Abstract
Various possible causes of proventriculitis include virus, bacteria, fungus, protozoans, nematodes, biogenic amines and excessive copper sulphate. In the present case, parasites were found in the lumen of the proventriculus, gizzard and duodenum of a poultry bird. Characteristic features of the parasite were studied and confirmed as Ascaridia galli. An ulcerative proventriculitis evident as denuded superficial epithelium, sub-epithelial hemorrhages, infiltration of the inflammatory cells and fibrosis were seen at histopathology. Proventriculitis caused by A. galli has not been reported till date. Here, we report a case of ulcerative proventriculitis in a poultry bird caused by nematode, A. galli.
Keywords: Ascaridia galli, Light microscopy, Poultry bird, Ulcerative proventriculitis
Introduction
Proventriculitis may be possibly caused by either viruses (Marusak et al. 2012) viz., infectious bursal disease virus (Pantin-Jackwood et al. 2004), infectious bronchitis virus (Yu et al. 2001), adenovirus (Lenz et al. 1998), reovirus (Kouwenhoven et al. 1988; Lenz et al. 1998); bacteria (Huff et al. 2001); megabacteria (Henderson et al. 1988; Huchzermeyer et al. 1993); Zygomycosis (Jeffery et al. 1994), aspergillosis (Dorner et al. 1983); protozoan Cryptosporidium (Blagburn et al. 1991) and Toxoplasma gondii (Henderson et al. 1988), Dispharynx nasyta (Goble and Kutz 1945); biogenic amines like cadaverine, tryptamine and histamine (Harry et al. 1975, Poole 1994) or excessive copper sulfate (Bayyari et al. 1995). Ascaridia galli is perhaps the most common roundworm in all types of poultry and is distributed across the globe (Ackert 1931; Katakum et al. 2010). We report a rare case of parasitic ulcerative proventriculitis caused by A. galli in a poultry bird.
Materials and methods
An adult poultry bird was presented for post mortem to Poultry disease diagnostic laboratory, Department of Veterinary Pathology, GADVASU, Ludhiana. A thorough necropsy of the bird was conducted and lesions were recorded. The visible parasites were collected in the normal saline solution. They were cleared by keeping in sufficient amount of lactophenol in petridish and identified as per Soulsby (1986). Provetriculus, gizzard and intestine were collected in 10 % neutral buffered formalin and processed as per routine histopathological techniques (Luna 1968) and stained as 5 mm thick tissue sections with hematoxylin and eosin and processed for histopathology. Photographs were taken under Olympus microscope with DP25 camera system.
Results and discussion
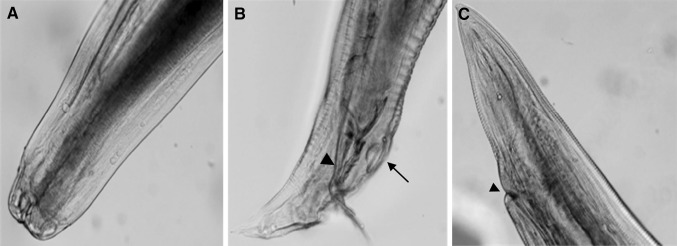
At post mortem examination, nematodes were found inside the lumen of the proventriculus, gizzard and duodenum (Fig. 1). Wall of proventriculus was thickened and mucosa of the proventriculus showed hemorrhagic areas and ulceration. Intestine showed pin point haemorrhages. Four male and six female worms were collected and were identified as A. galli. Adult worms were semi-transparent and yellowish white with male and female parasites ranging from 28–42 mm and 56–82 mm, respectively. Light microscopy revealed the presence of three prominent lips, one dorsally and two ventro-laterally located at the anterior end of the worm (Fig. 2a). Internal side of the lip was lined with fine dentines. Club shaped oesophagus without any posterior bulb were also observed (Fig. 2a). Outer surface of the dorsal lips showed cephalic papillae and sensory cephalic pores. The whole body cuticle except the lips was transversely straited or annulated (Fig. 2a–c). Distinguishing feature for both male and female parasites was the tail or the posterior part. A simple straight tail with a ventrally located anal opening was observed in female (Fig. 2c) while male have curved and pointed tail with a prominent circular precloacal sucker and well developed spicules (Fig. 2b) and also showed characteristic cuticular formation.
Fig. 1.
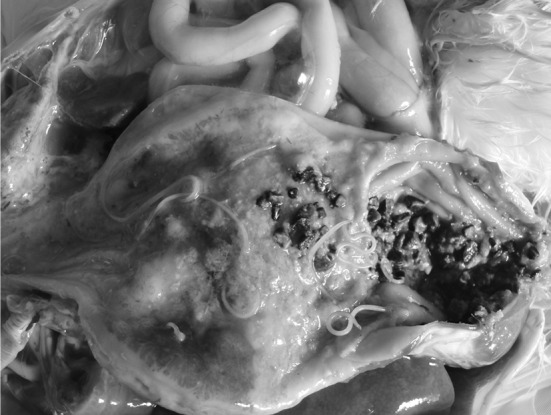
Proventriculus: hemorrhages and ulceration of the glandular mucosa with the presence of nematode
Fig. 2.
Light microscopic features of A. galli. a Light microscopic photograph of the anterior portion of the parasites showing prominent lips and oesophagus. b Posterior portion of the male showing two caudal spicules (arrow head) and circular pre-cloacal sucker (arrow). c Posterior end of the female showing straight and pointed tip tail and anal aperture (arrow head)
Ascaridia galli is the most common nematode of poultry (Ackert 1931; Katakum et al. 2010) having direct life cycle. Ramadan and AbouZnada (1992) have described the detailed morphological features of A. galli. In the present investigation, similar structural features for the parasite were recorded. Histopathologically, proventriculus revealed ulcerative proventriculitis characterized by denuded superficial epithelium, sub-epithelial hemorrhages and underlying fibrosis (Fig. 3). Lymphocytic and monocytic cell infiltration(s) in the underlying stroma was also observed. Few proventricular glands showed areas of fibrosis and mixed cell infiltration occasionally. No larvae per se discernible in the tissue. Secondary bacterial infection was observed in the ulcerated area.
Fig. 3.
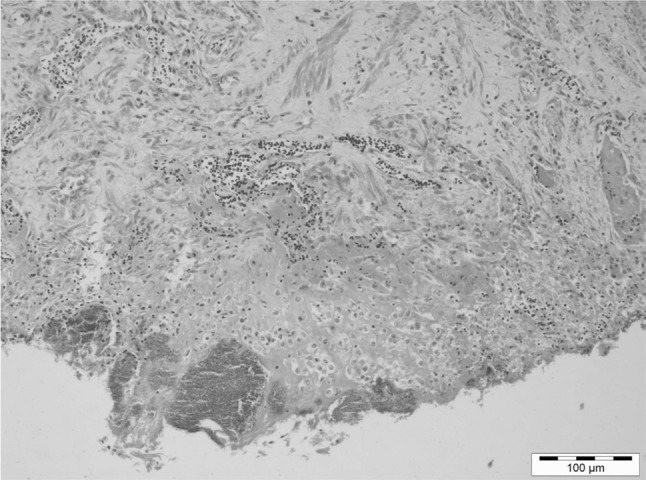
Proventriculus: denuded superficial epithelium, sub-epithelial hemorrhages and underlying fibrosis were observed along with lymphocytic and monocytic cell infiltration(s) in the underlying stroma. Secondary bacterial infection was also observed in the ulcerated area (H & E, Scale Bar 100 µm)
Parasitic proventriculitis is not a common observation in the poultry birds and has been reported by few researchers group. In chickens denuded mucosa and inflammatory changes in the proventriculus due to Cryptosporidium infection has been reported (Goodwin et al. 1996). Similarly, purulent necrotizing proventriculitis due to T. gondii was reported in chicken (Henderson et al. 1988). In several avian species, proventriculus infection with D. nasyta (Goble and Kutz 1945) and Tetrameres species (Norton and Ruff 2003) has been reported. However the current study reports parasitic proventriculus caused by A. galli which is a rare and unique finding till date.
Proventriculus acts as a nidus for hatching of the ingested infective eggs to larvae. The larvae were then localized and burrowed into the duodenal mucosa indicating an early mucosal phase (Luna-Olivares et al. 2012). The adult parasite lives in the lumen of the small intestine (Ackert 1931). Tissue-dwelling larvae cause extensive destruction and erosion of the epithelium as compared to that of adult worms. It also stimulates the proliferation of mucus secreting cells causing adhesion of villi (Ackert and Herrick 1928; Tugwell and Ackert 1952; Ikeme 1971a). Ikeme (1971b) reported that adult worms migrated up and down the intestinal lumen, when present in large numbers. Soulsby (1986) reported that burrowing of parasites in the intestinal mucosa reveals inflammatory lesions and focal hemorrhages.
References
- Ackert JE. The morphology and life history of the fowl nematode Ascaridia lineata (Schneider) Parasitology. 1931;23:360–379. doi: 10.1017/S0031182000013731. [DOI] [Google Scholar]
- Ackert JE, Herrick CA. Effects of the nematode Ascaridia lineata (Schneider) on growing chickens. J Parasitol. 1928;15:1–15. doi: 10.2307/3271596. [DOI] [Google Scholar]
- Bayyari GR, Huff WE, Beasley JN, Balog JM, Rath NC. The effect of dietary copper sulfate on infectious proventriculitis. Poult Sci. 1995;74:1961–1969. doi: 10.3382/ps.0741961. [DOI] [PubMed] [Google Scholar]
- Blagburn BL, Lindsay DS, Hoerr FJ, Davis JF, Giambrone JJ. Pathobiology of cryptosporidiosis (C. baileyi) in broiler chickens. J Protozool. 1991;38:25S–28S. [PubMed] [Google Scholar]
- Dorner JW, Cole RJ, Lomax LG, Gosser HS, Diener UL. Cyclopiazonic acid production by Aspergillus flavus and its effects on broiler chickens. Appl Environ Microbiol. 1983;46:698–703. doi: 10.1128/aem.46.3.698-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble FC, Kutz HL. The genus Dispharynx (nematoda: Acuariidae) in galliform and passeriform birds. J Parasitol. 1945;31:323–331. doi: 10.2307/3273088. [DOI] [PubMed] [Google Scholar]
- Goodwin MA, Hafner S, Bounous DI, Latimer KS, Player EC, Niagro FD, Campagnoli RP, Brown J. Viral proventriculitis in chickens. Avian Pathol. 1996;25:269–279. doi: 10.1080/03079459608419141. [DOI] [PubMed] [Google Scholar]
- Harry EG, Tucker JF, Larsen-Jones AP. The role of histamine and fish meal in the incidence of gizzard erosion and proventricular abnormalities. Br Poult Sci. 1975;16:69–78. doi: 10.1080/00071667508416161. [DOI] [PubMed] [Google Scholar]
- Henderson GM, Gulland FM, Hawkey CM. Haematological findings in budgerigars with megabacterium and trichomonas infections associated with ‘going light’. Vet Rec. 1988;123:492–494. doi: 10.1136/vr.123.19.492. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer FW, Henton MM, Keffen RH. High mortality associated with megabacteriosis of proventriculus and gizzard in ostrich chicks. Vet Rec. 1993;133:143–144. doi: 10.1136/vr.133.6.143. [DOI] [PubMed] [Google Scholar]
- Huff GR, Zheng Q, Newberry LA, Huff WE, Balog JM, Rath NC, Kim KS, Martin EM, Goeke SC, Skeeles JK. Viral and bacterial agents associated with experimental transmission of infectious proventriculitis of broiler chickens. Avian Dis. 2001;45:828–843. doi: 10.2307/1592863. [DOI] [PubMed] [Google Scholar]
- Ikeme MM. Observations on the pathogenicity and pathology of Ascaridia galli. Parasitology. 1971;63:169–179. doi: 10.1017/S003118200007949X. [DOI] [PubMed] [Google Scholar]
- Ikeme MM. Effects of different levels of nutrition and continuing dosing of poultry with Ascaridia galli eggs on the subsequent development of parasite populations. Parasitology. 1971;63:233–250. doi: 10.1017/S0031182000079555. [DOI] [PubMed] [Google Scholar]
- Jeffrey JS, Chin RP, Shivaprasad HL, Meteyer CU, Droual R. Proventriculitis and ventriculitis associated with zygomycosis in ostrich chicks. Avian Dis. 1994;38:630–634. doi: 10.2307/1592090. [DOI] [PubMed] [Google Scholar]
- Katakam K, Nejsum P, Kyvsgaard N, Jorgensen C, Thamsborg SM. Molecular and parasitological tools for the study of Ascaridia galli population dynamics in chickens. Avian Pathol. 2010;39:81–85. doi: 10.1080/03079451003599284. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven B, Vertomment M, Goren E. Investigations into the role of reovirus in the malbsorption syndrome. Avian Pathol. 1988;17:879–892. doi: 10.1080/03079458808436510. [DOI] [PubMed] [Google Scholar]
- Lenz SD, Hoerr FJ, Ellis AC, Toivio-Kinnucan MA, Yu M. Gastrointestinal pathogenicity of adenoviruses and reoviruses isolated from broiler chickens in Alabama. J Vet Diagn Invest. 1998;10:145–151. doi: 10.1177/104063879801000205. [DOI] [PubMed] [Google Scholar]
- Luna LG. Manual of histologic staining methods of the armed forces institute of pathology. 3. New York: McGraw-Hill; 1968. [Google Scholar]
- Luna-Olivares LA, Ferdushy T, Kyvsgaard NC, Nejsum P, Thamsborg SM, Roepstorff A, Iburg TM. Localization of Ascaridia galli larvae in the jejunum of chickens 3 days post infection. Vet Parasitol. 2012;185(2–4):186–193. doi: 10.1016/j.vetpar.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Marusak RA, West MA, Davis JF, Fletcher OJ, Guy JS. Transmissible viral proventriculitis identified in broiler breeder and layer hens. Avian Dis. 2012;56:757–759. doi: 10.1637/10216-042412-Case.1. [DOI] [PubMed] [Google Scholar]
- Norton RA, Ruff MD. Nematodes and acantocephalans. In: Saif YM, editor. Diseases of poultry. 11. IA: Iowa State Press Ames; 2003. pp. 937–938. [Google Scholar]
- Pantin-Jackwood MJ, Brown TP, Kim Y, Huff GR. Proventriculitis in broiler chickens: effects of immunosuppression. Avian Dis. 2004;48:300–316. doi: 10.1637/7099. [DOI] [PubMed] [Google Scholar]
- Poole DR. Proceedings of the 43rd western poultry disease conference. CA: Sacramento; 1994. Biogenic amines: an update; pp. 40–42. [Google Scholar]
- Ramadan HH, AbouZnada NY. Morphology and life history of Ascaridia galli in the domestic fowl that are raised in Jeddah. J King Abdulaziz Univ Sci. 1992;4:87–99. doi: 10.4197/Sci.4-1.9. [DOI] [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domestic animals. 7. London: Ballière Tindall; 1986. pp. 163–164. [Google Scholar]
- Tugwell RL, Ackert JE. On the tissue phase of the life cycle of the fowl nematode Ascaridia galli (Schrank) J Parasitol. 1952;4:277–288. doi: 10.2307/3273760. [DOI] [PubMed] [Google Scholar]
- Yu L, Jiang Y, Low S, Wang Z, Nam SJ, Liu W, Kwangac J. Characterization of three infectious bronchitis virus isolates from china associated with proventriculus in vaccinated chickens. Avian Dis. 2001;45:416–424. doi: 10.2307/1592981. [DOI] [PubMed] [Google Scholar]