Abstract
Descriptive morphology of multifocal hepatic cysts found in eight of forty five (17.78 %) Wistar rats sacrificed during pharmacological studies related to herbal formulations was studied. The creamish to white cysts were of varying sizes, ranging from 3–8 mm in diameter. Morphological studies of these cysts depicted the presence of metacestodes of Taenia taeniaeformis i.e. Cysticercus fasciolaris inside them. The scolex of metacestode revealed four suckers and rostellum armed with two distinct rows of characteristic pen knife shaped hooks (characteristics of taeniid cestodes). The average size of large hooks was 392.92 ± 10.12 µ and that of small hooks was 240.64 ± 14.26 µ. The average size of suckers was 304.36 ± 12.33 µ. Histopathology of hepatic tissue surrounding the cysts revealed zones of fatty change, inflammation, granulation tissue and metaplasia. However, the histopathology of stomach and small intestines didn’t show any significant lesions.
Keywords: Cysticercus fasciolaris, Hepatic cysts, Histopathology, Metacestode, Wistar rats
Introduction
Cysticercus fasciolaris is a metacestode stage of the adult tapeworm Taenia taeniaeformis, found in the small intestine of felines and related carnivores (stoats, lynx etc.) and is cosmopolitan in distribution. The intermediate hosts (rodents and occasionally lagomorphs and humans) get infected by consumption of feed or water contaminated with eggs. Thus, the larval stage called as strobilocercus develops in hepatic parenchyma, which acts as infective stage for definitive host (Soulsby 1982). This metacestode in literature has also been referred with various names such as bladderworm, Hydatigera fasciolaris, Strobilocercus fasciolaris and Taenia crassicolis (McCoy 1909; Hsu 1979; Kass et al. 1993). The definitive host i.e. cat and other felines get infected by the consumption of infected intermediate host’s liver (Singla et al. 2009). After the consumption, the wall of the cysts and posterior portion of strobila get digested and the scolex gets attached to the wall of the intestine of the definitive host, which attains maturity within few weeks (Hsu 1979). There are numerous reports and experimental studies related to this metacestode infecting rat, mice (Singla et al. 2008, 2013) and other lagomorphs but certain sporadic cases of zoonoses with C.fasciolaris have been reported from different parts of the world in past, especially in Argentina, Czechoslovakia, Denmark, Taiwan and Sri Lanka (Miyazaki 1991; Ekanayake et al. 1999). The metacestode stage besides causing significant hepatic destruction, also results in fibrosarcomas as well as gastroenteropathy in intermediate host (Kumar et al. 2006; Singla et al. 2008). Therefore, the main objective of this study was an efficient diagnosis based on morphology and histopathological study of liver and gastrointestinal tract of Wistar rats.
Materials and methods
Specimen collection
Three months old forty five Wistar rats were sacrificed for pharmacological studies, following the guidelines of Institutional Animal Ethics Committee. Different body organs of all the rats were examined thoroughly for the presence of cysts or other abnormalities and eight out of forty five rats showed the presence of multiple hepatic cysts, whereas other vital organs of these rats were found unaffected on gross examination. These liver specimens were thus collected and then fixed in 10 % neutral buffered formalin for further examination and studies. Along with liver specimens, the specimens of stomach and duodenum of the affected rats were also collected for histopathological studies.
Morphological studies
Morphological examination involving average number of cysts and size of the cysts was carried out grossly. The cysts were then dissected carefully for detection of presence or absence of the larval stages.
Histopathological processing
Tissue samples from infected livers, containing the cysts of C. fasciolaris, was collected and processed for histopathological studies as per the method of Luna and Lee (1968). The samples of the stomach and duodenum were also processed in similar way. Five micron thick sections were prepared and stained with haematoxylin and eosin stain for the histopathological studies.
Staining of larvae
The cyst wall was dissected carefully for the retrieval of the metacestode, which was then pressed between two slides and then fixed in 10 % neutral buffered formalin. After complete overnight washing, the specimen was stained in borax carmine and then transferred to clearing agent i.e. cedar wood oil and finally mounted in dextrine plasticised xylene (DPX) (Meyer and Oslen 1975). The development of scolices and complete arrangement of the rostellar hooks and suckers was studied as per method described by Meyer and Oslen (1975).
Statistical analysis
Data was analysed by SPSS 16 software and was expressed as mean ± standard deviation.
Results
Morphological studies
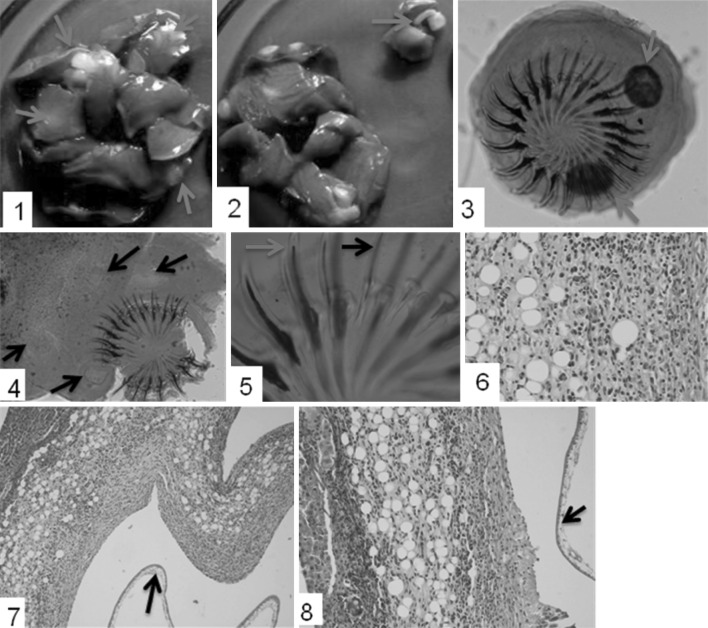
Hepatic parenchyma was mainly involved and multiple cysts, involving all lobes of liver were detected (Fig. 1 1, 2). The cysts were creamish to white in colour and ranged from 3–8 mm in diameter. Results are depicted as under (Table 1).
Fig. 1.
1 Multifocal hepatic cysts (arrows) involving all the lobes. 2 Incised section of the cyst depicting strobilocercus/metacestode (arrow). 3, 4 Mature scolex of metacestode with two distinct rows of large and small hooks with presence of lateral suckers (arrows). 5 Comparative representation of large (blue arrow) and small (black arrow) hooks. 6 Inflammatory reaction around the cyst with eosinophils and fatty changes. H E × 40X. 7, 8 Hepatic lesions possessing fibrous tissue, inflammatory cells and fatty change or metaplasia around the parasitic cyst. There is presence of healthy tissue, zone of inflammation and cyst wall (arrow). H E × 10X. (Color figure online)
Table 1.
Description of various cysts observed in rat liver
| S.N. | Number of rats | Number of cysts | Average relative size of cysts (in mm) (Mean ± S.D.) |
|---|---|---|---|
| 1. | 5 | Less than 10 | Small (3.8 ± 1.08) |
| 2. | 3 | More than 10 | Large (6.7 ± 1.22) |
The cysts with size ranging more than 5 mm showed presence of developed scolices, whereas, most of the cysts smaller than this size showed absence of mature scolices. The mature scolices were large, possessed four lateral suckers. Rostellum was found armed with two distinct rows of pen knife shaped hooks. A total of 42 hooks distinctly large and small (21 each) were present (Fig. 1 3, 4, 5). Mean size of large hooks was 392.92 ± 10.12 µ and that of small hooks 240.64 ± 14.26 µ.
Histopathological studies
The detailed microscopic study on tissue sections revealed fatty changes, extensive infiltration of inflammatory cells (lymphocytes, macrophages and eosinophils), and proliferation of fibrous connective tissue or metaplasia (Fig. 1 6). The lesions were of varying degree and clear zones containing healthy hepatic tissues adjacent to affected areas surrounding the cysts were also observed (Fig. 1 7, 8). Hepatocytes in the affected zones revealed fatty changes. But, there was no evidence of hepatic sarcoma as well as gastroenteropathy contrary to the findings of Al- Jashamy et al. (2004). In addition, the zone of granulation tissue infiltrated with lymphocytes was observed around certain cysts.
Discussion
An incidence of C. fasciolaris infection in Wistar rats was recorded to be 17.78 % (8/45). All the rats used in the study were of the same age group, but showed different grades of infection. Such variation observed may be due to infection at different time intervals. As the rats were kept in completely hygienic conditions thus the possible access of infection to breeder’s colony could be the cause of infection. During gross morphological studies, the number of cysts and their relative sizes were variable among different rats, which could be attributed to the time of exposure to the infection. With less than one month old infection, only few immature cysts without development of scolex targeting few hepatic lobes could develop, whereas in case of the infections older than one month, variable number of cysts ranging more than 10 could be easily detected throughout the liver parenchyma, including all the lobes as described by Hanes (1995). Difference in size of the cysts could be attributed to the time span of the infection as seen in the present study in five rats small immature cysts were present where as in 3 rats large mature cysts were seen. Though in some of the previous studies fibrosarcoma was reported, but in the present study it was not seen. Previous reports indicated that increase in post infection time interval during the developments of the larval stages increases the chances of fibrosarcoma in liver (Al-Jashamy et al. 2004; Armando et al. 2007). Fibrosarcomas may results due to carcinogenic substances released by cysticerci (Dunning and Curtis 1946), or due to a nonspecific foreign body response (Hanes 1995).
In the present study, the rostellum on the scolex contained two rows of characteristic pen knife shaped hooks with equally numbered large and small hooks in all cases, whereas, certain workers (Al-Jashamy and Islam 2007) had reported 30–40 hooks in rostellum of metacestode.
Histopathological studies revealed infiltration of mononuclear cells, which mainly comprised of lymphocytes, macrophages and occasional eosinophils, which is in corroboration with the observation of Singla et al. (2003) and Al-Najjar et al. (2009). But, there were no evidences of hepatic sarcoma as well as gastroenteropathy in the present study. Some of the previous studies revealed that host connective tissue capsule may give rise to sarcomas in older animals typically 12–15 months post-infection (Al-Jashamy and Islam 2007; Al-Najjar et al. 2009). This could be attributed to lower age group i.e. of 3 months of age of Wistar rats. The lesions around the cysts depicting infiltration of mononuclear cells and eosinophils indicated acute nature, whereas, granulation tissue with infiltration of fibroblasts and some lymphocytes indicated chronic nature.
Acknowledgments
Authors are highly obliged to Dr. V.K. Dumka, Professor, Department of Veterinary Pharmacology & Toxicology as well as to Professor- cum- Head, Department of Veterinary Pharmacology and Toxicology for sparing the specimens from the sacrificed animals for the present study.
Contributor Information
Aman Dev Moudgil, Email: moudgil.aman@gmail.com.
Lachhman Das Singla, Email: ldsingla@gmail.com.
References
- Al- Jashamy K, El Salihi K, Sheikh A, Saied H (2004) Cysticercosis in rat infected with C. fasciolaris. In: Proceedings of 9th National conference on medical sciences. University Sciences Malaysia, Kubang Kerian, pp 185
- Al-Jashamy K, Islam MN. Morphological study of Taenia taeniaeformis scolex under scanning electron microscopy using hexamethyldislazane. Ann Microbiol. 2007;7:80–83. [Google Scholar]
- Al-Najjar SS, Kadhimand FS, Abdalrziak NA. Parasitological and Pathological study of the Cysticercus fasciolaris naturally infested white mice. Al Anbar J Vet Sci. 2009;2:43–47. [Google Scholar]
- Armando R, Irizarry-Rovira Alexander W, Matthew B. Taenia taeniaeformis induced metastatic hepatic sarcoma in a pet rat (Rattus norvegicus) J Exot Pet Med. 2007;16:45–48. doi: 10.1053/j.jepm.2006.11.008. [DOI] [Google Scholar]
- Dunning WF, Curtis MR. Multiple peritoneal sarcoma in rats from intraperitoneal injection of washed, ground Taenia larvae. Cane Res. 1946;6:668–670. [PubMed] [Google Scholar]
- Ekanayake S, Warnasuriya ND, Samarakoon PS, Abewickrama H, Kuruppuarachchi ND, Dissanaike AS. An unusual ‘infection’ of a child in Sri Lanka with Taeniataeniaeformis of the cat. Ann Trop Med Parasitol. 1999;93:869–873. doi: 10.1080/00034989957871. [DOI] [PubMed] [Google Scholar]
- Hanes MA. Fibro sarcomas in two rats arising from hepatic cysts of Cysticercusfasciolaris. Vet Pathol. 1995;32:441–444. doi: 10.1177/030098589503200418. [DOI] [PubMed] [Google Scholar]
- Hsu CK. The laboratory rat. In: Baker HG, Lindsey JR, Weisbroth SH, editors. Biology and disease. New York: Academic Press; 1979. pp. 314–315. [Google Scholar]
- Kass PH, Barnes WG, Spangler WL, Chomel BB, Culbertson MR. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. Am Vet Med Assoc. 1993;203:396–405. [PubMed] [Google Scholar]
- Kumar JM, Reddy PL, Aparna V, Srinivas G, Nagarajan P, Venkatesan R, Sreekumar C, Sesikaran B. Strobilocercus fasciolaris infection with hepatic sarcoma and gastroenteropathy in a Wistar colony. J Vet Parasitol. 2006;141:362–367. doi: 10.1016/j.vetpar.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Luna HT, Lee G (1968) Manual of histological staining methods of the armed forces. Institute of pathology. 3rd ed. Plackiston Division McGraw Hill Book co. New York Toronto, London and Sydney
- McCoy GW. A preliminary report of tumors found in wild rats. J Med Res. 1909;21:283. [PMC free article] [PubMed] [Google Scholar]
- Meyer CM, Oslen WO. Essentials of parasitology. Dubuque: W. M. C Brown Company Publishers; 1975. [Google Scholar]
- Miyazaki I. Helminthic zoonoses. Tokyo: International Medical Foundation of Japan; 1991. p. 494. [Google Scholar]
- Singla LD, Singla N, Prasad VR, Sandhu BS, Singh J (2003) Occurrence and pathomorphological observations of Cysticercus fasciolaris in lesser bandicoot rats in India. In: Proceedings of Rats, Mice and People: Rodent Biology and Management. Australian Centre for International Agricultural Research, Canberra, pp 43–46
- Singla LD, Singla N, Parshad VR, Juyal PD, Sood NK. Rodents as reservoirs of parasites in India. Integr Zool. 2008;3:21–26. doi: 10.1111/j.1749-4877.2008.00071.x. [DOI] [PubMed] [Google Scholar]
- Singla LD, Aulakh GS, Sharma R, Juyal PD, Singh J. Concurrent infection of Taenia taeniaeformis and Isospora felis in a stray kitten: a case report. Vet Med. 2009;54(2):81–83. [Google Scholar]
- Singla N, Singla LD, Gupta K, Sood NK. Pathological alterations in natural cases of Capillaria hepatica infection alone and in concurrence with Cysticercus fasciolaris in Bandicota bengalensis. J Parasit Dis. 2013;37(1):16–20. doi: 10.1007/s12639-012-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. London: Baillere Tindall; 1982. [Google Scholar]