Abstract
Malaria is hyper-endemic in Ghana. Haematological alterations in the disease pathology may offer complimentary criteria to improve clinical and microscopy diagnosis. Our primary outcome was to evaluate haematological parameters in children with Plasmodium falciparum infections and report their predictive risk and diagnostic performance for malaria infections in Ghana. Haematological data, including thin and thick blood films were examined for children less than 12 years of age in a multicenter-based active case finding approach. Haematological changes were common in P. falciparum infected children and more pronounced in severe malaria cases. More so, a unit increase in parasiteamia increased the odds for severe malaria infection by 93 % [OR, 95 % CI: 1.93 (1.28–2.91); P value = 0.02]. In multivariate regression, low haemoglobin was a significant haematological change in predicting P. falciparum infections [OR, 95 % CI: 3.20 (1.26–7.09); P value = 0.001]. Low haemoglobin levels <11 g/dl was the most reliable indicator for P. falciparum infections [with a sensitivity of (64 %), specificity (71 %), positive predictive value (83 %) and likelihood ratio (2.2)]—even when evaluated in combination with leucocytosis, lymphocytopaenia and high neutrophil counts >7,500 µL. In malaria endemic settings, low haemoglobin concentration (<11 g/dl) in children with febrile illness should prompt a more diligent search for the malarial parasite to limit the misuse and abuse of anti-malarial drugs.
Keywords: Malaria, Severe, Uncomplicated, Parasitaemia, Plasmodium falciparum
Introduction
Malaria is hyper-endemic in Ghana with Plasmodium falciparum accounting for about 90–98 % of the deaths and morbidity associated with the disease (Ghana Health Service (GHS) 2009). Consequently, empirical therapy is pervasive especially in resource-poor settings where the challenge of clinical microbiology services appears formidable. Moreover, symptoms of bacterial or viral infections often mirror malaria and are wrought with significant misuse of anti-malaria drugs (Bell et al. 2005; Peeling et al. 2008). When a disease pathology occur in circulation, it is natural to speculate that it will have an impact on systemic biological signals of the human host. During the course of malaria infection, parasites and red blood cells come under oxidative stress and the host system responds in an attempt to protect the red blood cells (RBCs) (Narsaria et al. 2012). Indeed, it is becoming increasingly clear that many disease pathologies modulate mitochondrial energetics in leucocytes as early warning signs for bioenergetics dysfunction (Guha et al. 2006; Dey et al. 2009). This opens up the possibility that circulating leukocytes, particularly lymphocytes and neutrophils could serve as biomarkers for oxidative stress, and as an index of inflammation in relation to malaria parasiteamia (Maina et al. 2010; Olliaro et al. 2011).
Haematological alterations in the malaria pathology may offer complimentary criteria to improve clinical and microscopy diagnosis by prompting a more diligent search for the malarial parasite to limit the misuse and abuse of anti-malarial drugs in Ghana. The present study examined the occurrence and significance of haematological changes in children with P. falciparum malaria in Ghana, West Africa. Our primary outcome was to evaluate haematological patterns in children with P. falciparum infections compared with those uninfected from the same population. In this study, we report the predictive risk and diagnostic performance of haemoglobin, total white blood cells (TWBC), neutrophils, and lymphocytes for malaria infections in children.
Methods
Study settings
The study was conducted in four conveniently selected primary care health centers (Princess Marie Louis Hospital, Achimota Hospital, Amasaman Hospital, and Ussher polyclinic) in Accra, Ghana. Accra, is the capital city of Ghana, and a cosmopolitan metropolis with an estimated urban population of 2.269 million as of 2012 (GSS 2012). Malaria transmission is perennial with distinct seasonal patterns, but highest during the rainy season from April to October, followed by dry season from November to March. The peak malaria transmission season coincides with the period of major rains while the dry season has low rates of malaria infection (Koram et al. 2003).
Study design
We conducted a case–control, multicenter-based study with children participants selected prospectively from June 2012 to March 2013 at the study sites. We used primary care centers to minimise the spectrum bias of referral hospitals contributing more severe cases with discrepant proportion of haematological alterations. An active case-finding network was organized with visits to participating centers to identify and interview the cases before any treatment was applied. Physician-identified children presenting with symptoms consistent with malaria [history of fever with or without chills (axillary temperature >37.5 °C, sweating, headache] and referred to the laboratory for a malaria tests were evaluated for inclusion in the study after informed consents were obtained from parents or guardians. Routinely, children were assessed by physicians, who documented the findings of clinical examinations, using the national guidelines for case management of malaria in Ghana, updated to reflect standard WHO recommendations (WHO: Severe falciparum malaria 2000; Ghana Health Service (GHS) 2009). Patients and guardians were requested to assist in the provision of information for the study. Laboratory records of children enrolled were also reviewed. The study included incident cases with P. falciparum confirmed infections and controls of similar age, without parasiteamia, assigned from the general population that selected the cases. This study was approved by the Ethical and Protocol Review Committee of University of Ghana Medical School College of Health Sciences.
Exclusion criteria
Children with history of antimalarial treatment during the last 2 weeks or other treatments for the recent febrile illness or sickling positive were excluded to limit bias for false negatives and resolution of haematological alterations. Also, patients identified with malnutrition, infectious diseases such as upper respiratory tract infections and typhoid or any cause of anaemia other than malaria were excluded from the study.
Sample collection
About 2–3 mL venous blood was drawn into EDTA-coated syringes, distributed into sterile test tubes, and placed immediately on ice. Routinely, all FBCs were performed in parallel with thin and thick film microscopy by separate operators blinded to the results of the other assays.
Microscopy
Two thin and thick films were prepared from whole-blood specimens and stained with 3 % Giemsa as outlined by the WHO Basic Malaria Microscopy Document, 2009. Parasite densities were estimated with thick film (Adu-Gyasi et al. 2012). Thin films were examined to confirm the species identification on the thick film. Blood films were examine with ×100 oil immersion lens such that at least 100 ocular fields were examined before slides were declared negative.
Parasite density estimations
Parasite densities were determined with absolute WBC counts as a ratio of P. falciparum counts relative to 200 WBC in thick films. Five hundred WBC were counted where less than nine parasites were counted after counting against 200 WBC. For P. falciparum counts ≥100 parasites per thick smear high power field, parasite counts were confirmed in thin films (against 2,000 red blood cells) and recalculated with 200 WBC. Parasite density/µL was calculated as (Number of parasites counted/WBC counted) × WBC count/μL of participant’s whole blood. At least 100 high power fields were examined before thick films were described negative.
Full blood counts
Using an automated haematology analyzer (Sysmex KX-21 N, Japan), full blood counts (FBC) of children participants were performed within an hour of sample collection according to manufacturer’s instructions. The Analyzer provided data on WBCs, RBCs and haemoglobin levels. The machine provided an immediate hardcopy readout of haematological results with time and date. Commercial controls and quality assurance checks were performed daily in accordance with manufacturer’s recommendations.
Validation
Microscopists assessing blood slides, and data analysts remained unaware of case–control allocation until the end of the study. Whole blood samples were re-examined and cross-checked at Microbiology Reference Laboratory at Korle-Bu Teaching Hospital for P. falciparum infections by expert microscopists without reference to results of previous microscopy. Likewise, all blood slides with discrepant results, defined as >50 % difference in parasite density or a positive versus negative result between the readers, were subjected to cross-checking microscopy for quality assurance. Expert cross-checking microscopy was regarded decisive.
Statistical analyses
Data from interviews and parasitological investigations were captured into Microsoft Excel to generate a database, and exported into Statistical Package for Social Sciences (SPSS, Version 20.0) for data editing and statistical analyses. Continuous data were compared using student’s t-test, analyses of variance (ANOVA) or Mann–Whitney U test, (respectively for normalised and non-parametric distributions), with point estimates of statistical significance indicated with 2 tailed P values < 0.05. Categorical data were compared across study parameters using χ2 and Fisher’s exact tests. Correlations were assessed with Pearson coefficient or Spearman’s rho where appropriate. The association between haematological parameters and patient characteristics as predictors of P. falciparum infections was quantified by odds ratio (OR) with 95 % confidence interval (CI). From univariate analyses, variable with a P value < 0.05 were analysed in a multivariate logistic regression models to identify independent risk factors.
Predictive accuracy of the models was evaluated by Hosmer and Lemeshow goodness-of-fit test with P value > 0.05 suggesting that the model predicts accurately on average. The area under the receiver operating characteristic (ROC) Curve >0.7 was used to analyse the discriminatory capability of P. falciparum infected children and controls (Kleinbaum and Klein 2010). Diagnostic performance of haematological parameters was assessed with sensitivity, specificity, predictive values and odds ratios with precision at 95 % confidence intervals.
Results
Overall, 150 children aged 6 months to 12 years were recruited between June 2012 and March 2013. A hundred and five children (70 %) had mono-infections with P. falciparum. Forty-five (30 %) enrollees, uninfected with Plasmodium species, were engaged as controls. Males constituted 64 % (n = 29/45) and 51.4 % (n = 56/105) of P. falciparum infected children and controls respectively. The mean age of children was 4.46 (95 % CI, 3.9–4.9) but without significant differences between P. falciparum infected children and controls (P value = 0.27, F = 1.59).
Plasmodium falciparum parasiteamia
Among P. falciparum-infected children, the mean parasitaemia was 40,673 parasites/µL (95 % CI: 33,58–54,73) of blood. Whilst approximately a third (n = 45/150; 30 %) of the children had no P. falciparum infection as determined by light microscopy, none of the children had P. falciparum parasiteamia <500 parasites/µL of blood (37.9 %), and 23 (15.3 %) had a parasitaemia ranging between 500 and 10,000 parasites/µL of blood. More than half (n = 80/150; 53.3 %) of the recruited children had a parasite count >10,000 parasites/µL of blood. Haematological changes were determined for the non-malaria infected group (controls) and compared across the study population stratified by severe (n = 81/150, 54 %) or uncomplicated malaria (n = 24/150, 16 %) as previously described—severe malaria was the presence of asexual parasitaemia and at least one of the following: altered consciousness (change of behaviour, confusion, delirium, coma persisting for over 30 min after convulsion), repeated generalized convulsions (fits) 2 or more in 24 h, inability to take fluids, repeated profuse vomiting, extreme pallor (severe anaemia; haematocrit <15 % or Hb <5 g/dl), signs of hypoglycemia (sweating, pupil dilation, abnormal breathing, coldness, blood, sugar-<40 mg/dl. or 2.2 mmol/L), signs of renal failure (passing very little urine), signs of haemoglobinuria (dark or cola-colored urine), circulatory collapse or shock (cold limbs, weak rapid pulse), difficulty in breathing or pulmonary oedema, spontaneous unexplained heavy bleeding (Disseminated intravascular coagulation), marked jaundice (yellowish coloration of the eyes), prostration including generalised weakness, such that the patient cannot walk or sit without assistance, hyperpyrexia (axillary temperature >38.5 °C), and hyperparasitaemia (reported as “1–10 parasites per HPF,” “3+” or more). Malaria cases with a history of fever within the last 2–3 days, or found to have fever on exam (axillary temperature >38.5 °C or rectal temperature >37.5 °C), in the absence of signs of severe disease were deemed uncomplicated (WHO: Severe falciparum malaria 2000; Ghana Health Service (GHS) 2009).
Haematological parameters
Table 1 describes the haematological parameters of malaria parasiteamic children versus those of non-malaria infected controls. Mean values for haemoglobin (Hb) and lymphocyte counts were significantly lower for the parasiteamic group compared with the non-infected controls (P value = 0.021, and <0.001 respectively). In both parameters, Bonferroni post hoc comparisons indicated significant mean variance between children suffering from severe malaria and uninfected controls. Conversely, the mean neutrophil count was significantly elevated in the malaria parasiteamic group. (P value = 0.024, and <0.001 respectively). There was no significant difference in means of total white blood cell counts (TWBC) between the parasiteamic and the non-P. falciparum infected controls.
Table 1.
Haematological parameters of children of malaria and non-malaria-infected children
| Variables | Mean (95 % CI)a | P value | Bonferonni post-hocc | ||
|---|---|---|---|---|---|
| Malaria | Controls (45) | ||||
| Uncomplicated (n = 24) | Severe (n = 81) | ||||
| Age (months) | 5.35 (3.8–6.9) | 4.49 (3.5–5.9) | 3.96 (3.0–4.9) | 0.207 | – |
| Hemoglobin (g/dl) | 9.92 (8.9–11.0) | 10.02 (9.5–10.5) | 11.08 (10.7–11.5) | 0.021 | 0.024; Severe vrs non-malaria |
| TWBCb (×103/uL) | 10.08 (5.4–14.8) | 8.91 (8.1–9.8) | 8.85 (7.4–10.4) | 0.663 | – |
| Lymphocytes (×103/uL) | 41.9 (33.7–50.2) | 36.58 (32.9–40.2) | 50.1 (44.0–56.2) | <0.001 | <0.001; Severe vrs non-malaria |
| Neutrophil (×103/uL) | 58.0 (49.8–63.5) | 63.5 (59.9–67.2) | 49.9 (43.8–56.0) | <0.001 | <0.001; Severe vrs non-malaria |
| Parasiteamia (×103/uL) | 5.81 (4.64–6.79) | 55.56(42.8–68.2) | <0.001 | – | |
a CI confidence interval
b TWBC total white blood cells
c vrs versus
Haemoglobin
Anaemia was defined as haemoglobin level <11 g/dl and further classified as severe if Hb <5 g/dl. Sixty seven (63.8 %) of the malaria-infected children had anaemia with 4(3.8 %) being severe compared to 13 (28.9 %) in the non-malaria infected group, who all had mild aneamia (P value < 0.002, Z ratio = 3.29). Two patients (1.9 %) had Hb <5 g/dl in the presence of hyper-parasitaemia (>200,000 P. falciparum/μL) and were categorized as suffering from severe malaria anaemia.
White blood cells
Leucocytosis was defined as total white cell count >17,000/μL and was observed in similar proportions amongst the malaria-infected group and controls (4.4 % vs. 3.8 %, P value = 0.856, Z ratio = 0.812). There was poor correlation between TWBC and parasiteamia (P value = 0.591, r = 0.044). However leukocyte components (neutrophils and lymphocytes) showed significant correlations with parasite density (P value = 0.001, r = 0.260 and −0.260 respectively)
Haematological predictors of P. falciparum infection
A multinomial logistic regression was applied for the dependent outcomes “severe malaria”, “uncomplicated malaria” and “non-malaria”, but consistently yielded an area under the ROC curve less than 0.7. As a result, subsequent analysis were conducted in binary logistic regressions with the dependent variable stratified according to malaria versus controls. In bivariable analyses (Table 2), respiratory distress and pallor were significantly associated with P. falciparum infections. Neutrophil counts, lymphocyte counts and haemoglobin concentrations were significant associated with malaria infections. Also, malaria infected children were more likely to have anaemia compared to controls [OR, 95 % CI = 4.34 (2.04–9.26); P value < 0.0001]. The results of the final multivariate analyses are presented in Table 3 for models 1 and 2. The Hosmer and Lemeshow goodness-of-fit tests suggested satisfactory fits for both models respectively (Chi square = 3.817, 8.645; degree of freedom (df) = 8, 8; P value = 0.873, 0.373; predictive ability = 76.0, 79.3 %). When applied to haematological parameters only (model 1), anaemia was the strongest and only independent risk factor associated with P. falciparum infections [OR, 95 % CI: 3.20 (1.26–7.09); P value = 0.001]. Model 2 was adjusted to include significant patient characteristics and this did influence the results. Patients with P. falciparum infection significantly suffered from respiratory distress 21 times more than non-malaria infected children [OR, 95 % CI: 20.95 (4.27–103.08); P value < 0.001]. And although the odds of being anaemic was significantly reduced by having respiratory distress [OR, 95 % CI: 0.069 (0.025–0.192); P value = 0.01], haemoglobin levels in such patients was not significantly associated with respiratory distress [OR, 95 % CI: 1.148 (0.900–1.467); P value = 0.267].
Table 2.
Binary logistic regression for possible predictors of P. falciparum infection among children in Ghana
| Variables (number) | P. falciparum infection | OR (95 % CI) | P value | |
|---|---|---|---|---|
| Yes (105) | No (45) | |||
| Male gender | ||||
| Yes (67) | 51 | 16 | 0.58 (0.28–1.20) | 0.142 |
| No (83) | 54 | 29 | Ref. | |
| Age (SD) | 4.69 (3.11) | 3.96 (3.02) | 1.09 (0.96–1.22) | 0.19 |
| Age group | 0.617 | |||
| 0–3 (67) | 44 | 23 | 0.55 (0.11–0.25) | 0.473 |
| 4–7 (58) | 17 | 41 | 0.69 (0.13–3.67) | 0.662 |
| 8–11 (16) | 3 | 13 | 1.24 (0.17–9.25) | 0.832 |
| ≥12 (9) | 2 | 7 | Ref. | |
| Patient characteristicsa | ||||
| Respiratory distress | ||||
| Yes (57) | 55 | 2 | 23.65 (5.44–102.71) | <0.001 |
| No (93) | 50 | 43 | Ref. | |
| Pallor | ||||
| Yes (81) | 68 | 13 | 4.52 (2.12–9.66) | <0.001 |
| No (69) | 37 | 32 | Ref. | |
| Convulsion | ||||
| Yes (15) | 15 | 0 | – | – |
| No (135) | 90 | 45 | ||
| Vomiting | ||||
| Yes (89) | 89 | 0 | – | – |
| No (61) | 16 | 45 | ||
| Fever | – | – | ||
| Yes (150) | 105 | 45 | ||
| No (0) | 0 | 0 | ||
| Diarrhoea | ||||
| Yes (28) | 28 | 0 | – | – |
| No (122) | 77 | 45 | ||
| Prostration | ||||
| Yes (54) | 54 | 0 | – | – |
| No (96) | 51 | 45 | ||
| Haematological parameters | ||||
| Haemoglobin (SD) × g/dl | 10.00 (2.43) | 11.08 (1.39) | 0.77 (0.64–0.93) | 0.007 |
| Anaemia | ||||
| Yes (80) | 67 | 13 | 4.34 (2.04–9.26) | 0.0001 |
| No (70) | 38 | 32 | Ref. | |
| TWBC (SD) × 103/µL | 9.17 (6.25) | 8.85(4.95) | 1.01 (0.95–1.07) | 0.756 |
| Leucocytosis | ||||
| Yes (6) | 4 | 2 | 0.83 (0.15–4.72) | 0.835 |
| No (144) | 101 | 43 | Ref. | |
| Neutrophil count (SD) × 103/µL | 6.29 (1.73) | 4.98 (2.01) | 1.04 (1.01–1.06) | <0.001 |
| Neutropenia | ||||
| Yes (0) | 0 | 0 | – | – |
| No (150) | 105 | 45 | ||
| Lymphocyte count (SD) × 103/µL | 3.81 (1.72) | 5.01 (2.03) | 0.965 (0.945–0.984) | <0.001 |
| Lymphocytopaenia | ||||
| Yes (27) | 23 | 4 | 2.87 (0.93–8.86) | 0.066 |
| No (123) | 82 | 41 | Ref. | |
OR (95 % CI) odds ratio at 95 % confidence interval, SD standard deviation, TWBC total white blood cell count, Ref. reference group
aPhysician identified patient characteristics based on WHO, sever falciparum report, 2009
Data are presented as the absolute number of children with the exception of age, haemoglobin, TWBC, neutrophil counts, and lymphocyte counts which are listed as mean values with SD, ORs are unavailable for continuous variables or categorical variables with no events in one or more cells, Respiratory distress, presence of alar flaring, intercostals or subcostal chest recession, use of accessory muscles of respiration, or abnormally deep respiration; Prostration, inability to sit or drink/eat, although normally able to do so
Table 3.
Final multivariable logistic regression of hematological changes in P. falciparum infections amongst Ghanaian children
| Variable | level | Adjusted odds ratio | 95 % CI | P value |
|---|---|---|---|---|
| Model 1: Adjusted for haematological parameters only | ||||
| Haemoglobin × g/dl | 1 g/dl increase | 0.848 | 0.689–1.014 | 0.121 |
| Aneamia | Yes/no | 3.20 | 1.262–7.098 | 0.001 |
| Model 2: Haematological changes adjusted for confounding patient characteristics | ||||
| Respiratory distress | Yes/no | 20.950 | 4.268–103.082 | <0.001 |
| Haemoglobin × g/dl | 1 g/dl increase | 0.799 | 0.628–1.015 | 0.066 |
95 % CI odds ratio at 95 % confidence interval
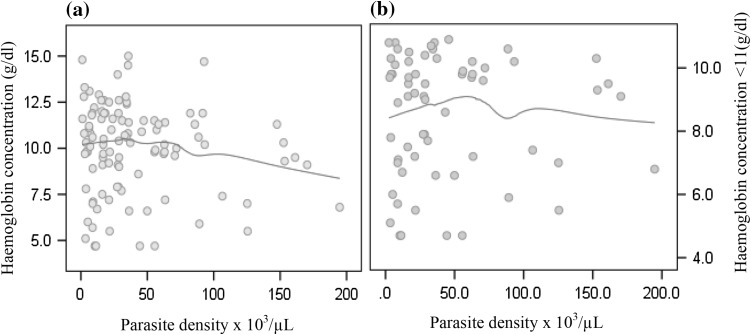
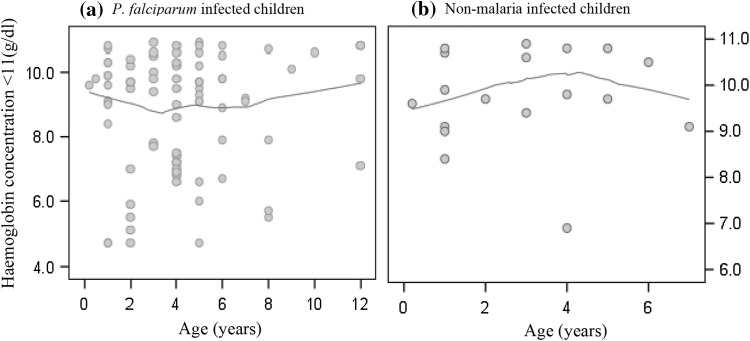
Given haemoglobin <11 g/dl as significant predictor of P. falciparum infections, we used local polynomial regression to explore the relation between haemoglobin and P. falciparum parasiteamia. In Fig. 1a, haemoglobin concentration was highest towards children with 40,000 parasites per µL of blood and steadily decreased with increasing parasiteamia. A similar trend was observed when the distribution was limited to anaemic patients (Fig. 1b) but bivariate correlation suggested non-significant negative linear relationships with Pearson’s coefficients of −0.05., −0.122 at P value = 0.971, and 9.217 respectively. Parasiteamia was not significantly associated with the risk of having haemoglobin levels <11 g/dl [OR, 95 % CI: 0.995 (0.985–1.004); P value = 0.255]. Figure 2a shows the tendency of haemoglobin to increase in P. falciparum infected anaemic children older than 4 years in contrast to that observed among the non-malaria infected children (Fig. 2b).
Fig. 1.
Profile of haemoglobin concentration in (a) all children, and (b) aneamic children across parasite densities in Ghanaian children infected with P. falciparum. Haemoglobin (Y-axis, g/dl) by parasiteamia ×103 µL (X-axis) with Tricube kernel smooth fit line generated with local polynomial regression at 60 % of points to fit
Fig. 2.
Haemoglobin versus age distribution of (a) P. falciparum infected and (b) non-malaria infected aneamic children with Tricube kernel smooth fit line generated with local polynomial regression at 60 % of points to fit
Performance of haematological parameters as indicators of P. falciparum infections
Table 4 displays the differential diagnostic performance of haematological parameters compared to microscopy for P. falciparum infections in children. Discrepancies were observed across the parameters and per criteria. First, leucocytosis, lymphocytopaenia and high neutrophil counts >7,500 µL demonstrated good specificities (96, 91 and 82 % respectively) and positive predictive tests (except for TWBC, 67.0 %) but lacked sensitivity (≤30.0 % in all cases), and had poor odds ratios and kappa agreement (<0.1 in all cases) to accurately diagnose malaria. Haemoglobin <10 g/dl had lower specificity (74 %) but good sensitivity (64.0 %), good positive predictive test (83 %) and likelihood ratio (2.20). Low haemoglobin recorded the strongest and statistically significant Kappa agreement, albeit weak (K = 0.117, P value = 0.001). Children with haemoglobin <10 g/dl were significantly up to 4.34 times (P value = 0.001) more likely to have malaria than children with normal haemoglobin (Table 2). A combination of low haemoglobin with leucocytosis, or lymphocytopaenia or high neutrophil counts >7,500 µL were not better indicators of malaria.
Table 4.
Diagnostic performance of hematological changes in malaria infected children
| Variable | Percent sensitivity (95 % CI) | Percent specificity (95 % CI) | Percent predictive tests (95 % CI) | Likelihood ratios (95 % CI) | Cohen’s Kappa (P value) | OR (95 % CI) | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| Hb <10 g/dl | 64.0 (0.54–0.73) | 71.0 (0.554–0.83) | 83.0 (0.73–0.90) | 45.0 (0.33–0.57) | 2.20 (1.36–3.57) | 0.50 (0.38–0.66) | 0.117 (0.001) | ref. Table 2 |
| TWBC >1,7000 µL | 4.0 (0.01–0.10) | 96.0 (0.84–0.99) | 67.0 (0.24–0.94) | 29.0 (0.23–0.38) | 0.863 (0.16–5.51) | 1.01 (0.97–1.05) | 0.004 (0.856) | ref. Table 2 |
| Neutrophil count >7,500 µL | 30.0 (0.22–0.40) | 82.0 (0.67–0.91) | 80.7 (0.63–0.90) | 33.5 (0.25–0.43) | 1.71 (0.85–3.420 | 0.84 (0.74–0.96) | −0.055 (0.107) | ref. Table 2 |
| Lymphocyte <2,000 µL | 21.4 (0.15–0.31) | 91.1 (0.77–0.97) | 85.4 (0.65–0.95) | 33.1 (0.25–0.42) | 2.46 (0.92–2.71) | 0.85 (0.77–0.95) | 0.031 (0.057) | ref. Table 2 |
| Hb <10 g/dl + TWBC >17,000 µL | 40.1 (0.01–0.1) | 100.0 (90.2–100) | 100.0 (0.39–100) | 30.8 (23.5–31.2) | –a | .96 (92.5–99) | – | –a |
| Hb <10 g/dl + Neutrophil count >7,500 µL | 15.3 (0.0900.23) | 88.1 (0.75–0.95) | 76.5 (0.52–0.90) | 31.2 (0.23–0.39) | 1.37 (0.53–3.51) | 0.95 (0.87–1.04) | – | 0.69 (0.23–2.03) P value = 0.613 |
| Hb <10 g/dl + Lymphocytes <2,000 µL | 10.8 (0.6–0.180 | 93.1 (0.80–98) | 78.3 (0.48–0.94) | 30.2 (0.23–0.39) | 1.57 (0.46–5.4) | 0.95 (0.89–1.03) | – | 0.61 (0.16–2.31) P value = 0.556 |
OR (95 %) CI odds ratio at 95 % confidence interval, Hb haemoglobin, TWBC total white blood cell count
aLikelihood ratios and odds ratios were not calculated for contingency tables with 0 in any of the cells
Discussion
The current study recorded an overall high crude prevalence (n = 105/150, 70 %) of P. falciparum infections in children ≤12 years reporting with clinical symptoms suspicious of malaria. The results also showed significant numbers (n = 81/150, 54 %) of severe P. falciparum infections and indicate a fair index of the prevalence of the disease among children with malaria attending the hospital (Agyapong et al. 2009). Two observation merit attention. First, haematological abnormalities were common in P. falciparum infected children and more pronounced in severe malaria cases, perhaps due to the higher levels of parasite density in this group. Children with severe malaria had higher mean parasiteamia than children without malaria (55.56 × 102 µL versus 5.81 102 µL; difference 49.70 × 102 µL; 95 % CI: 26.3–73.07 × 102 µL; P value < 0.001). More so, a unit increase in parasiteamia increased the odds for severe malaria infection by 93 % [OR, 95 % CI: 1.93 (1.28–2.91); P value = 0.02]. Second, we observed a significant reduction in haemoglobin concentration and proportion of anaemia (Table 2) in P. falciparum infected children compared to those not infected—with severe anaemia only seen in a minority (n = 4/105, 3.8 %) of the children infected with malaria but none in the controls. Because, malaria-induced immune response contributes to accelerated lysis of both parasitized and non-parasitized red cells, which in turn results in increased erythropoiesis under the influence of a combination of haemolytic mechanisms, anaemia is a common complications in P. falciparum infected children (Wickramasinghe and Abdalla, 2000; Owusu-Agyei et al. 2002). We noted severe malarial anaemia as the most common form of severe malaria (n = 2/4, 50 %) encountered. Indeed, in areas of high P. falciparum transmission such as is observed in Ghana, severe malarial anaemia is most pervasive in children with severe malaria (Oduro et al. 2007; Osafo-Addo et al. 2008).
In this study, anaemia compared to leucocytosis, lymphocytopaenia and high neutrophil counts >7,500 µL, was an important haematological risk factor for malaria infections and was associated with a non-significant decrease in haemoglobin concentration of 0.832 g/dl [95 % CI: (0.689–1.014); P value = 0.121]. However, haemoglobin concentrations in anaemic children was not significantly associated with P. falciparum parasiteamia, and thus may not be a marker for disease severity. When adjusted for patient characterisccs, respiratory distress was significantly more likely to be present in P. falciparum infections but none of the occurrences could be defined as malaria-associated acute respiratory distress syndrome. Each incident of respiratory distress corresponded to a decrease in haemoglobin concentration of 0.799 g/dl [95 % CI: (0.628–1.015); P value = 0.066] (Table 3). The findings are consistent with studies on clinical and laboratory manifestations reported in other endemic settings (Oduro et al. 2007; Van den Steen et al. 2013).
Varying performance standards for haematological parameters have been reported by workers in Africa and elsewhere (Erhart et al. 2004; Lathia and Joshi 2004; Taylor et al. 2008; Maina et al. 2010). Examining the full repertoires of haematological changes in Plasmodium infections appears practically daunting. Most workers have looked at patelets, monocytes, haemoglobin, total white blood cell counts, neutrophils and lymphocytes (Erhart et al. 2004; Quintó et al. 2006; Olliaro et al. 2011), and a few have reported on eosinophils and basophils (Maina et al. 2010). Irrespective of the measured parameters, low haemoglobin <11 g/dl, low platelets <1.5 × 104 µL, high monocytes counts >1.5 × 103 µL and their combinations thereof, have had superior predictive capacity for P. falciparum infections in Sub-Sahara Africa (Quintó et al. 2006; Oduro et al. 2007; Adedapo et al. 2007; Olliaro et al. 2011). In this study, we identified low haemoglobin levels, as the most reliable haematological change in predicting P. falciparum infections in children from this study—even when evaluated in combination with leucocytosis, lymphocytopaenia and high neutrophil counts >7,500 µL. Aneamic children were 4.34 times [95 % CI: (2.04–9.26); P value = 0.001] more likely to have malaria than children with normal haemoglobin (Table 2). In areas with high incidence of P. falciparum infections, an ideal predictive parameter must have the greatest ability to select true positives without scoring false negatives. The overall sensitivity (64 %), specificity (71 %) and positive predictive value (83 %) of low haemoglobin <11 g/dl makes it the most sensitive indicator for malaria in this study. With such predictive efficacy, 29 % of malaria negative cases would receive anti-malarial drugs, but would also result in 36 % of true malaria cases not being treated (Table 4). This has underlining public health implications. For remote and resource poor settings in malaria endemic areas with some capacity to perform limited blood differentials, the margins of over-treatment that may be acceptable, using haematological criteria to compliment malaria diagnosis, compared to empirical clinical diagnosis must be carefully considered in light of the evidence available (Gérardin et al. 2002; Maina et al. 2007).
There are some noteworthy limitations of this study that should be discussed briefly. First, the rather few participants (105 P. falciparum infected versus 45 non-malaria children; range, 6 months-12 years). Perhaps, a more large-scale survey is much more likely to be with little bias for severe malaria. Second, the absence of comparative data on widely reported monocytes and platelets detracts from the strength of this study (Erhart et al. 2004; Quintó et al. 2006; Olliaro et al. 2011). Nevertheless, the strong association of low haemoglobin <11 g/dl in patients with P. falciparum infection, as compared to the others argues for recognition of low haemoglobin as an important predictor of malaria in febrile patients.
In conclusion, low haemoglobin concentration (< 11 g/dl) was a significant hematological alteration in children with febrile illness that increases the probability of malarial infection. In malaria endemic settings, the presence of such indicator may heighten the suspicion for malaria particularly when low parasite densities render microscopy and RDTs sub-optimal. Subsequently, a more diligent search for the malarial parasite will inform appropriate therapy to limit the misuse and abuse of anti-malarial drugs.
Acknowledgments
We thank the study participants and their parents/guardians for providing us with the samples to work with. Also, staff of Medical Laboratory Science Department, School of Allied Health Science, University of Ghana, are thanked for their technical support. The study was supported by a Grant from the University of Ghana Research Fund (No. URF/4/007/2011–2012).
References
- Adedapo AD, Falade CO, Kotila RT, Ademowo GO. Age as a risk factor for thrombocytopenia and anaemia in children treated for acute uncomplicated falciparum malaria. J Vector Borne Dis. 2007;44:266–271. [PubMed] [Google Scholar]
- Adu-Gyasi D, Adams M, Amoako S, Mahama E, Nsoh M, Amenga-Etego S, Baiden F, Asante KP, Newton S, Owusu-Agyei S. Estimating malaria parasite density: assumed white blood cell count of 10,000/μL of blood is appropriate measure in Central Ghana. Malaria J. 2012;11:238. doi: 10.1186/1475-2875-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agyapong RNK, Duwiejua M, Bio FY, Woode E, Ansah C, Owusu-Daaku FT, Buabeng KO. Characterization and Treatment of Severe Malaria in Hospitalized Children at a Ghanaian District Hospital. Open Trop Med J. 2009;2:39–44. doi: 10.2174/1874315300902010039. [DOI] [Google Scholar]
- Bell DR, Jorgensen P, Christophel EM, Palmer KL. Malaria risk: estimation of the malaria burden. Nature. 2005;437:E3. doi: 10.1038/nature04179. [DOI] [PubMed] [Google Scholar]
- Dey S, Guha M, Alam A, Goyal M, Bindu S, Pal C, Maity P, Mitra K, Bandyopadhyay U. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic Biol Med. 2009;46:271–281. doi: 10.1016/j.freeradbiomed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Erhart LM, Yingyuen K, Chuanak N, Buathong N, Laoboonchai A, Miller RS, Meshnick SR, Gasser RA, Jr, Wongsrichanalai C. Hematological and clinical indices of malaria in a semi-immune population of Western Thailand. Am J Trop Med Hyg. 2004;70:8–14. [PubMed] [Google Scholar]
- Gérardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–691. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- Ghana Health Service (GHS) Guidelines for case management of malaria in Ghana. Ghana: Ministry of Health; 2009. [Google Scholar]
- Ghana Statistical Service (GSS) (2012) 2010 Population and housing census: summary report of final results
- Guha M, Kumar S, Choubey V, Maity P, Bandyopadhyay U. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20:E439–E449. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M. Assessing discriminatory performance of a binary logistic model: ROC curves. In: Kleinbaum DG, Klein M, editors. Logistic regression—a self-learning text. 3. Edinburgh: Churchill Livingstone; 2010. pp. 346–386. [Google Scholar]
- Koram KA, Owusu-Agyei S, Fryauff DJ, Anto F, Atuguba F, Hodgson A, Hoffman SL, Nkrumah FK. Seasonal profiles of malaria infection, anaemia, and bed net use among age groups and communities in northern Ghana. Trop Med Int Health. 2003;8(9):793–802. doi: 10.1046/j.1365-3156.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- Lathia TB, Joshi R. Can hematological parameters discriminate malaria from nonmalarious acute febrile illness in the tropics? Indian J Med Sci. 2004;58(6):239–244. [PubMed] [Google Scholar]
- Maina RN, Walsh D, Gaddy C, Hongo G, Waitumbi J, Otieno L, Jones D, Ogutu BR. Impact of Plasmodium falciparum infection on haematological parameters in children living in Western Kenya. Malaria J. 2010;9(Suppl 3):S4. doi: 10.1186/1475-2875-9-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsaria N, Mohanty C, Das BK, Mishra SP, Prasad R. Oxidative stress in children with severe malaria. J Trop Pediatr. 2012;58:147–150. doi: 10.1093/tropej/fmr043. [DOI] [PubMed] [Google Scholar]
- Oduro AR, Koram KA, Rogers W, Atuguba F, Ansah P, Anyorigiya T, Ansah A, Anto F, Mensah N, Hodgson A, Nkrumah F. Severe falciparum malaria in young children of the Kassena-Nankana district of northern Ghana. Malar J. 2007;6:96. doi: 10.1186/1475-2875-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro P, Djimdé A, Dorsey G, Karema C, Mårtensson A, Ndiaye J, Sirima SB, Vaillant M, Zwang J. Hematologic parameters in pediatric uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Am J Trop Med Hyg. 2011;85(4):619–625. doi: 10.4269/ajtmh.2011.11-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafo-Addo AD, Koram KA, Oduro AR, Wilson M, Hodgson A, Rogers WO. HLA-DRB1-04 allele is associated with severe malaria in northern Ghana. Am J Trop Med Hyg. 2008;78:251–255. [PubMed] [Google Scholar]
- Owusu-Agyei S, Fryauff DJ, Chandramohan D, Koram KA, Binka FN, Nkrumah FK, Utz GC, Hoffman SL. Characteristics of severe anemia and its association with malaria in young children of the Kassena-Nankana District of northern Ghana. Am J Trop Med Hyg. 2002;67:371–377. doi: 10.4269/ajtmh.2002.67.371. [DOI] [PubMed] [Google Scholar]
- Peeling RW, Smith PG, Bossuyt MMP. A guide for diagnostic evaluations. Nature Rev Microbiol. 2008;8:S2–S6. doi: 10.1038/nrmicro1522. [DOI] [PubMed] [Google Scholar]
- Quintó L, Aponte JJ, Sacarlal J, Espasa M, Aide P, Mandomando I, Guinovart C, Macete E, Navia MM, Thompson R, Menéndez C, Alonso PL. Haematological and biochemical indices in young African children: in search of reference intervals. Trop Med Int Health. 2006;11:1741–1748. doi: 10.1111/j.1365-3156.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Widjaja H, Basri H, Ohrt C, Taufik T, Tjitra E, Baso S, Fryauff D, Hoffman SL, Richie TL. Changes in the total leukocyte and platelet counts in Papuan and non-Papuan adults from northeast Papua infected with acute Plasmodium vivax or uncomplicated Plasmodium falciparum malaria. Malar J. 2008;7:59. doi: 10.1186/1475-2875-7-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen PE, Deroost K, Deckers J, Van Herck E, Struyf S, Opdenakker G. Pathogenesis of malaria-associated acute respiratory distress syndrome. Trends Parasitol. 2013;29(7):346–358. doi: 10.1016/j.pt.2013.04.006. [DOI] [PubMed] [Google Scholar]
- WHO: Severe Falciparum Malaria World Health Organization, communicable diseases cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- Wickramasinghe SN, Abdalla SH (2000) Blood and bone marrow changes in malaria. In: Bailliere’s clinical haematololgy, Vol 13. Harcourt Pub Ltd, p 277–299 [DOI] [PubMed]