Abstract
The coproculture study on Strongyle infection of goats was carried out in small holder farmers kept under semi-intensive management system in Balaghat, Narsinghpur and Chhindwara district, Madhya Pradesh, during the period from July 2011 to February 2012. Copro-culture of the samples positive for Strongyle infection revealed Haemonchus sp., Trichostrongylus sp., Oesophagostomum sp., Strongyloides sp., and Bunostomum sp. in a decreasing order in goats. The larvae of Haemonchus sp. (61.63 %) and Strongyloides sp. (7.50 %) were highest in Balaghat, Trichostrongylus (18.13 %) in Narsinghpur, while Oesophagostomum sp. (10.50 %) and Bunostomum sp. (5.75 %) were in Chhindwara district. The finding of this study indicates that, even though subclinical in nature, Strongyle infection are one of the major problems that could hamper health and productivity and there is need for design a programme to minimize and control Strongyle infection in goats in the study area.
Keywords: Copro-culture, Goat, Larvae, Madhya Pradesh, Strongyle
Introduction
Goats in India possess an estimate of 140.5 million (Livestock census 2007). The poor animal production and management coupled with infectious and parasitic disease had lead to reduce productivity of small ruminants (Hailelul 2002). Parasitic infections cause a serious health threat and limit the productivity of livestock due to the associated morbidity and mortality (Nwosu et al. 2007; Singh et al. 2014). Small ruminants managed under semi- intensive production systems are extremely susceptible to the effect of wide ranges of endoparasites (Abebe and Esayas 2001). Strongyle infection of goat is responsible for economic losses through reduced productivity and increased mortality (Perry et al. 2002). The loss through reduced productivity is related to reduction of food intake, stunted growth, reduced work capacity, cost of treatment and control of nematodes (Pedreira et al. 2006; Odoi et al. 2007; Chaudary et al. 2007). The effect of infection by Strongyle infection varies according to the parasite concerned, the degree of infection and other risk factors such as species, age, season and intensity of worm burden. Hence, it is imperative to investigate the level of the parasitism, the type of Strongyle and the associated risk factors those make the small ruminants susceptible to the wide host range of Strongyle infection in an area, in order to devise effective control measure and monitor their outcome properly, so that the purpose of this study to determine the prevalence and severity of Strongyle infection, establish the relationship between risk factors of Strongylosis and their prevalence and identify the most prevalent Strongyle in small ruminants in different district of Madhya Pradesh, India.
Materials and methods
The faecal samples positive for Strongyle infection in a month were, pooled district wise and culture in the laboratory by glass tumbler (300 ml capacity) method as per the procedure of Roberts and Sullivan (1949). A drop of preserved sediment containing larvae was placed on a glass slide, mixed with a drop of Lugol’s iodine or aqueous Safranin and then examined under dry magnifications of the compound microscope after applying a cover slip over the preparation. 100 L3 parasites were counted and identification of Strongyle larvae was done with the help of the key and plates provided by Ministry of Agriculture, Fishery and Food (1971).
Results and discussion
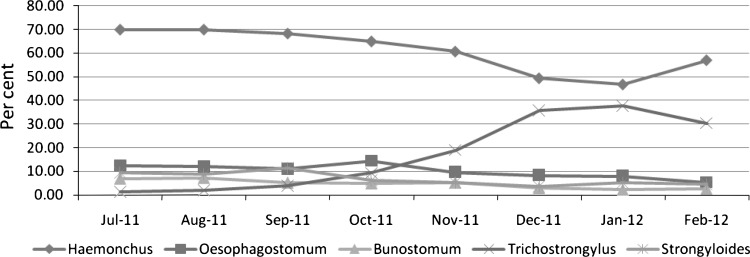
The larvae of Strongyle nematodes recovered from coprocultural examination, were identified up to the level of genera to which they belonged. The month wise generic composition of nematode larvae revealed that Haemonchus sp. was predominant during July–September, Trichostrongylus sp. during October–February, Oesophagostomum sp. during July–August and in October month, Strongyloides sp. July–October while Bunostomum sp. was predominant during July–November month (Fig. 1). The overall composition of the coprocultural larvae revealed that Haemonchus (60.88 %) was the most predominant followed by Trichostrongylus sp. (17.42 %), Oesophagostomum sp. (10.13 %), Strongyloides sp. (6.83 %). and Bunostomum sp. (4.75 %), while district wise, Balaghat had the higher prevalence of Haemonchus (61.63 %) and Strongyloides (7.50 %), Chhindwara for Oesophagostomum (10.50 %) and Bunostomum (5.25 %) and in Narsinghpur had predominant infection of Trichostrongylus. (18.13 %) (Table 2). The infection with gastro-intestinal nematodes was observed throughout the study period and well agreement with (Nginyi et al. 2001; Githigia et al. 2005), suggested hot and humid tropical environment to be provide favourable condition for the development of various species of Strongyle nematodes viz., Haemonchus contortus, Trichostrongylus sp., Oesophagostomum sp., Strongyloides and Bunostomum, who further indicated severity of infection to be influenced by weather conditions to a large extent. The characteristic features of Strongyle larvae are shown in Table 1. The contribution of agro ecology and climatic parameters proposed to play an important role in the development and survivility of infective stages of Strongyle nematodes on pasture. The collective predominance of Haemonchus sp. to be found on copro-culture in the present study and well agreement with those reported by Parihar et al. 1996; Faizal et al.1999; Githigia et al. 2005. However, highest prevalence of Haemonchus sp. might be due to high biotic potential to acquire faster resistant than other nematodes. The occurrence of higher prevalence of Strongyle nematodes in this region, animals were kept confined to small animal houses, during night in a muddy-floored house that provided favourable condition for the development in large no. and transmission of infective larvae.
Fig. 1.
Month wise mean generic composition (%) of nematode larvae in goat of M. P.
Table 2.
Mean generic composition (%) of nematode larvae in goat of Madhya Pradesh (M.P.) district nematode larvae
| District | Nematode larvae | ||||
|---|---|---|---|---|---|
| Haemonchus | Oesophagostomum | Bunostomum | Trichostrongylus | Strongyloides | |
| Balaghat | 61.63 | 9.50 | 4.75 | 16.63 | 7.50 |
| Narsinghpur | 60.38 | 10.38 | 4.25 | 18.13 | 6.88 |
| Chhindwara | 60.63 | 10.50 | 5.25 | 17.50 | 6.13 |
| Over all | 60.88 | 10.13 | 4.75 | 17.42 | 6.83 |
Table 1.
Characteristic features of strongyle nematode larvae recovered from copro-culture
| S. no. | Name of larvae recovered | Key identification features |
|---|---|---|
| 1 | Haemonchus | Slender larva, tail of seath of medium length tapering to a point and often kinked. Tail of seath very short, conical |
| 2 | Trichostrongylus | Small larva bearing one or two tuberosities or indistinctly rounded |
| 3 | Oesophagostomum | Larva of medium size, 32 pentagonal gut cells, lumen of gut wavy |
| 4 | Strongyloides | Without seath, oesophagus nearly half the length of the body with seath, oesophagus less than ¼ the length of the body |
| 5 | Bunostomum | Very small larva with 16 guts cells |
Conclusion
The overall composition of the coprocultural larvae in the entire three districts viz., Balaghat, Narsinghpur and Chhindwara of Madhya Pradesh, India revealed that Haemonchus was the predominant nematode, followed by Trichostrongylus, Oesophagostomum, Strongyloides and Bunostomum. These parasites are responsible for causing heavy losses due to reduced production, morbidity and mortality. However, suggested that the proper deworming schedule of animals, when conditions are more favourable for development and survival of Strongyle larvae on the pasture. Rotational grazing pattern is used at interval and avoid infected herd with healthy herd. Moreover, proper pasture and animal management could improve the control of gastrointestinal nematode infections in goat in small holder farmer.
Acknowledgments
The authors are highly thankful to the Dean, College of Veterinary Science & Animal Husbandry, (NDVSU), Jabalpur, laboratory assistant and farmers, who providing the facilities required for conducting research work.
References
- Abebe W, Esayas G. Survey of ovine and caprine intestinal helminthosis in eastern part of Ethiopia during the dry season of the year. Rev Vet Med. 2001;152(5):379–384. [Google Scholar]
- Chaudary FR, Khan MFU, Qayyum M. Prevalence of Haemonchus contortus in naturally infected small ruminants grazing in the Photohar area of Pakistan. Pak Vet J. 2007;27(2):73–79. [Google Scholar]
- Faizal ACM, Rajapakse RPVJ, Jayasinghe SR, Rupasinghe V. Prevalence of coccidial and gastrointestinal nematode vs. weight gains in treated goats in the dry area of Sri Lanka. Small Rumin Res. 1999;34:21–25. doi: 10.1016/S0921-4488(99)00037-1. [DOI] [Google Scholar]
- Githigia SM, Thamsborg SM, Maingi N, Munyua WK. The epidemiology of gastrointestinal nematodes in Goats in the low potential areas of Thika District Kenya. Bull Anim Health Prod. 2005;53(1):5–12. [Google Scholar]
- Hailelul N (2002) Study on prevalence of GIT helminthes of small ruminants in and around Wolayta Soddo, southern Ethiopia. DVM Thesis, Faculty of veterinary medicine, Addis Ababa University, Debre-Zeit, Ethiopia. pp 353
- Livestock census (2007) 18th All India Livestock Census, Dept. of Animal Husbandry & Dairying Ministry of Agriculture, GOI
- Ministry of Agriculture, Fisheries and Food (1971) Manual of Veterinary Parasitological techniques. Technical Bulletin 18, MAFF, London
- Nginyi JM, Duncan JL, Mellor DJ, Stear MJ, Wanyangu SW, Bair RK, Gatongi PM. Epidemiology of parasitic gastro-intestinal nematode infections of ruminants on small holder farms in Central Kenya. Res Vet Sci. 2001;70:33–39. doi: 10.1053/rvsc.2000.0438. [DOI] [PubMed] [Google Scholar]
- Nwosu CO, Madu PP, Richards WS. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Vet Parasitol. 2007;144:118–124. doi: 10.1016/j.vetpar.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Odoi A, Gathuma JM, Gachuiri CK, Omore A. Risk factors of gastrointestinal nematode parasite infections in small ruminants kept in smallholder mixed farms in Kenya. BMC Vet Res. 2007;3(6):1186–1746. doi: 10.1186/1746-6148-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MG, Manohar GS, Pathak KML, Kumar D (1996) Prevalence of gastrointestinal parasitosis in goats in and around Ramsar, Rajasthan. VIIIth National Congress of Veterinary Parasitology, Hissar. Oct 9–11, p 36
- Pedreira J, Silva AP, Andrade RS, Suarez JL, Arias M, Lomba C, Diaz P, Lopez C, Banos PD, Morrondo P. Prevalence of gastrointestinal parasites in sheep and parasite control practices in North-West Spain. Prev Vet Med. 2006;75:56–62. doi: 10.1016/j.prevetmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Perry BD, Randolph TF, McDermott JJ, Sones KR, Thornton PK. Investing in animal health research to alleviate poverty. Nairobi: International Livestock Research Institute (ILRI); 2002. p. 148. [Google Scholar]
- Roberts FHS, Sullivan PJO. Methods for EPG counts and larval cultures for strongyles infesting the gastrointestinal tract of cattle. Aust J Agric Res. 1949;1:99–103. doi: 10.1071/AR9500099. [DOI] [Google Scholar]
- Singh AK, Verma AK, Neha, Tiwari R, Karthik K, Dhama K, Singh SV. Trends and advances in vaccines against protozoan parasites of veterinary importance: A review. J Biol Sci. 2014;14(2):95–109. doi: 10.3923/jbs.2014.95.109. [DOI] [Google Scholar]