Abstract
Schistosomiasis is one of the most prevalent parasitic infections worldwide. Praziquantel is the drug of choice for treatment of schistosomiasis for its high efficacy. The present work was carried out on 160 mice to evaluate the therapeutic effect of mefloquine on experimental schistosomiasis mansoni. Mice were classified into 3 groups; group I (20 infected non-treated mice), group II included 60 infected mice which were further divided into group IIm (20 mice treated with 400 mg/kg mefloquine), group IIp (20 mice treated with 1,000 mg/kg/2 days praziquantel) and group IIpm (20 mice treated with 200 mg/kg mefloquine and 500 mg/kg praziquantel), group III included 80 non-infected mice subdivided into group IIIn (20 non-treated mice), group IIIm (20 mice treated with 400 mg/kg mefloquine), group IIIp (20 mice treated with 1,000 mg/kg/2 days praziquantel), group IIIpm (20 mice treated with 200 mg mefloquine and 500 mg praziquantel). Mefloquine significantly reduced worm burden, tissue egg load, number of liver granulomas and increased the percent of dead ova within granulomas. Combination of mefloquine and praziquantel gave better curative effects than praziquantel or mefloquine given alone.
Keywords: Scistosoma mansoni, Mefloquine, Praziquantel
Introduction
Schistosomiasis has been estimated to infect more than 207 million people with 779 million people at risk of infection (Steinmann et al. 2006). The most important species of Schistosoma that infect humans are S. japonicum, S. mansoni and S. hematobium (Gryseels et al. 2006).
Schistosomiasis results in both acute granulomatous injury and chronic fibrotic injury to the human host’s organs (Smith and Christie 1986). Early detection and treatment of schistosomiasis in humans greatly helps in prevention of late manifestations (Nicolls 2008). The drug widely used in population based morbidity control programs is praziquantel (Utzinger et al. 2011).
Praziquantel does not prevent re-infection and is inactive against juvenile Schistosomes (Stelma et al. 1995). Such juvenile Schistosomes will develop into adults and start laying eggs which means failure of treatment with praziquantel (Sabah et al. 1986).
In some African countries, several Schistosome strains with lower sensitivity to praziquantel have been identified raising the possibility of resistance (Ismail et al. 1999 and Gryseels et al. 2001). A possible way to delay the development of drug resistance is the use of combination therapy (Cui and Su 2009)
Therefore, the effective drug control of schistosomiasis in the future requires the identification of new and effective schistosomicidal compounds (Sanderson et al. 2002).
Mefloquine is an orally administered drug used in treatment and prophylaxis against all forms of malaria (WHO 2010). Therapeutic effects of racemic mefloquine were assessed in Schistosoma mansoni-infected mice by recording worm burden, status of egg maturation and viability and intestinal mast cell recruitment (Van Nassauw et al. 2008). They concluded that mefloquine significantly reduced egg production in Schistosoma mansoni-infected mice, suggesting a therapeutic potency in schistosomiasis therapy and also exerted a significant pro-inflammatory effect on the intestine.
Since large parts of Africa are co-endemic for malaria and schistosomiasis (Keiser and Utzinger 2007), it was recommended to test mefloquine for its potential antischistosomal activities.
Materials and methods
Type of the study: case control study
The study was carried out in Medical Parasitology and Pathology Departments in Faculty of Medicine, Cairo University and Parasitology Department in Theodor Bilharz Research Institute (TBRI).
Experimental animals
Male CD1 Swiss albino mice (n = 160, age = 6 weeks, weight ~45 g), were bred and maintained under conventional conditions (temperature ~25 °C, humidity 70 %) at the experimental animal research unit of Schistosome Biological Supply Program at Theodor Bilharz Research Institute (Giza, Egypt). Animals were fed a standard commercial pelleted diet.
Drugs
Praziquantel tablets (Distocide, EIPICO, El-Asher Men Ramadan, Egypt) were crushed, administered orally as a suspension in 2 % cremophore-E1 (Sigma-Aldrich Chemical Co., St. Louis, MO) (Fallon and Doenhoff 1994) to obtain a stock solution of 50 mg in 100 ml. Praziquantel was given to mice in a dose of 1,000 mg/kg divided in half and given on two consecutive days.
Mefloquine (Mephaquin) tablets were obtained from (Mepha Ltd., Aesch-Basel, Switzerland, lot 0850074) and were administered orally as a fresh suspension in 7 % (v/v) Tween-80 and 3 % (v/v) ethanol in a dose of 400 mg/Kg orally once (Keiser et al. 2009).
Induction of Schistosoma infection
Biomphalaria alexandrina snails were infected by miracidia, left in dechlorinated water under ceiling illumination for 3–5 h at 25–27 °C. The fluid volume was adjusted to contain the desired number of cercariae; 60 ± 10 cercariae/0.2 ml. Mice were infected by S. mansoni cercariae subcutaneously (Peters and Warren 1969). Drugs were given to mice 6 weeks after infection using esophageal tube.
Experimental design
Mice included in this study (n = 160) were classified into 3 groups; group I (infected non-treated, n = 20), group II which was further subdivided into group IIm (infected treated with 400 mg/kg mefloquine, n = 20), group IIp (infected treated with praziquantel 1,000 mg/kg/2 days, n = 20), group IIpm (infected treated with 200 mg/kg mefloquine and 500 mg/kg praziquantel, n = 20), group III(non-infected group) including group IIIn (control group, n = 20), group IIIm(naïve mice treated with 400 mg/kg mefloquine, n = 20), group IIIp (naïve mice treated with 1,000 mg/kg/2 days praziquantel, n = 20), group IIIpm (naïve mice treated with 200 mg/g mefloquine and 500 mg/kg praziquantel, n = 20).
All mice were sacrificed by cervical dislocation under general anaesthesia 8 weeks after infection. Sacrificed mice were decapitated and subjected to porto-mesenteric perfusion to collect adult Schistosomes from hepatic portal and mesenteric veins to assess worm burden (Yolles et al. 1974).
Parasitological study
Small intestines of sacrificed mice were cut into fragments (1 cm in length) and were compressed between a slide and a coverslip. S. mansoni eggs (immature, mature and dead) were counted in 3 fragments to calculate the mean number of eggs in each developmental stage (Pellegrino et al. 1962).
Other fragments of small intestines and livers of sacrificed mice were weighed and incubated in 5 % KOH solution at 37 °C for 24 h to digest tissues. Three samples of the digest (0.25 ml each) were examined using a hemocytometer to calculate the average egg count in the 3 samples (Cheever 1969)
Histopathological study
Liver samples from all groups were fixed in 10 % formalin solution and embedded in paraffin wax. Five sections were taken from each liver, stained with hematoxylin and eosin (Bancroft and Steven 1975) and Masson Trichrome stain (Masson 1929). The prepared slides were microscopically examined to assess the number, diameter (using ocular micrometer), type of granulomas (cellular, fibrous, fibrocellular) and state of egg (intact or degenerated).
Mean granuloma number was calculated by dividing the total number of granulomas by number of fields examined while the mean diameter of liver granuloma was calculated by dividing the sum of vertical and transverse diameters by 2 (Mahmoud and Warren 1974).
Statistical analysis of data
Results were collected, tabulated and statistically analyzed using the statistical package SPSS version 12. Data were tabulated as mean and standard deviation (SD) for quantitative variables and percent for qualitative variables. ANOVA was used to detect significance in the quantitative variables, and P values < 0.05 were considered as statistically significant.
Ethical considerations
The experimental animal studies were managed in accordance with international valid guidelines and animals were maintained under convenient conditions at the animal house in TBRI.
Results
The effects of praziquantel, mefloquine and combined drug administration on worm burden were assessed. The highest decrease in total worm burden was achieved after administration of praziquantel and mefloquine in group IIpm, followed by group IIp (infected treated with praziquantel), then group IIm (infected treated with mefloquine) as shown in Table 1. The difference in total worm burden between infected treated groups (IIp, IIm, IIm) and group I (infected untreated) was statistically significant (p value < 0.001).
Table 1.
Effect of praziquantel, mefloquine and combined treatment on worm burden
| Animal groups | Worm burden (mean ± SD) | Total worm | % of total worm burden reduction | |||
|---|---|---|---|---|---|---|
| Immature worm | Male worm | Female worm | Couple worm | |||
| Group I | 0 | 6.33 ± 2.42 | 2.5 ± 1.22 | 7.5 ± 2.51 | 23.83 ± 4.99 | |
| Group IIm | 0 | 3.28 ± 2.56 | 1.42 ± 1.27 | 0 | 4.71 ± 3.49 | 80.23 |
| Group IIp | 1 ± 2.44 | 0 | 0.33 ± 0.81 | 0 | 2.5 ± 6.12 | 89.51 |
| Group IIpm | 0 | 0 | 0 | 0 | 0 | 100 |
Group I infected untreated, group IIm infected treated with 400 mg/kg mefloquine, group IIp infected treated with praziquantel 1,000 mg/kg/2 days, group IIpm infected treated with 200 mg/kg mefloquine and 500 mg/kg, SD standard deviation
The effect of the tested drugs on oogram pattern in small intestine was assessed as shown in Table 2. In group I, the percentages were 49.6 ± 3.29 % for immature ova, 42.8 ±1.92 % for mature ova and 7.6 ±1.52 % for dead ova. In group IIm, the mean percentages were 1.43 ±3.78 for immature ova, 9.71 ±9.29 % for mature ova and 88.86 ±12.97 % for dead ova. These values were statistically significant (p value < 0.001) when compared to their corresponding values in group I.
Table 2.
Effect of praziquantel, mefloquine and combined treatment on oogram pattern in small intestine
| Animal groups | % Egg developmental stages ± SD | ||
|---|---|---|---|
| Immature ova | Mature ova | Dead ova | |
| Group I | 49.6 ± 3.29 | 42.8 ± 1.92 | 7.6 ± 1.52 |
| Group IIm | 1.43 ± 3.78 | 9.71 ± 9.29 | 88.86 ± 12.97 |
| Group IIp | 2.5 ± 6.12 | 2.33 ± 4.80 | 95 ± 11.29 |
| Group IIpm | 0 | 0 | 100 |
Group I infected untreated, group IIm infected treated with 400 mg/kg mefloquine, group IIp infected treated with praziquantel 1,000 mg/kg/2 days, group IIpm infected treated with 200 mg/kg mefloquine and 500 mg/kg, SD standard deviation
In group IIp, the mean percentages of S. mansoni eggs were 2.5 ±6.12 % for immature ova, 2.33 ±4.80 % for mature ova and 95 ±11.29 % for dead ova with statistical significance (p value < 0.001) when compared to group II. In group IIpm, the mean percentage of immature ova was 0 %, mature ova 0 % and dead ova 100 %. These values were statistically significant (p value < 0.001) when compared to group II. Liver sections of mice included in group III (non-infected) showed no eggs.
The effect of praziquantel, mefloquine and combined drug administration on tissue egg load was assessed by egg count per gram of liver or intestinal tissue as shown in Table 3. In group I, the mean hepatic and intestinal egg load/g tissue were 28,965 ±6,103 and 18,827 ±4,477 respectively and the mean total tissue egg load was 47,792.
Table 3.
Effect of the praziquantel, mefloquine and combined treatment on tissue egg load
| Animal groups | Mean number of ova/g ± SD | Mean total ova count | % Reduction of total ova count | |
|---|---|---|---|---|
| Liver | Intestine | |||
| Group I | 28,965 ± 6,103 | 18,827 ± 4,477 | 47,792 | |
| Group IIm | 14,562 ± 7,928 | 3,082 ±742 | 17,644 | 63.08 |
| Group IIp | 7,463 ± 1,135 | 986 ± 750 | 8,449 | 82.32 |
| Group IIpm | 2,147 ± 463 | 1,061 ± 554 | 3,208 | 93.28 |
Group I infected untreated, group IIm infected treated with 400 mg/kg mefloquine, group IIp infected treated with praziquantel 1,000 mg/kg/2 days, group IIpm infected treated with 200 mg/kg mefloquine and 500 mg/kg, SD standard deviation
In group IIm, the mean hepatic egg load was 14,562 ±7,928 while the intestinal egg load was 3,082 ±742. The mean total tissue egg load was 17,644 with percentage of reduction 63.08 %. In group IIp, the mean hepatic and intestinal egg load/g tissue were 7,463 ±1,135 and 986 ±75 respectively. The mean total tissue egg load was 8449 with percentage of reduction of 82.32 %. In group IIpm, the total tissue egg load was 3,208 (hepatic 2,147 ±463.3; intestinal 1,061 ±554.32) with a percentage of reduction of 93.28 %. All the previous results were statistically significant (p value < 0.001) when compared to group I. Tissue egg load was not shown in mice included in group III.
In group IIIn (normal control mice), hepatocytes were arranged radially in plates in respect to the central vein of each lobule as shown in Fig. 1. No apparent pathological changes were seen in liver sections of mice included in groups IIIm, IIIp and IIIpm following administration of drugs.
Fig. 1.
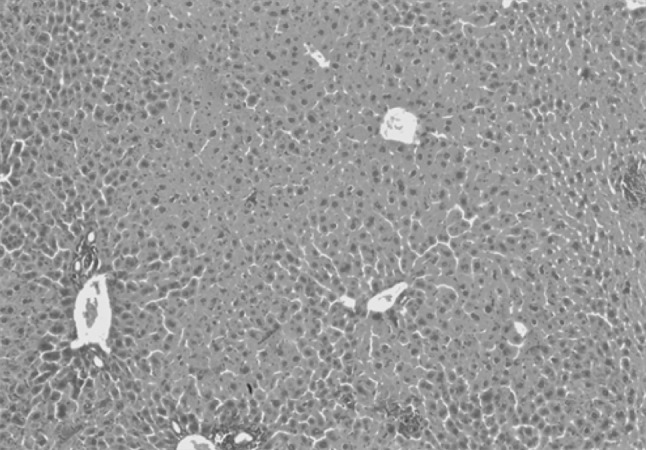
Liver section of group IIIn stained with H&E (×100)
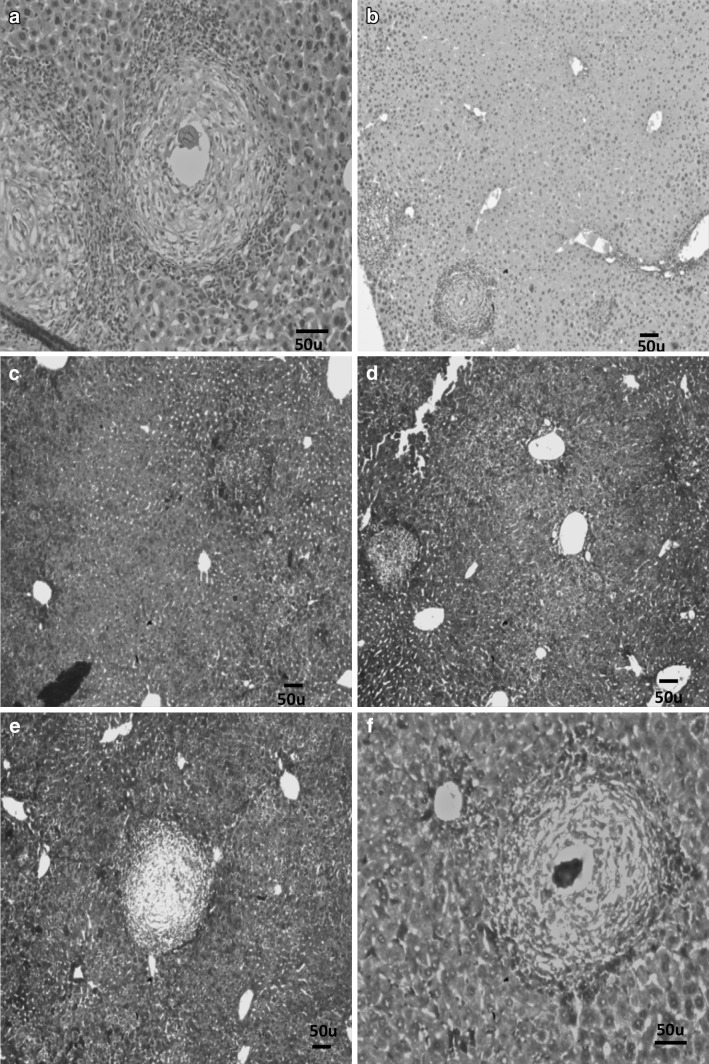
Liver sections of mice included in group I showed worms surrounded by cellular infiltration in portal tracts with many granulomas (75 % fibrocellular and 25 % cellular) as shown in Figs. 2 and 3. The granulomas consisted of intact ova containing miracidium surrounded by histiocytes, lymphocytes, eosinophils, neutrophils, macrophages, plasma cells and fibroblasts. The mean number of granulomas in successive fields (10 × 10), the mean granuloma diameter and percentages of reduction in the mean granuloma diameter detected in liver sections from all groups of mice included in this study are summarized in Table 4. There was a significant reduction in the mean granuloma number and diameters in groups IIm, IIp and IIpm when compared to their corresponding values in group I (p value < 0.001).
Fig. 2.
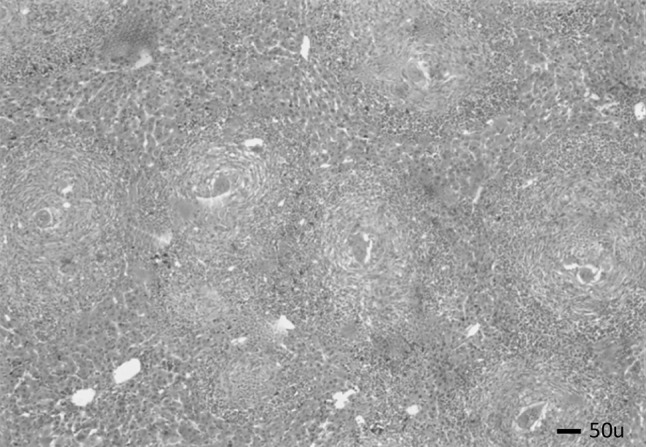
Liver section of group I stained with H&E showing fibrocellular granulomas (×40)
Fig. 3.
a Liver section of group I stained with H&E showing a cellular granuloma (×100), b Liver section of group IIm stained with H&E showing decreased number and diameter of granulomas (×40), c Liver section of group IIm stained with Masson trichrome stain showing decreased number and diameter of granulomas (×40), d Liver section of group IIp stained with H&E showing decreased number and diameter of granulomas (×40), e Liver section of group IIp stained with Masson trichrome stain showing decreased number and diameter of granulomas (×40), f Liver section of group IIpm stained with Masson trichrome stain showing fibrosed granuloma with dead ovum in its centre (×100)
Table 4.
The effect of treatment with mefloquine, praziquantel and combined mefloquine and praziquantel on granuloma number and diameter in mice groups
| Animal groups | Number of granuloma in successive power fields (10 × 10) | % Reduction of number of granulomas | Granuloma diameter in µm | % of reduction of mean granuloma diameter | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Group I | 13 | 4.7 | 427.5 | 97.5 | ||
| Group IIm | 5.7 | 0.3 | 56.15 | 297.5 | 82.5 | 30.41 |
| Group IIp | 4.8 | 0.6 | 63 | 230 | 54.2 | 46.19 |
| Group IIpm | 8.28 | 2.48 | 36.31 | 378 | 43.5 | 11.58 |
Group I infected untreated, group IIm infected treated with 400 mg/kg mefloquine, group IIp infected treated with praziquantel 1,000 mg/kg/2 days, group IIpm infected treated with 200 mg/kg mefloquine and 500 mg/kg, SD standard deviation
There was also a significant increase in percentage of fibrocellular granulomas and percentage of degenerated ova with reduction in percentage of intact ova in the treated groups (p value < 0.001) as demonstrated in Table 5.
Table 5.
The effect of treatment with mefloquine, praziquantel and combined mefloquine and praziquantel on granuloma type and the state of the eggs within the granulomas in the infected groups of mice
| Animal groups | % of types of granuloma | % of the state of eggs | |||
|---|---|---|---|---|---|
| Cellular | Fibrocellular | Fibrous | Intact | Degenerated | |
| Group I | 25 | 75 | 0 | 95 | 5 |
| Group IIm | 12 | 88 | 0 | 3 | 97 |
| Group IIp | 0 | 100 | 0 | 5 | 95 |
| Group IIpm | 0 | 100 | 0 | 15 | 85 |
Group I infected untreated, group IIm infected treated with 400 mg/kg mefloquine, group IIp infected treated with praziquantel 1,000 mg/kg/2 days, group IIpm infected treated with 200 mg/kg mefloquine and 500 mg/kg, SD standard deviation
Discussion
Schistosomiasis, caused by blood flukes of the genus Schistosoma, is an important cause of morbidity and mortality (Gryseels et al. 2006). Several laboratory studies have demonstrated promising antischistosomal properties of mefloquine (Keiser et al. 2011).
In the present study, mefloquine displayed antischistosomal properties with considerable potential for reducing worm burden and hence the mortality of schistosomiasis. Worm burden reduction after mefloquine treatment could be attributed to many factors as interference with worm nutrition. Mefloquine inhibits crystallization of haem into dark brown pigment which is haemozoin (Ncokazi and Egan 2005; Correa Soares et al. 2009). This crystallization constitutes a major detoxification process in Schistosomes acting as a preventive antioxidant defense (Oliveira et al. 2002). Thus, mefloquine can lead to prevention of transport of essential nutrients to the parasite and accumulation of harmful substances inside its body.
The occurrence of ultra-structural degeneration and dilatation of the gut with focal desquamation of the gut epithelium of treated Schistosome worms as reported by (Zhang et al. 2009; Xiao and Zhang 2010) may hinder the efficacy of blood intake by the worms which is essential to complete its development and sexual maturation (Lawrence 1973).
Mefloquine treatment resulted in degenerative tegumental changes of Schistosomes in the form of swelling, ulceration and degenerative change of the suckers affecting their strength. Sluggish fixation of the worms in their place can make them easily swept by the blood stream. Additionally, weak tegumental surface of the treated worms facilitates attack of worms by the host immune cells as schistosomal tegument is one of the most important immune evasion strategies of Schistosoma (Xiao and Zhang 2010; Manneck et al. 2010).
In accordance with our results, a single oral dose of mefloquine exerted significant worm burden reduction in hamsters infected with S. japonicum at various intervals post-infection as reported by Xiao et al. (2009).
Administration of mefloquine in a dose of 400 mg/kg caused a reduction in the number of total mature by 67.7 % with an 80.3 % reduction in the number of mature females (El-Lakkany et al. 2011). Nwaka and Hudson (2006) found a greater than 80 % reduction in worm burden using the adult S. mansoni model and five consecutive mefloquine doses given intraperitoneally or subcutaneously. Keiser et al. (2009) showed that a single dose of mefloquine (200 or 400 mg/kg) administered orally to mice infected with either young developing or adult S. mansoni (Liberian strain) resulted in a high or complete reduction of the total and female worm burden.
Keiser et al. (2011) stated that treatment of S. mansoni-infected mice with 50, 100 or 200 mg/kg mefloquine resulted in total worm burden reductions of 44.1, 64.0 and 93.4 % respectively.
Similarly, single oral doses of 200 or 400 mg/kg of mefloquine administered to mice infected with S. mansoni and S. japonicum resulted in high or complete total and female worm burden reduction of 72.3–100 %. Single oral dose of 200 mg/kg mefloquie given to S. mansoni infected mice achieved total worm burden reduction of 80.4 % (Keiser et al. 2010b).
Similar results were obtained in a randomized, exploratory open-label trial (Keiser et al. 2010a). The authors reported high cure rates of 61 % and egg-reduction rates of >95 % of S. haematobium infected children.
A statistically significant total and female worm burden reductions ranging between 65.4 and 100 % were achieved by Manneck et al. (2010) and Manneck et al. (2011) which strengthened the evidence of the anti-schistosomal properties of mefloquine derivatives on S. mansoni infected mice.
On the other hand, administration of mefloquine 100 mg/kg combined with praziquantel 100 mg/kg made a statistically significant difference of female worm burdens between praziquantel group and combined treatment group only (Xiao et al. 2010).
A single oral dose of mefloquine at 150 mg/kg to S. mansoni infected mice had no effect on the worm burden (Van Nassauw et al. 2008). Similarly, there was no significant reduction of total and female worm burdens following administration of single oral dose of 100 mg/kg mefloquine given to S. japonicum infected mice as reported by Xiao et al. (2011). This may be attributed to the lower doses of the drug and hence the weak effect.
There was a statistically significant reduction in worm burden following combined treatment with praziquantel and mefloquine (100 % reduction and p value < 0.001) indicating the synergistic effect when combined treatment with both drugs is given to infected mice.
Similar results were reported when mefloquine and praziquantel were administered simultaneously at doses of 50 mg/kg each, low total (25.9 %) and female (29.0 %) worm burden reductions were observed, which were even lower than worm burden reductions observed with mefloquine (50 mg/kg) alone. When both drugs were given at 100 mg/kg simultaneously, high total and female worm burden reductions of 86.0 and 86.9 % respectively were reported by Keiser et al. (2011).
Regarding the oogram pattern, the antischistosomal chemotherapy is considered effective against S. mansoni infection when the oogram pattern shows disappearance of about 50 % or more of the mature ova or increased the percentage of dead ova or if there was complete absence of one or more of the immature stages (Pellegrino et al. 1962).
In the present work, mefloquine therapy caused a significant increase in the percentages of dead eggs. The mechanism underlying the reduction in egg number may be related to interference with the reproductive ability of the Schistosomes in addition to interference with the excretion of metabolic wastes of adult worms (Van Nassauw et al. 2008).
A significant reduction in the number of eggs in the early developmental stages following treatment of S. mansoni infected mice with single oral dose of 150 mg/kg of mefloquine 8 weeks post-infection was recorded by Van Nassauw et al. (2008).
Our findings were similar to El-Lakkany et al. (2011) who reported that mefloquine in doses of 200 and 400 mg/kg caused a significant increase in the percentages of dead eggs to 67.25 and 84.29 % respectively in S. mansoni infected mice.
The significant increase in the percentage of dead ova following praziquantel treatment reported in our study was similar to El-Lakkany et al. (2011). The authors stated that treatment of schistosomiasis mansoni with 200 and 1,000 mg/kg of praziquantel caused a significant decrease in percentages of dead ova to be 84.38 and 96.13 % respectively.
Treatment of S. mansoni infected mice with single oral dose of 200 mg/kg of mefloquine and 500 mg/kg of praziquantel resulted in a significant increase in percentage of dead eggs to be 100 % in the present study. Similarly, there were complete loss of immature eggs and increase in the percentages of dead eggs to be 94.22 and 99.25 % in the groups treated with praziquantel and mefloquine in low and high doses respectively (El-Lakkany et al. 2011).
Schistosomiasis causes histopathological changes in the liver of the host (Sadun and Williams 1966; Cheever and Andrade 1967). Schistosome eggs are deposited in the pre-sinusoidal spaces of the liver inducing a granulomatous inflammatory reaction around these eggs (Andrade 1965; Dunn et al. 1979; El-Meneza et al. 1989).
In the present work, mefloquine treatment caused a statistically significant reduction in the granulomas diameter (30.41 %) while praziquantel reduced the granuloma diameter by 46.19 %. In accordance with these results, El-Lakkany et al. (2011) stated that treatment with mefloquine or praziquantel alone reduced the diameters of the granulomas by 26.26 and 28.08 % respectively.
In our study, combined treatment with mefloquine and praziquantel caused significant reduction of the granulomas diameter. In agreement with our results, a significant reduction in the granuloma diameters following treatment with low and high doses mefloquine and praziquantel was reported by El-Lakkany et al. (2011).
Tissue granuloma around Schistosoma eggs is a delayed hypersensitivity reaction induced by egg-secreted soluble antigens (Warren and Boros 1975). Elimination of parasites following specific treatment of schistosomiasis causes a slight reduction in hepatic fibrosis (Homeida et al. 1991).
Thus, the small sized granulomas detected in liver sections of treated mice can be explained by the loss of the ability of the eggs to induce inflammatory reaction. The treated worms lay poorly developed eggs with reduced or even no ability to induce granuloma formation or (Warren 1996).
Decreased number of the granulomas was in accordance with diminished egg counts in liver and intestinal tissues and could be attributed to the elimination of adult worms by drug administration and consequent decreased egg deposition leading to sustained diminution of eggs induced immunopathology (Pearce 2005).
Morcos et al. (1985) demonstrated that praziquantel treatment resulted in partial reversal of liver fibrosis in S. mansoni infected mice. Improvements and/or resolutions of schistosomal-induced periportal thickening/fibrosis in praziquantel treated models have also been demonstrated by (Frenzel et al. 1999; Berhe et al. 2008). It is theorized that the beneficial effects are likely related to the clearance of schistosomal worms and subsequent reduction of egg deposition. Praziquantel is believed to exert antifibrotic effects by affecting (decreasing) activation of hepatic stellate cells through inhibition of profibrotic gene expression (Liang et al. 2011).
References
- Andrade ZA. Hepatic schistosomiasis morphological aspects. In: Propper H, Schifner F, editors. Progress in liver disease. New York: Grune and Stratton; 1965. pp. 228–242. [PubMed] [Google Scholar]
- Bancroft JD, Steven A, editors. Histopathological stains and their diagnostic uses. Edinburgh: Churchill Living Stone; 1975. pp. 7–46. [Google Scholar]
- Berhe N, Myrvang B, Gundersen SG. Reversibility of schistosomal periportal thickening/fibrosis after praziquantel therapy: a twenty-six month follow-up study in Ethiopia. Am J Trop Med Hyg. 2008;78:228–234. [PubMed] [Google Scholar]
- Cheever AW. Quantitative comparison of the intensity of Schistosoma mansoni infection in man and experimental animals. Trans R Soc Trop Med Hyg. 1969;63:781–792. doi: 10.1016/0035-9203(69)90122-9. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Andrade ZA. Pathological lesions associated with S. mansoni infection in man. Trans R Soc Trop Med Hyg. 1967;61:626–639. doi: 10.1016/0035-9203(67)90125-3. [DOI] [PubMed] [Google Scholar]
- Correa Soares JB, Menezes D, Vannier-Santos MA, Ferreira-Pereira A, Almeida GT, Venancio MT. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PloS Negl Trop Dis. 2009;3(7):1–16. doi: 10.1371/journal.pntd.0000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Su XZ. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther. 2009;7:999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Kamel R, Kamel IA, Biampica L, El-Kholy A, Hait PK. Liver collagen synthesis in schistosomiasis mansoni. J Gastroenterol. 1979;76:978–982. [PubMed] [Google Scholar]
- El-Lakkany NM, Seif el-Din SH, Sabra AA, Hammam OA (2011) Pharmacodynamics of mefloquine and praziquantel combination therapy in mice harboring juvenile and adult Schistosoma mansoni. Mem Inst Oswaldo Cruz Rio J 106(7):814–822 [DOI] [PubMed]
- El-Meneza SE, Olds GR, Kresina TF, Mahmoud AAF. Dynamics of hepatic connective tissue matrix constituents during murine S. mansoni infection. Hepatology. 1989;9:50–56. doi: 10.1002/hep.1840090108. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis, resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- Frenzel K, Grigull L, Odongo-Aginya E, Ndugwa CM, Loroni-Lakwo T, Schweigmann U, Vester U, Spannbrucker N, Doehring E. Evidence for a long-term effect of a single dose of praziquantel on Schistosoma mansoni-induced hepatosplenic lesions in northern Uganda. Am J Trop Med Hyg. 1999;60:927–931. doi: 10.4269/ajtmh.1999.60.927. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Mbaye A, de Vlas SJ, Stelma FF, Guisse F. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infection in Senegal due to resistance? An overview of the evidence. Trop Med Int Health. 2001;6:864–873. doi: 10.1046/j.1365-3156.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Homeida MA, Tom I, Nash T, Bennett JL. Association of the therapeutic activity of praziquantel with the reversal of Symmers’ fibrosis induced by Schistosomamansoni. Am J Trop Med Hyg. 1991;45:360–365. doi: 10.4269/ajtmh.1991.45.360. [DOI] [PubMed] [Google Scholar]
- Ismail M, Botros S, Metwally A, William S, Farghally A. Resistance to praziquantel: direct evidence for Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr Opin Infect Dis. 2007;20:605–612. doi: 10.1097/QCO.0b013e3282f19ec4. [DOI] [PubMed] [Google Scholar]
- Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J. Mefloquine: an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3(1):1–11. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, N’Guessan NA, Adoubryn KD, Silué KD, Vounatsou P, Hatz C. Efficacy and safety of mefloquine, artesunate, mefloquine–artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50(9):1205–1213. doi: 10.1086/651682. [DOI] [PubMed] [Google Scholar]
- Keiser J, Vargas M, Doenhoff MJ. Activity of artemether and mefloquine against juvenile and adult Schistosoma mansoni in athymic and immunocompetent NMRI mice. Am J Trop Med Hyg. 2010;82(1):112–114. doi: 10.4269/ajtmh.2010.09-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Manneck T, Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J Antimicrob Chemother. 2011;66(8):1791–1797. doi: 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
- Lawrence JD. The ingestion of red blood cells by Schistosoma mansoni. J Parasitol. 1973;59:60–63. doi: 10.2307/3278572. [DOI] [PubMed] [Google Scholar]
- Liang YJ, Luo J, Yuan Q, Zheng D, Liu YP, Shi L, Zhou Y, Chen AL, Ren YY, Sun KY, Sun Y, Wang Y, Zhang ZS. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One. 2011;6:e20247. doi: 10.1371/journal.pone.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AEF, Warren KS. Anti-inflammatory effects of tartar emetic and niridazole: suppression of Schistosome egg granuloma. J Immunol. 1974;112:222–228. [PubMed] [Google Scholar]
- Manneck T, Haggenmüller Y, Keiser J. Morphological effects and tegumental alterations induced by mefloquine on Schistosomula and adult flukes of Schistosoma mansoni. Parasitology. 2010;137(1):85–98. doi: 10.1017/S0031182009990965. [DOI] [PubMed] [Google Scholar]
- Manneck T, Braissant O, Ellis W, Keiser J. Schistosoma mansoni: antischistosomal activity of the four optical isomers and the two racemates of mefloquine on Schistosomula and adult worms in vitro and in vivo. Exp Parasitol. 2011;127(1):260–269. doi: 10.1016/j.exppara.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Masson P (1929) Some histological methods. Trichrome stain and preliminary technique. Bull intern Assoc Med 12:75
- Morcos SH, Khayyal MT, Mansour MM, Saleh S, Ishak EA, Girgis NI, Dunn MA. Reversal of hepatic fibrosis after praziquantel therapy of murine schistosomiasis. Am J Trop Med Hyg. 1985;34:314–321. doi: 10.4269/ajtmh.1985.34.314. [DOI] [PubMed] [Google Scholar]
- Ncokazi KK, Egan TJ. A Colorimetric high-throughput beta-haematin inhibition screening assay for use in the search of antimalarial compounds. Anal Biochem. 2005;338:306–319. doi: 10.1016/j.ab.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Nicolls DJ. Characteristics of schistosomiasis in travelers reported to the GeoSentinel Surveillance Network 1997–2008. Am J Trop Med Hyg. 2008;79:927. [PubMed] [Google Scholar]
- Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- Oliveira MF, Timm BL, Machado EA, Miranda K, Attisa M. On the pro-oxidant effects of haemozoin. FEBS Lett. 2002;512:139–144. doi: 10.1016/S0014-5793(02)02243-3. [DOI] [PubMed] [Google Scholar]
- Pearce EJ. Priming of the immune response by Schistosome eggs. Parasite Immunol. 2005;27(7–8):265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- Pellegrino J, Oliveira CA, Faria J, Cumha AS. New approach to the screening of drugs in experimental Schistosoma mansoni in mice. Am J Trop Med Hyg. 1962;11:201–215. doi: 10.4269/ajtmh.1962.11.201. [DOI] [PubMed] [Google Scholar]
- Peters AP, Warren KS. A rapid method of infecting mice & other laboratory animals with Schistosoma mansoni subcutaneous injection. J Parasitol. 1969;55:558–563. doi: 10.2307/3277297. [DOI] [Google Scholar]
- Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- Sadun EH, Williams JS. Biochemical aspects of schistosomiasis mansoni in mice in relation to worm burdens and duration of infection. Exp Parasitol. 1966;18:266–273. doi: 10.1016/0014-4894(66)90025-7. [DOI] [Google Scholar]
- Sanderson L, Bartlett A, Whitfield PJ. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult Schistosomes and their egg production. J Helminthol. 2002;76:241–247. doi: 10.1079/JOH2002116. [DOI] [PubMed] [Google Scholar]
- Smith JH, Christie JD. The pathobiology of Schistosoma haematobium infection in humans. Hum Pathol. 1986;17:333–345. doi: 10.1016/S0046-8177(86)80456-7. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systemic review, meta-analysis and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Stelma FF, Talla I, Sow S, Kongs A, Niang M, Polman K. Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am J Trop Med Hyg. 1995;53:167–170. doi: 10.4269/ajtmh.1995.53.167. [DOI] [PubMed] [Google Scholar]
- Utzinger J, N’Goran KE, Caffrey CR, Keiser J. From innovation to application: social–ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl. 1):121–137. doi: 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Van Nassauw L, Stephen T, Joeri V, Jean-Pierre T, Jozef V. Schistosomicidal activity of the antimalarial drug, mefloquine, in Schistosoma mansoni-infected mice. Travel Med Infect Dis. 2008;6(5):253–258. doi: 10.1016/j.tmaid.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Warren KS. The pathogenesis of clay-pipe-stem cirrhosis in mice with chronic schistosomiasis. Am J Path. 1996;49:477–489. [PMC free article] [PubMed] [Google Scholar]
- Warren KS, Boros DL (1975) The Schistosome egg granuloma: a form of cell-mediated immunity. In: Furth RV (ed) Mononuclear phagocytes in immunity, infection and pathology, vol 92. Blackwell Scientific Publications, Oxford, p 112
- World Health Organization (2010) Guidelines for the treatment of malaria, 2nd edn. WHO Library Cataloguing-in-Publication Data
- Xiao SH, Zhang CW. Further observation on histopathological alterations of adult Schistosoma japonicum harbored in mice following treatment with mefloquine at a smaller single dose. Parasitol Res. 2010;107(4):773–781. doi: 10.1007/s00436-010-1928-5. [DOI] [PubMed] [Google Scholar]
- Xiao SH, Mei JY, Jiao PY. Further study on mefloquine concerning several aspects in experimental treatment of mice and hamsters infected with Schistosoma japonicum. Parasitol Res. 2009;106(1):131–138. doi: 10.1007/s00436-009-1640-5. [DOI] [PubMed] [Google Scholar]
- Xiao SH, Xue J, Shen B. Tegumental alterations of adult Schistosoma japonicum harbored in mice treated with a single oral dose of mefloquine. Chin J Parasitol Parasit Dis. 2010;28(1):1–7. [PubMed] [Google Scholar]
- Xiao SH, Mei JY, Jiao PY. Effect of mefloquine administered orally at single, multiple, or combined with artemether, artesunate or praziquantel in treatment of mice infected with Schistosoma japonicum. Parasitol Res. 2011;108:399–406. doi: 10.1007/s00436-010-2080-y. [DOI] [PubMed] [Google Scholar]
- Yolles TK, Moore DV, De Guisti D, Ripsam CL, Meleney HE. A technique for perfusion of laboratory animals for the recovery of Schistosomes. J Parasitol. 1974;33:419–426. doi: 10.2307/3273678. [DOI] [PubMed] [Google Scholar]
- Zhang CW, Xiao SH, Utzinger J, Chollet J, Keiser J, Tanner M. Histopathological changes in adult Schistosoma japonicum harbored in mice treated with a single dose of mefloquine. Parasitol Res. 2009;104(6):1407–1416. doi: 10.1007/s00436-009-1341-0. [DOI] [PubMed] [Google Scholar]