Abstract
A study was conducted to determine the prevalence of microfilariasis and analysis of diagnostic methods in buffaloes at Veterinary Poly Clinic, Gudiwada with relation to season, age and breed for a period of 1 year (March 2011–February 2012).Out of 1,222 blood smears examination, only 123 samples were found positive for microfilariasis with a prevalence rate of 10.06 %. Highest prevalence was observed in monsoon (12.54 %) followed by summer (9.18 %) and least in winter (7.05 %). Buffaloes above 9 years age were at high risk with a prevalence rate of 12.5 %, followed by 3–9 years (10.7 %) and below 3 years age (6.09 %) respectively. Breed wise prevalence of microfilariasis was higher in graded murrah buffaloes 10.87 % compared to non-descripts (6.19 %). Three commonly used diagnostic techniques viz. Modified Knott’s Technique, thick blood smear examination and wet film examination were done to evaluate comparative sensitivity/efficacy. Among these techniques, 100 % efficacy was observed in Modified Knott’s Technique and 86.99 % in thick blood smear examination followed wet film examination 78.04 % respectively.
Keywords: Microfilaria, Buffalo parasites, Modified Knotts Technique
Introduction
Buffaloes are one of the economically important animal species in most of the Asian countries including India (Liu et al. 2009). This animal species is vulnerable to most of the diseases caused by infectious agents and parasites. Among the parasitic infections, microfilariasis is an important infection, especially in coastal belt of Andhra Pradesh and is caused by larval stages of genera Setaria, Onchocerca, Stephanofilaria and Parafilaria of the family Filariidae. In India microfilariasis in bovines has been reported from different parts like Andhra Pradesh, Uttar Pradesh, Orissa, Madhya Pradesh and Tamilnadu (Lakshmi Rani et al. 2009). Microfilariasis appears to be an emerging disease particularly in costal districts of Andhra Pradesh (Venu et al. 2000). Microfilariasis inflicts a great economic loss on the part of the farmer due to gradual deterioration in general body condition of the animal, marked decline in milk yield, (Fig.1) prolonged convalescent period and cost of treatment. Gudiwada Veterinary Poly Clinic (VPC) is located at costal district of Krishna and has a potential tract of buffalo population and is identified as an endemic area for microfilariasis. Hence, the present study was under taken to find out the incidence of microfilariasis in buffaloes and to compare the sensitivity/efficacy of different established diagnostic techniques to demonstrate the microfilaria in blood samples
Fig. 1.
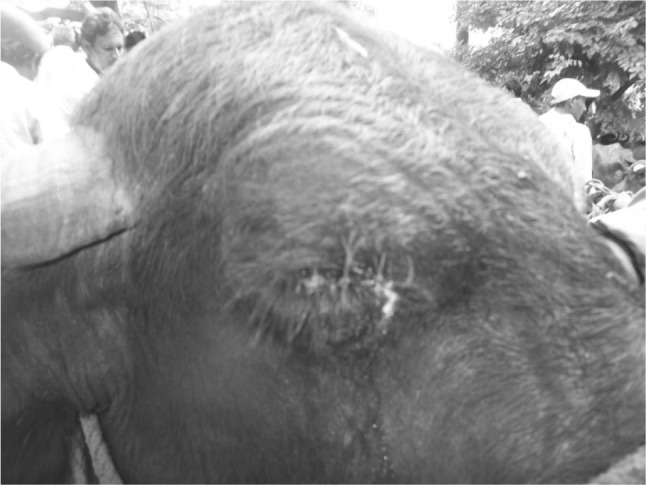
Photograph of buffalo showing thick ocular discharges
Materials and methods
The present study was carried out for a period of 12 months (between March 2011 and April 2012) at VPC, Gudiwada, Krishna district of Andhra Pradesh. Buffaloes exhibiting symptoms like pyrexia with ocular and nasal discharges, sudden drop in milk production, edema of hind limbs, weakness and dehydration were suspected for the disease and were selected for blood the sample collection. The blood samples (1,222) from different age groups, breeds were collected in a clean and sterile ethylene diamine tetra acetic acid coated glass vials. These blood samples were examined on the same day by wet film method, thick blood smear examination and Modified Knott’s Technique to evaluate the more sensitive/effective methods of the study (Fig. 2).
Fig. 2.

Photo micro graph showing Microfilariae in modified Knott’s technique
Wet blood film examination
A drop of wet blood was placed on a non greasy microscopic slide, covered with a cover slip and examined under low magnification for presence of microfilaria. Same procedure was repeated for five times to confirm the diagnosis (Table 1).
Table 1.
Prevalence of microfilariasis based on season, age and breed during the year March 2011–February 2012
| S. no | Parameter | No of animals screened | No of positive sample | Percentage of incidence (%) |
|---|---|---|---|---|
| 1 | Season | |||
| Summer | 392 | 36 | 9.18 | |
| Monsoon | 518 | 65 | 12.54 | |
| Winter | 312 | 22 | 7.05 | |
| 2 | Age (years) | |||
| <3 | 312 | 19 | 6.09 | |
| 3–9 | 542 | 58 | 10.7 | |
| >9 years | 368 | 46 | 12.5 | |
| 3 | Breed | |||
| G.M | 1,012 | 110 | 10.87 | |
| N.D | 210 | 13 | 6.19 | |
Thick blood smear examination
Thick blood smears were made on a non greasy microscopic slide, air dried at room temperature, and after dehaemoglobinisation the slides were fixed in methanol for 1 min, later they were air dried and stained with Giemsa-staining solution (1:20) for 30 min. Stained slides were washed in running tap water, air dried and screened for presence of microfilariae.
Modified Knott’s technique
The procedure was done as described by Vihan (1991). In a 15 ml centrifuge tube 1 ml blood and 9 ml 2 % formalin was mixed gently and centrifuged at 2,000 rpm for 10 min. The supernatant fluid was discarded and equal amount of 1:1,000 Methylene Blue staining solution was added to the bottom sediment, mixed thoroughly with an applicator stick. From that aliquot a drop of sediment was taken on a non greasy microscopic slide, covered with a cover slip and examined under 10× and 40× for the presence of microfilariae.
Results and discussion
Out of 1,222 blood samples examined, only 123 were found positive for microfilariasis with an over all prevalence of 10.06 %. The present prevalence record was higher than the reports of Pavan Kumar et al. (2004) and Bhaskara Rao et al. (2005) who have reported 3.227.46 and 8.49 % from Gudiwada (Krishna District), Tanuku (West Godavari District) and Kakinada (East Godavari District) of coastal Andhra Pradesh respectively. This variation might be attributable to different agro climatic conditions, managemental practices, vector density, natural infection and number of animals screened. Same opinion was expressed (this also opined) by Venu et al. (2000). Seasonal prevalence was highest in monsoon 12.54 % (July–October) followed by summer 9.18 % (March–June) and winter 7.05 % (November–February). This observation is in agreement with the findings of Pavan Kumar et al. (2004) and contrary to the reports of Venu et al. (2000) and Bhaskara Rao et al. (2005) who reported peak prevalence in summer followed by monsoon and winter seasons. Age wise prevalence was highest in above 9 years (12.5 %) followed by 3–9 years (10.7 %) and lowest in below 12 years (6.09 %). The findings are in accordance with the observations of Pavan Kumar et al. (2004), Bhaskara Rao et al. (2005) and Venu et al. (2000). The higher rate of infection in above 9 years might be due to frequent and prolonged exposure to insect vectors. Breed wise prevalence was higher in graded murrah buffaloes (10.87 %) compared to non-descriptive animals (6.19 %). These observations are similar with that of Satish and Rao (1996) and Kumar et al. (1993). As graded murrah buffaloes are high milk yielders than nondescripts, lactation stress might be the major contributing factor which makes them susceptible to the microfilariasis.
Out of 1,222 blood samples, only 123 blood samples were found positive for microfilariasis by Modified Knott’s Technique, 107 by thick blood smear examination and 96 blood samples by wet blood film examination. Comparatively Modified Knott’s Technique is highly sensitive/effective (100 %) than thick blood smear examination (86.99 %) and wet film examination (78.50 %) respectively. The observations recorded in this communication were in accordance with the report of Pavan Kumar et al. (2004), and vice versa made by Satish and Rao (1996) and Lahitte et al. (1986). The higher efficacy of Modified Knott’s technique might be due to heavy sample size, concentration of samples, dehaemoglobinization processes which makes the smear more clear for visualization of the microfilariae with out interference of erythrocytes (Table 2). Similar findings were observed by Pavan Kumar et al. (2005), Bino Sundar and Ravindran (2010). Comparison of different stains 0.1 % methylene blue and Giemsa stains were found to be the best for morphological studies of microfilariae Bino Sundar and Ravindran (2010). Morphologically the microfilariae observed in the present study were sheathed, long slender with blunt anterior and tapering posterior ends. They were assumed in different shapes, some are folded, curved, straight and coiled as described by Bino Sundar and Ravindran (2010). Wet film examination revealed live microfilariae with different motility pattern. The present study communicates that seasonal prevalence of microfilariasis is more in monsoon (12.54 %), especially in graded murrah buffaloes with highest risk in age groups above 9 years age (12.5 %) in Coastal districts of Andhra Pradesh. The concentration techniques like Modified Knott’s technique, thick blood smear examination were proved to be more sensitive, rapid and easy to perform at field level.
Table 2.
Evaluation of sensitivity of diagnostic procedures for microfilariasis (1,222 samples)
| S. no | Diagnostic technique | No of positives out of 123 cases | Comparison of efficacy (%) |
|---|---|---|---|
| 1 | Wet blood film | 96 | 78.04 |
| 2 | Thick blood smear | 107 | 86.99 |
| 3 | Modified Knott’s technique | 123 | 100 |
Contributor Information
V. Samatha, Email: samathaxavier@gmail.com
Ch. Jyothisree, Email: chellivet@rediffmail.com
K. Ramesh Babu, Email: ramvety@gmail.com.
References
- Bhaskara Rao R, Patro D, Hafeez Md. Prevalence of microfilariasis in buffaloes in East Godavari District of Andhra Pradesh. Indian Vet J. 2005;82:824–825. [Google Scholar]
- Bino Sundar ST, Ravindran R. Comparision of various methods for detection of microfilariae of Seteria in the blood of cattle. Tamilnadu J Vet Anim Sci. 2010;6(1):45–48. [Google Scholar]
- Kumar M, Joshi HC, Garg SK. Prevalence of Seteiriticen-I in buffaloes of Kumaon region. Indian J Vet Res. 1993;2:1–6. [Google Scholar]
- Lahitte JD, Davoust B, Dorchies P (1986) Comparative study of five methods of detecting microfilariae. Rev Med Vet 137:639–6644. Helminth. Abst. 2099, 1986
- Lakshmi Rani N, Syaama Sundar N, Jayabal L, Ramadevi V. Microfilariosis associated with epistaxsis in a she buffalo. Buffalo Bull. 2009;28(4):171–172. [Google Scholar]
- Liu Y, Li F, Liu W, Dai RS, Tan YM, Lin RQ, He DS, Zhu XQ (2009) Prevalence of helminths in Water buffaloes in Hunan Province, China. Trop Anim Health Prod 41:543–546 [DOI] [PubMed]
- Pavan Kumar C, Sreedevi B, Venkatareddy T, Nalini Kumari K (2004) Epidemiological studies on bovine microfilariasis in costal districts of Andhra Pradesh. J Parasit Dis 28(1):17–22
- Pavan Kumar C, Sreedevi B, Venkatareddy T, Nalini Kumari K. Analysis of methods for the diagnosis of microfilaraemia in buffaloes and cattle. J Vet Parasitol. 2005;19(12):107–110. [Google Scholar]
- Satish U, Rao D (1996) Clinico therapeutic studies on microfilariasis in buffaloes(Biihualus buhulis). Ph.D.Thesis.Acharya NG Ranga Agricultural University, Rajendranagar, Hyderabad
- Venu R, Radhakrishnamurthy P, Sreedevi C. Prevalence of microfilariasis in graded murrah buffaloes in west Godavari District of Andhra Pradesh. Indian Vet J. 2000;77:272–273. [Google Scholar]
- Vihan VS. Modern veterinary laboratory techniques in clinical diagnosis. 1. Delhi: CBS Publishers and Distributors; 1991. [Google Scholar]


