Abstract
Neurocysticercosis is an important parasitic disease of the central nervous system and constitutes a public health challenge for most of the developing world. Radiological findings may be quite variable. A 50 year old man presented with recent onset generalized tonic–clonic seizures. CT scan revealed a lobulated cystic lesion in the right fronto-temporal lobe compressing the lateral and third ventricle and upper brainstem. Clinico-radiological diagnosis of right fronto-temporal space occupying lesion with possibility of cystic glioma was made. He underwent craniotomy with cyst decompression and excision of cyst wall. Histopathology showed features of Racemose variant of Neurocysticercosis. This is an uncommon variant of neurocysticercosis characterized by extraparenchymal involvement, an aggressive clinical course and requiring surgical management. This case is being presented because of its rare occurrence and potential diagnostic difficulties on clinico-radiological grounds.
Keywords: Racemose, Neurocysticercosis, Variant, Radiology
Introduction
Cysticercosis is a zoonosis caused by larval stage of Taenia solium and is endemic in India. Human cysticercosis result from fecal to oral contamination with taenia solium eggs from human tapeworm carrier (Mittal and Mittal 2011).
Nervous system is commonly involved in 60–90 % cases and is called Neurocysticercosis (NCC). NCC most commonly infects the brain parenchyma. However, it may occur in extraparenchymal locations like subarachanoid, cisternal, intraventricular or may be mixed, and are usually of racemose type. This variant of NCC is rarely seen in India (Sharma et al. 2013).
Racemose NCC is often difficult to diagnose on computed tomography (CT) as the cyst membrane is thin and the fluid is isodense with the cerebrospinal fluid, it may present with only subtle asymmetry of ventricles or enlargement of cisterns. Uninflamed extraparenchymal cysticerci may not be visible on CT. MRI is an accurate technique to assess the degree of infection, the location, and the evolutionary stage of the parasites. However, it may also sometimes, only reveal subtle indirect findings (Mittal and Mittal 2011; Garcia et al. 2003, 2002).
Racemose NCC differs from more common cysticercus cellulose in being larger and multiloculated cystic lesions and usually lacks a scolex. The racemose variant forms a large translucent vesicle, several vesicles may aggregate together to form appearance of bunch of grapes (hence the name). As vesicles grow, they may also give an infiltrative appearance (Sharma et al. 2013).
Absolute diagnostic criteria, includes histological demonstration of the parasite, cystic lesions with scolex on neuroimaging, or direct visualisation of cysts (Garcia et al. 2002; Del Brutto et al. 2001).
Racemose NCC have higher morbidity and mortality because of parasite growth, intracranial hypertension, arachnoiditis, blockage of cerebrospinal-fluid pathways, hydrocephalus, and other complications (Garcia et al. 2003).
The case is presented because of rarity of racemose variant of neurocysticercosis, especially in India. The potential diagnostic dilemma due to a variety of clinical presentations depending on its location as observed on imaging studies, and the importance of pathological examination in providing diagnosis in such cases.
Case report
A 50 year old man presented with chief complaints of episodes of generalized tonic–clonic seizure for 6 days. He gave history of weakness of both lower limbs, difficulty in speech and headache of 8 days duration. There was no history of vomiting or any visual complaints preceding the onset of symptoms. He was vegetarian by diet. General physical examination was normal. Fundus examination did not show papilledema. Glasgow coma scale on admission was E4V5M6. The hemogram and biochemical parameters were within normal limits.
Contrast enhanced CT scan of brain showed a lobulated cystic lesion measuring 7.7 × 6 × 5.4 cm in right fronto-temporal lobe with calcified focus in its wall. Lateral ventricle, third ventricle and upper brainstem were compressed. Midline shift was seen towards left side. Basal cisterns were partially defined. Cerebellum, fourth ventricle, sellar and parasellar regions were normal. A possibility of cystic glioma was made, with differential diagnosis of arachanoid cyst or porencephalic cyst. (Figure 1).
Fig. 1.
CT scan showing lobulated CSF density like lesion
He underwent right frontal craniotomy with decompression of cyst. The cyst wall was excised and sent for histopathological examination.
Pathological findings
Grossly, multiple greyish white, membranous tissue bits with few fluid filled vesicular structures all together measuring 4 × 3 × 0.5 cm.
Microscopic examination showed a small area of poorly preserved glial tissue and ventricular lining epithelium with adjacent parasitic cyst. The cyst wall showed a cuticle layer and loose myxoid layer. However, scolex could not be appreciated (Fig. 2). Aggregates of lymphocytes and area of hemorrhage were seen. A diagnosis of Racemose variant of Neurocysticercosis was made in correlation with clinico-radiological findings.
Fig. 2.
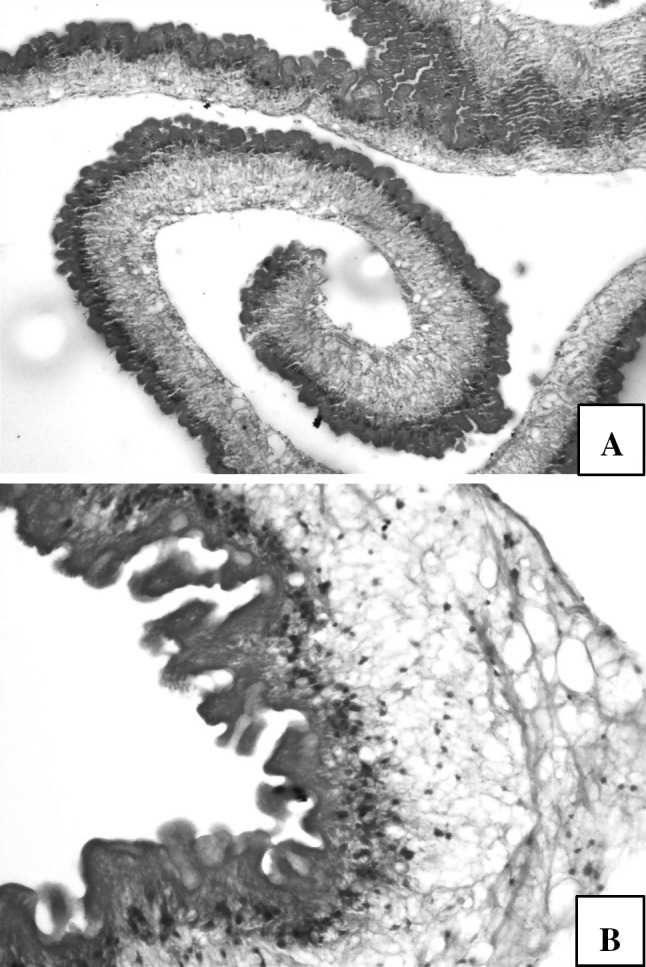
a Cyst wall (H&E, × 100) b Outer cuticular, middle loose myxoid layer and inner layer with invaginations (H&E, × 400)
The post-operative period was uneventful and he was given a course of albendazole for 2 weeks and started on antiepileptics. Repeat CT scan 1 month following surgery revealed encephalomalacia with peripheral gliosis in right frontal region, causing prominence of right frontal horn with confluent hypodense area in right perifrontal horn region, suggesting post-operative sequel. He is currently on regular follow up and is asymptomatic.
Discussion
Cysticercus racemose was first described by Virchow in 1860. The racemose form of neurocysticercosis is a collection of transient membranes forming a cluster like a bunch of grapes, thought to be a forme fruste of cysticercus cellulosae. Few authors consider that it may be due to a different variety of cestode. viz. sterile coenurus of Taenia multiceps, or Taenia serialis, or an aberrant cysticercus of Taenia solium (Ghosh et al. 1999).
Epileptic seizures are the commonest presentation of NCC, as in the present case and may represent the primary or sole manifestation of the disease. In endemic regions, recent onset of seizures in otherwise healthy teenage, young adult, or middle-aged individuals strongly suggests NCC (Garcia et al. 2002).
After entering the central nervous system, cysticerci are viable and elicit few inflammatory changes in the surrounding tissues. Cysticerci may remain for a long time in this stage, protected by the blood–brain barrier and active immune-evasion mechanisms by the cysticerci. After a variable and unknown time the parasite degenerates with associated immune-mediated inflammation. Cysticerci cause symptoms because of mass effect or by blocking the circulation of cerebrospinal fluid, but most symptoms in NCC are the direct result of the inflammatory process that accompanies cyst degeneration. The clinical manifestations depend on load, type, size, location, stage of development of the cysticerci, and the host’s immune response against the parasite (Garcia et al. 2003).
Neurocysticercosis has been classified according to location into subarachnoid-cisternal, parenchymal, intraventricular, and spinal forms. On the basis of clinical presentation and imaging findings, it is classified into active and nonactive forms. Noncystic (non-active) NCC is asymptomatic with negative imaging findings. However, in vesicular NCC (active), a fully grown cyst or a cluster of cysts called racemose form is frequently found at the subarachnoid space near the gray matter–white matter junction or in the basal ganglia, cerebellum, brainstem, cisterns, or ventricular system (Kimura-Hayama et al. 2010).
Imaging findings vary with development stage of NCC or host response. On the basis of radiologic findings, NCC is divided into five stages: noncystic, vesicular, colloidal vesicular, granular nodular, and calcified nodular. However, such evolution of cyst is not seen in racemose NCC (Sharma et al. 2013).
CT findings that may be helpful in differentiating between cellulose and racemose cysts are: cellulose cysts are located intra-axially or parenchymal, while racemose cysts are frequently seen in basal cisterns, particularly suprasellar area as in the present case or cerebellopontine angle. These may frequently present as space occupying lesions or may appear as multiloculated cysts resembling “bunches of grapes” (Garcia et al. 2003).
CT has been claimed to have sensitivity and specificity of over 95 % for the diagnosis of NCC, although CT images are rarely pathognomonic for this disease. The differential diagnosis of parenchymal neurocysticercosis is tuberculosis, mycosis, toxoplasmosis, abscess, early glioma, Arteriovenous malformation and metastasis which present as enhancing lesions. Racemose cysticercosis commonly presents as single or multiple non-enhancing cystic lesions, and needs to be differentiated from hydatid disease, arachnoid cysts, porencephaly, cystic astrocytoma and colloid cyst of third ventricle. (Garcia et al. 2003, 2002).
Subarachanoid and intraventricular racemose cyst show typical budding of the vesicular wall on gross inspection. Microscopic findings are typical and diagnostic of racemose variant of NCC. It is characterized by abnormal growth of cystic membranes followed by degeneration of scolex. The proliferating connective tissue encircles the characteristic bladder wall of the parasite, as was observed in the present case. As the cyst degenerates granulomatous ependymitis and gliosis are the only residual histological findings (Ghosh et al. 1999).
Therapeutic measures include antiparasitic drugs, surgery, and symptomatic medications. As inflammation is the conspicuous accompaniment corticosteroids represents the primary form of therapy. Albendazole is considered the antiparasitic drug of choice for NCC because of its better penetration into CSF and because its concentration is not affected when administered along with corticosteroids (Garcia et al. 2002).
Extra-parenchymal cysticercosis is associated with poor prognosis and requires aggressive approach. Surgery plays an important role in the management of these cases. Cyst extirpation and drainage is indicated for racemose cysticercosis (Garcia et al. 2003).
In conclusion, Racemose variant of NCC is a rare presentation, especially in India. It has an aggressive behavior compared to intraparenchymal lesions. Histopathological demonstration of the parasite confirms the disease. Therefore, it should be kept in the clinico-radiological differential diagnosis of extraparenchymal cystic space occupying lesions of the brain.
References
- Del Brutto OH, Rajshekhar V, White AC, Tsang VCW, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–183. doi: 10.1212/WNL.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Evans CAW, Nash TE, Takayanagui OM, White C, Botero D, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002;15:747–756. doi: 10.1128/CMR.15.4.747-756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Gonzalez AE, Evans CAW, Gilman RH. Taenia solium cysticercosis. Lancet. 2003;16:547–556. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Dubey TN, Prabhakar S. Brain parenchymal, subarachanoid racemose, and intraventricular cysticercosis in an Indian man. Postgrad Med J. 1999;75:164–167. doi: 10.1136/pgmj.75.881.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Hayama ET, Higuera JA, Corona- Cedillo R, Chavez-Maciis L, Perochena A, Quiroz-Rojas L, et al. Neurocysticercosis: radiologic-pathologic correlation. RadioGraphics. 2010;30:1705–1719. doi: 10.1148/rg.306105522. [DOI] [PubMed] [Google Scholar]
- Mittal P, Mittal G. Intraventricular and subarachanoid racemose cysticercosis. Trop Parasitol. 2011;1(2):111–112. doi: 10.4103/2229-5070.86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Modi M, Lal V, Prabhakar S, Bhardwaj A, Sehgal R. Reversible dementia as a presenting manifestation of racemose neurocysticercosis. Ann Indian Acad Neurol. 2013;16:88–90. doi: 10.4103/0972-2327.107706. [DOI] [PMC free article] [PubMed] [Google Scholar]



