Public Health Nutrition. 2008;11:914–923. doi: 10.1017/S1368980008002371. [DOI] [PubMed] [Google Scholar]
- 21.Merrill, R. M., Aldana, S. G., Greenlaw, R. L., Diehl, H. A., Salberg, A., & Englert, H. (2008). Can newly acquired healthy behaviors persist? An analysis of health behavior decay. Preventing Chronic Disease, 5(1). Available at http://www.cdc.gov/pcd/issues/2008/jan/07_0031.htm. Accessibility verified February 17, 2016. [PMC free article] [PubMed]
- 22.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 23.Bandura A. Self-efficacy: the exercise of control. New York: Freeman; 1997. [Google Scholar]
- 24.Kruger J, Blanck HM, Gillespie C. Dietary and physical activity behaviors among adults successful at weight loss maintenance. International Journal of Behavioral Nutrition and Physical Activity. 2006;3:17. doi: 10.1186/1479-5868-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira PJ, Silva MN, Coutinhon SR, et al. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity. 2010;18:725–735. doi: 10.1038/oby.2009.281. [DOI] [PubMed] [Google Scholar]
- 26.Baranowski T, Anderson C, Carmack C. Mediating variable framework in physical activity interventions. How are we doing? How might we do better? American Journal of Preventive Medicine. 1998;15:266–279. doi: 10.1016/S0749-3797(98)00080-4. [DOI] [PubMed] [Google Scholar]
- 27.Galesic M, Bosnjak M. Effects of questionnaire length on participation and indictors of response quality in a web survey. Public Opinion Quarterly. 2009;73:349–360. doi: 10.1093/poq/nfp031. [DOI] [Google Scholar]
- 28.Kristal AR, Beresford SAA, Lazovich D. Assessing change in diet-intervention research. American Journal of Clinical Nutrition. 1994;59(Suppl):S185–S189. doi: 10.1093/ajcn/59.1.185S. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Agriculture. MyPlate and historical food pyramid resources. Available at http://fnic.nal.usda.gov/dietary-guidance. Accessibility verified February 17, 2016.
- 30.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. American Journal of Epidemiology. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 31.Mares-Perlman JA, Klein BEK, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. Journal of Nutrition. 1993;123:489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 32.Godin G. The Godin-Shephard Leisure-time Physical Activity Questionnaire. Health and Fitness Journal of Canada. 2011;4(1):18–22. [Google Scholar]
- 33.Jetté M, Sidney K, Blumchen G. Metabolic equivalents (METs) in exercise testing, exercise prescription and evaluation of functional capacity. Clinical Cardiology. 1990;13:555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 34.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Science. 1985;10:141–146. [PubMed] [Google Scholar]
- 35.Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and Science in Sports and Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using Caltrac accelerometer and five questionnaires. Medicine & Science in Sports & Exercise. 1994;26:376–382. [PubMed] [Google Scholar]
- 37.McNair DM, Heuchert JWP. Profile of Mood States technical update. North Tonawanda: Multi-Health Systems; 2009. [Google Scholar]
- 38.Arnow B, Kenardy J, Agras WS. The Emotional Eating Scale: the development of a measure to assess coping with negative affect by eating. International Journal of Eating Disorders. 1995;18:79–90. doi: 10.1002/1098-108X(199507)18:1<79::AID-EAT2260180109>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 39.Saelens BE, Gehrman CA, Sallis JF, Calfas KJ, Sarkin JA, Caparosa S. Use of self-management strategies in a 2-year cognitive-behavioral intervention to promote physical activity. Behavior Therapy. 2000;31:365–379. doi: 10.1016/S0005-7894(00)80020-9. [DOI] [Google Scholar]
- 40.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. Journal of Consulting and Clinical Psychology. 1991;59:739–744. doi: 10.1037/0022-006X.59.5.739. [DOI] [PubMed] [Google Scholar]
- 41.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Research Quarterly for Exercise and Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 42.McAuley E, Mihalko SL. Measuring exercise-related self-efficacy. In: Duda JL, editor. Advances in sport and exercise psychology measurement. Morgantown: Fitness Information Technology; 1998. pp. 371–390. [Google Scholar]
- 43.Annesi JJ, Unruh JL, Marti CN, Gorjala S, Tennant G. Effects of The Coach Approach intervention on adherence to exercise in obese women: assessing mediation of social cognitive theory factors. Research Quarterly for Exercise and Sport. 2011;82:99–108. doi: 10.1080/02701367.2011.10599726. [DOI] [PubMed] [Google Scholar]
- 44.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 45.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- 46.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American Journal of Epidemiology. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 47.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- 48.Hayes AF. Beyond Barron and Kenny: statistical mediation analysis in the new millennium. Communications Monographs. 2009;76:408–420. doi: 10.1080/03637750903310360. [DOI] [Google Scholar]
- 49.Palmeira AL, Markland DA, Silva MN, et al. Reciprocal effects among changes in weight, body image, and other psychological factors during behavioral obesity treatment: a mediation analysis. International Journal of Behavioral Nutrition and Physical Activity. 2009;6:9. doi: 10.1186/1479-5868-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burton P, Smit HJ, Lightowler HJ. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–197. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Gerber BS, Stolley MR, Thompson AL, Sharp LK, Fitzgibbon ML. Mobile phone text messaging to promote healthy behaviors and weight loss maintenance: a feasibility study. Health Informatics Journal. 2009;15:17–25. doi: 10.1177/1460458208099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obesity Reviews. 2009;11:306–321. doi: 10.1111/j.1467-789X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 53.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. Journal of Clinical Psychiatry. 2004;65:634–651. doi: 10.4088/JCP.v65n0507. [DOI] [PubMed] [Google Scholar]
- 54.Baranowski T. Advances in basic behavioral research will make the most contributions to effective dietary change programs at this time. Journal of the American Dietetic Association. 2006;106:808–811. doi: 10.1016/j.jada.2006.03.032. [DOI] [PubMed] [Google Scholar]


