Abstract
The present study have been conducted to evaluate the anthelmintic activity of crude aqueous and crude methanolic leaf extracts of Murraya koenigii. Infection of ruminants with gastro-intestinal (GI) parasite has become a worldwide problem. The parasite causes economic losses in a variety of ways. Previously sheep producers relied heavily on anti-parasitic drugs to control gastro-intestinal parasites of the flocks. But due to misuse of these drugs the parasites become resistant to drugs. Thus created interest in studying medicinal plants as an alternative source of controlling the GI parasites. Adult motility assay (AMA) and egg hatch assay (EHA) have been done for in vitro study, and faecal egg count reduction (FECR) assay have been done for in vivo study. The in vitro study revealed anthelmintic effects of M. koenigii on Haemonchus contortus as evident from their paralytic condition and/or death at eight hour post exposure in different concentrations (12.5, 25, 50 mg/ml) of aqueous and methanolic extracts which exhibit to be dose-dependent. Aqueous and methanolic extracts of M. koenigii were found to have low percent inhibitory effect on egg hatching. It may be concluded that M. koenigii showed significant anthelmintic activity.
Keywords: Anthelmintics, Murraya koenigii, Ruminants, Haemonchus contortus
Introduction
Gastrointestinal parasites cause a major constraint for the breeding of small ruminants throughout the world. This problem explains the current interest in search of alternative solutions in order to reduce the parasites by chemotherapy. In particular, there is renewed interest in veterinary drugs and plants with anthelmintic properties in both temperate and tropical countries (Hammond et al. 1997; Waller et al. 2001).
Compared with other GI nematode parasites, H. contortus is a highly pathogenic parasite of live stock and is capable of causing acute disease and high mortality in small ruminants. Commercial anthelmintic drugs have been used for some decades throughout the world to minimize the losses caused by helminth infections. However, the threats of anthelmintic resistance, availability, and high cost especially to farmers of low income group have led to the need of other alternative control methods (Baker et al. 1992).
Tandon et al. (1997) studied in vitro anthelmintic activity of root tuber extract of Flemingia vestita against Paramphistomum sp. The treatment of gastro-intestinal parasites with the crude extract (50 mg/ml) in PBS revealed complete immobilization of the trematodes in about 43 min. Akhtar et al. (1999) studied the anthelmintic activity of Chenopodium album against sheep nematodes. The aqueous and ethanolic extracts of C. album showed significant percentage of egg reduction.
Athanasiadou et al. (2000) studied the possible direct anthelmintic effect of a condensed tannins (CTs) extract (Quebracho extracted from Schinopsis) on the population and fecundity of the T. colubriformis. In the treated sheep, the worm burdens and EPG of faeces per worm had been reduced by 30 % in comparison to control sheep. In 2001 the same authors studied the possible direct anthelmintic effects of CT from Quebracho towards different ovine GI nematode larvae (H. contortus, T. circumcinata and T. vitrinus). Brelin (2002) evaluated the Neem leaves (Azadirachta indica) as an alternative anthelmintic for helminth control. They observed that fresh neem leaves significantly reduced the numbers of H. contortus in the abomassum of the treated sheep.
Alawa et al. (2003) examined the in vitro anthelmintic activity of Vernonia amygdalina and Annona senegalensis extracts against H. contortus eggs and reported that the extract of V. amygdalina did not show any significant activity at concentrations up to 11.2 mg/ml. However, the extract of A. senegalensis showed significant (P < 0.001) reduction in egg hatch at a concentration of 7.1 mg/ml. Lateef et al. (2003) explored in vitro and in vivo anthelmintic activity of Adhatoda vasica in comparison to levamisole. The in vitro studies revealed anthelmintic effects (P ≥ 0.05) of crude aqueous extracts (CAE) and crude methanolic extracts (CME) of A. vasica on H. contortus as evident from their mortality.
Ademola et al. (2004) studied the in vitro and in vivo anthelmintic activity of ethanolic as well as aqueous extracts of Khaya senegalensis against GI nematodes of sheep and reported that nematode larvae decreased after treatment. Maphosa et al. (2010) evaluated the crude aqueous extracts of leaves of Aloe ferox and Leonotis leonurus and roots of Elephantorrhiza elephantine for anthelmintic activity. They reported that inhibition of egg hatching and larval development increased significantly (P < 0.05) with increasing concentrations of the crude extracts. E. elephantina and L. leonurus extracts had 100 % egg hatch inhibition at concentration as low as 2.5 and 1.25 mg/ml respectively, whereas extracts of A. ferox had 100 % inhibition at concentrations of 20 mg/ml. At the lowest concentration tested (0.625 mg/ml), E. elephantina inhibited egg hatching is greater than 96 % and this was comparable to albendazole at the same concentration. However, E. elephantina and L. leonurus inhibited totally the larval development at concentrations of 1.25 mg/ml. Jaliwala et al. (2011) conducted a study to evaluate the anthelminthic activity of Argemone mexicanain aqeous and alcoholic extracts. Both alcoholic and aqueous extracts has shown significant anthelmintic activity in comparison to standared drug albendazole.
The present study was designed to evaluate the in vitro and in vivo anthelmintic efficacy of crude aqueous and crude methanolic leaf extracts of M. koenigii upon gastrointestinal nematode parasites.
Materials and methods
Collection of plant materials
Fresh leaves of locally available medicinal plant namely, M. koenigii L. (Curry) (Fig. 1) were collected from the garden of the Department of Botany, University of Kalyani and Faculty of Agriculture, Bidhan Chandra Krishi Viswavidyalaya, Kalyani campus, West Bengal. The plants were identified based on their physical characteristics by the plant taxonomists. M. koenigii belonging to family Rutaceae is used as a spice for its characteristic flavour and aroma. It is reported to have anti- oxidant, anti-diabetic, anticarcinogenic, antidysenteric, stimulant, hypoglycaemic and antimicrobial activities (Khanu et al. 2000; Ningappa et al. 2008). Phytochemical investigations on this plant revealed the presence of carbazole alkaloid and couramin (Adebajo and Reisch 2000).
Fig. 1.

Photograph of M. koenigii
Preparation of plant extracts
Aqueous extract
The crude aqueous extracts of selected plant were prepared according to the method as described by Iqbal et al. (2006a). The powdered plant parts (500 g) were extracted with distilled water (2,000 ml) at 90–100 °C in a Soxhlet extractor for 8 h. The extracts were filtered, evaporated under reduced pressure of 22–26 mmHg in a vacuum rotary evaporator at 55 °C and the aqueous extract were stored at 4 °C for further use.
Methanolic extract
The powdered plant parts (500 g) were extracted with methanol (2,800 ml, Qualigens) at 60 °C in a Soxhlet extractor using for 8 h. The extracts were filtered, evaporated under reduced pressure of 22–26 mmHg in a vacuum rotary evaporator at 55 °C and methanolic extract were stored at 4 °C until used.
In vitro study
Parasite collection
Adult female parasites of H. contortus were collected from the abomasum of infected sheep from local abattoir. Then the worms were washed and crushed to liberate eggs. The eggs were then cultured in a plastic jar filled with sterilised sheep faeces for 8 days at room temperature. At the end of the eighth day, the infective (L3) larvae were harvested by rinsing the side of the culture jar with a drop of water. After overnight withdrawal of feed and water two sheep were administered orally with the third stage infective larvae (L3) at the dose rate of seven hundred L3/kg body weight using a 50-ml plastic syringe. These sheep served as H. contortus egg donors for the egg hatch assay trial.
Egg hatch assay (EHA)
Egg hatch assay was conducted following the procedure described by Coles et al. (2006). Approximately, fifty eggs were collected per tube; each tube contained 1 ml of phosphate buffer saline (PBS) and 1 ml of increasing concentrations of plant extracts (75, 150, 300, 600, 1,200 and 2,400 μg/ml) prepared with phosphate buffer saline (PBS). Each concentration was tested on five replicates. In addition, levamisole (0.125 mg/ml) was used as positive control and PBS as negative controls in the experiment. The tubes were covered, and the eggs were incubated for 48 h at 27 °C. Thereafter, the number of the first stage larvae (L1) present per tube was counted using a dissecting microscope. An inhibition percentage (%) of egg hatching was calculated for each extract concentration using the following modified formula of Coles et al. (1992):
where X1 is the number of eggs hatched in test extracts, and X2 is the respective number in Phosphate Buffer Saline (PBS) control.
Adult motility assay (AMA)
The abomasum of sheep slaughtered at the local abattoir were brought to the laboratory. Adult stages of H. contortus were manually picked up from the mucosal surface and the contents of the abomasum have been kept in phosphate buffer saline (PBS).The experiment was conducted according to Eguale et al. (2007). Fifty actively moving worms were placed in petri-dishes containing 12.5, 25 and 50 mg/ml of aqueous and methanolic extracts of plants in PBS and PBS alone for the control group in a total volume of 4 ml. Levamisole was used as the positive control. Five replications per each treatment concentration were employed. After 24 h, the plant extracts and levamisole were washed away and the parasites suspended in PBS for 30 min for possible recovery of parasite motility. The numbers of motile and immotile worms were counted. Motility and viability of the worms were assessed by gently prodding the worms using a pointed probe or forceps. The response was recorded either as live or dead condition. Observations were made on the motility or survivality of parasites at 0, 2, 4, 6 and 8 h post-exposure (PE).
Mortality index (MI): The mortality index have been calculated by adopting the following formula.
In vivo study
Animals and experimental design
A total twenty four sheep of both genders (one year of age) having naturally acquired gastro-intestinal (GI) nematode infection were collected. In vivo studies were performed using the faecal egg count reduction assay. All the sheep were pre-adapted to the pen conditions for 18 days prior to commencement of the study. The water, hay and feed were provided regularly to the experimental animals. The experiment continued for a period of 15 days post-treatments. Before the commencement of the experiment, the animals were confirmed positive with an infection of mixed gastro-intestinal (GI) nematode parasites by faecal examination using the standard parasitological procedures applicable to detection of nematode eggs in sheep faeces (Soulsby 1982). The animals used for the experiment were randomly divided into six treatment groups (i.e. Gr I, Gr II, Gr III, Gr IV, Gr V and Gr VI) of four animals each on the basis of faecal egg counts (mean ± S.E. of eggs per gram of faeces) and assigned to different treatments which were administered orally using a syringe. Group I received a single dose of crude methanolic extract (CME) at 1.0 g kg−1 body weight (bw). Group II received crude aqueous extract (CAE) at 1.0 g kg−1 body weight (bw). Group III received crude methanolic extract (CME) at 2.0 g kg−1 body weight (bw). Group IV received crude aqueous extract (CAE) at 2.0 g kg−1 body weight (bw). Group V received levamisole at 7.5 mg kg−1 body weight (bw) as positive control. Group VI received no treatment and served as negative control. Each group was isolated from other groups and no physical contact was possible between them from different treatment groups.
Laboratory procedure
To determine the faecal egg count reductions of gastro-intestinal (GI) nematode parasites in sheep, faecal samples of each animal in the respective treatment groups were collected directly from the rectum in the morning, starting from day zero and at days five, ten and fifteen post-treatment (PT). The faecal samples were homogenized so that the eggs were uniformly distributed throughout the faeces prior to counting. The total numbers of nematode eggs (faecal egg counts) were determined using McMaster egg counting technique (Soulsby 1982); with each egg counted representing fifty eggs per gram of faeces. Faecal egg count percent reduction (FECR %) was calculated using the following formula:
Phytochemical screening
Preliminary qualitative screenings for major secondary metabolites of the medicinal plant were conducted according to Debella (2002). The plant materials were screened for the presence of protein and amino acid, fatty acid, carbohydrate, tannin, saponin, alkaloid, flavonoid, steroid, triterpenoid, glycoside and phenol.
Statistical analysis
The statistical package-SPSS-10.0 was applied for the analysis of data and P < 0.05 was taken as the level of significance.
Results
The major secondary metabolites detected were tannin, saponin, phenol, triterpenoid and flavonoid (Table 1).
Table 1.
Phytochemical analysis of methanolic leaf extract of M. koenigii
| Protein and amino acid | – |
| Fatty acid | − |
| Carbohydrate | − |
| Tannin | + |
| Saponin | + |
| Alkaloid | − |
| Flavonoid | + |
| Steroid | − |
| Triterpenoid | + |
| Glycoside | − |
| Phenol | + |
+ indicates presence; − indicates absence
Adult motility assay (AMA)
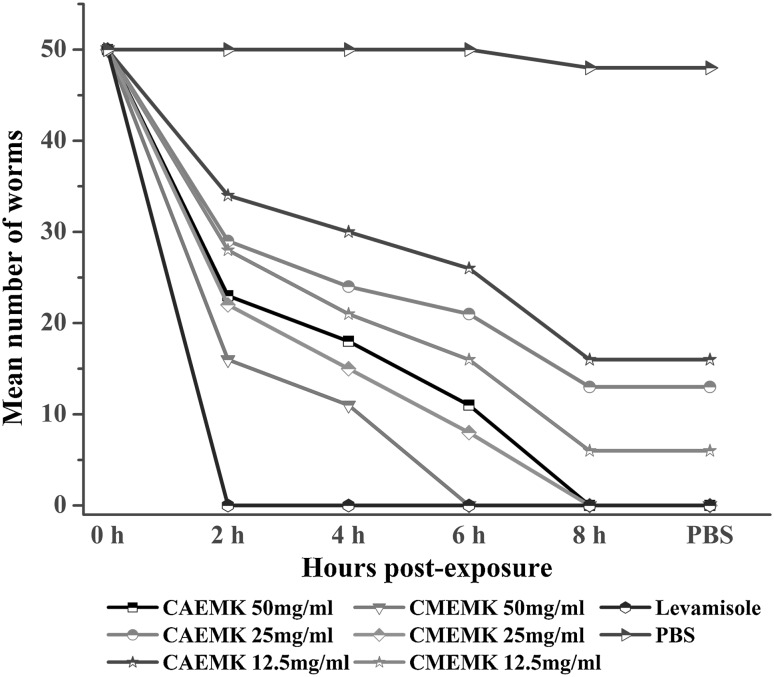
Effect of crude aqueous extracts and crude methanolic extracts of M. koenigii (CMEMK) was dose-dependent (Fig. 2). Highest mortality (100 %) of worms have been observed in eight hours post-exposure @ 50 mg/ml of crude methanolic extract. It resulted in mean percentage worm motility inhibition (%WMI) of 93.52 %, as observed after the worms were put in PBS for thirty minutes after exposure to different treatments. The percentage of worm mortality index of the crude methanolic extract and crude aqueous extract of M. koenigii (CAEMK) were hundred percent after eight hours post treatment. The methanolic extract of M. koenigii was more potent than the aqueous extract. There was hundred percent mortality of worms in levamisole (used as a reference drug) within two hours post-exposure. The result of in vitro anthelmintic activity of crude methanolic extract of M. koenigii as compared with levamisole was statistically non-significant, and was statistically significant when compared with PBS.
Fig. 2.
In vitro anthelmintic efficacy of crude extracts of M. koenigii on H. contortus
Egg hatch assay (EHA)
The crude aqueous and crude methanolic extracts of M. koenigii exhibited ovicidal effects; whereas, egg hatching was not affected by the distilled water (negative control). Inhibition of egg hatching (percent egg hatch) for H. contortus exposed to extracts of M. koenigii was low in comparison to eggs exposed to levamisole. Crude methanolic extract (LC50 = 2.25 mg/ml) was found higher inhibitory effects in comparison to that of aqueous extract (LC50 = 2.90 mg/ml) on egg hatching. The data of correlation of regression revealed a dose dependent response of plant extracts both for aqueous and methanolic. Lethal concentration 50 (LC50) for the inhibition of egg hatching have been shown in Table 2.
Table 2.
Regression values and correlation of regression of the effect of M. koenigii different extracts on egg hatching of H. contortus
| Treatment | LC50 | Regression values and correlation of regression |
|---|---|---|
| Methanolic extract of M. koenigii | 2.25 | y = −0.0002x + 4.6331, R2 = 0.9685 |
| Aqueous extract of M. koenigii | 2.90 | y = −0.0003x + 5.1328, R2 = 0.9141 |
| Levamisole | 1.80 | y = −0.2161x + 6.2452, R2 = 0.755 |
Faecal egg count reduction test (FECRT)
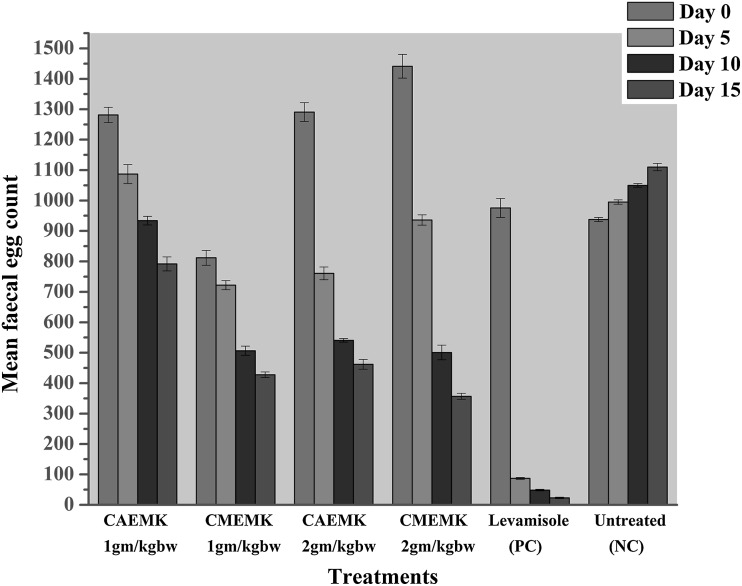
The mean eggs per gram counts (EPG) and percentage reduction in faecal egg counts of Bonpala sheep treated with different doses of the aqueous and methanolic extracts of M. koenigii and levamisole have been presented in Fig. 3. The in vivo anthelmintic activity (in terms of egg count percentage reduction) of methanolic extracts of M. koenigii in sheep infected with mixed species of GI parasites demonstrated significant anthelmintic activity of extracts tested. The result revealed that a gradual reduction in faecal egg count (%FEC) of experimental animals treated with both the aqueous and methanolic plant extracts and were significant (0 < 0.5) on day fifteen post-treatment from day zero pre-treatment.
Fig. 3.
Mean faecal egg counts and percentage reduction in egg counts for M. koenigii extracts
Discussion
The result of the present study suggested that the plant extracts exhibited significant in vitro and in vivo anthelmintic activity against H. contortus of sheep, and has the potential to contribute the control of gastro-intestinal parasites of sheep. As recorded in the recent study, larvicidal and ovicidal effects of some plants have also been reported earlier against H. contortus eggs and larvae (Ketzis et al. 2002; Assis et al. 2003; Hordegen et al. 2003; Maciel et al. 2006; Hordegen et al. 2006; Bizimenyera et al. 2006; Al-Shaibani et al. 2009). Molla and Bandyopadhyay (2013) reported anthelmintic activity of crude aqueous and methanolic extract of P. guajava against H. contortus. The study showed highest nematode mortality in the higher concentrations of methanolic extract rather than in aqueous extracts of the same concentration. This result could be explained by the fact that the methanolic extracts possess several chemical compounds, and many of them could display ovicidal activity. The total action of the extracts is a sum of the activities of their constituents (Rates 2001). The phytochemicals obtained from P. guajava were flavonoid, triterpenoid, fatty acid and tannin (Table 1).
Min and Hart (2003) postulated that the condensed tannin decrease the viability of the larval stages of the nematode parasites and also interfere with the parasite egg hatching and development to infective larval stage. The faecal egg count reduction test was the first test developed for evaluating anthelmintic efficacy and remains the one most widely used for routine diagnosis in commercial flocks and herds. The test provides an estimation of anthelmintic efficacy by comparing faecal egg counts from hosts treated or not, before and after treatment and thus evaluating faecal egg count reduction (Cabaret and Berrag 2004). Iqbal et al. (2006b) revealed significant in vitro anthelmintic activity of crude aqueous extracts and crude methanolic extracts of Swertia chirata against H. contortus. The ethanolic extract of seed of Melia azedarach was the most active on eggs (LC50 = 0.36 mg mL−1) and the leaf ethanol extract showed the best inhibition of larval development (LC50 = 9.18 mg mL−1) of H. contortus (Maciel et al. 2006). Alawa et al. (2003) reported that the extract of Annonasenegalensis showed significant (P < 0.001) reduction in egg hatch (H. contortus) at a concentration of 7.1 ml−1. The less effectiveness was observed for Spigelia anthelmia extracts on H. contortus egg hatching that was effective in 50 mg ml−1 concentration (Assis et al. 2003).
Githiori et al. (2003) observed significant reduction in faecal egg count of H. contortus of sheep after treatment with Albizia anthelmintica, Olea europaea, Annona squamosa and Ananas cosmosus water extracts. The efficacy of Myrisine africana, Albizia anthelmintica and Hilderbrantia sepalosa against nematode parasites (Haemonchus spp., Trichostrongylus spp., Oesophagostomum spp. and Moniezia spp.) was 77, 89.9, 90 and 100 % respectively (Gathuma et al. 2004).
Beriajaya et al. (1998) recorded the in vitro anthelmintic effect of different concentrations of Zingiber purpureum infusion (0, 2.5, 5, 10, 20, 40 % w/v) and extract (0, 0.5, 1, 2, 4 and 8 % w/v) on adult worms of H. contortus. Singh et al. (2004) also reported the in vitro anthelmintic efficacy of methanolic, chloroform and aqueous extracts of Allium sativum, Areca catuha, Azadirachta indica, Butea monosperma, Mallotus phillipensis, Psoralea corylifolia, Pygeumpersica, Scindapsus officinalis and Vernonia anthelminticd compared with fenbendazole against H. contortus of sheep.
Conclusion
Based on the results of the present experiment it may be concluded that M. koenigii possess adequate in vitro and in vivo anthelmintic activity against different parasitic stages including eggs and adults of gastro-intestinal nematode parasites of sheep. However, more detailed studies are needed to identify and evaluate the active components and the mechanisms of action of M. koenigii extracts and also studies on toxicity and evaluation of the effects.
Acknowledgments
One of the authors (SHM) is thankful to the University Grants Commission, New Delhi for providing a fellowship in the form of Maulana Azad National Fellowship to carry out the research work.
References
- Adebajo AC, Reisch J. Minor furocoumarins of Murraya koenigii. Fitoterap. 2000;71:334–337. doi: 10.1016/S0367-326X(99)00163-X. [DOI] [PubMed] [Google Scholar]
- Ademola IO, Fagbnemi BO, Idowu SO. Evaluation of the anthelmintic activity of Khaya senegalensis extract against gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet Parasitol. 2004;122:151–164. doi: 10.1016/j.vetpar.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Akhtar MS, Iqbal Z, Khan MN. Evaluation of anthelmintic activity of Chenopodium album (Bathu) against nematodes in sheep. Int J Agric Biol. 1999;1(3):120–124. [Google Scholar]
- Alawa CBI, Adamu AM, Gefu JO, Ajanusi OJ, Abdu PA, Chiezey NP, Alawa JN, Bowman DD. In vitro screening of two Nigerian medicinal plants (Vernonia amygdalina and Annona senegalensis) for anthelmintic activity. Vet Parasitol. 2003;113:73–81. doi: 10.1016/S0304-4017(03)00040-2. [DOI] [PubMed] [Google Scholar]
- Al-Shaibani IRM, Phulan MS, Shiekh M. Anthelmintic activity of Fumaria parviflora (Fumariaceae) against gastrointestinal nematodes of sheep. Int J Agric Biol. 2009;11:431–436. [Google Scholar]
- Assis LM, Bevilqua CML, Morais SM, Vieira LS, Costa CTC, Souza JAL. Ovicidal and larvicidal activity in vitro of Spigelia anthelmia Linn. extracts on Haemonchus contortus. Vet Parasitol. 2003;117:43–49. doi: 10.1016/j.vetpar.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Athanasiadou S, Kyriazakis I, Jackson F, Coop RL. Consequences of long-term feeding with condensed tannin on sheep parasitized with Trichostrongylus colubriformis. Int J Parasitol. 2000;3:1025–1033. doi: 10.1016/S0020-7519(00)00083-7. [DOI] [PubMed] [Google Scholar]
- Baker RL, Reynolds L, Lahlou KA, Rege JE, Bekele T, Mukassa M, Rey B (1992) Prospects of breeding for resistance to endoparasites in small ruminants in Africa. In: Proceedings of the second conference of small ruminant research network
- Beriajaya P, Murdiati TB, Herawaty M. Anthelmintic effect of Zingiber purpureum infusion and extract on adult worms of Haemonchus contortus in vitro. J Ilmu Ternak Dam Vet. 1998;3:277–282. [Google Scholar]
- Bizimenyera ES, Githiori JB, Eloff JN, Swan GE. In-vitro activity of Peltophourum africanum Sond. (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubrifrmis. Vet Parasitol. 2006;142(3–4):336–343. doi: 10.1016/j.vetpar.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Brelin D. Evaluation of the neem tree (Azadirachta indica) as an alternative anthelmintic for helminth control in small ruminants in Malaysia. Minor field studies. Uppsala: Swedish University ofAgricultural Sciences; 2002. p. 31. [Google Scholar]
- Cabaret J, Berrag B. Faecal egg count reduction test for assessing anthelmintic efficacy: average versus individually based estimations. Vet Parasitol. 2004;121:105–113. doi: 10.1016/j.vetpar.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Coles GC, Buer C, Borgsteede FHM, Gerts S, Klei TR, Taylor MA, Waller PJ. World association for the advancement of veterinary parasitology (WAAP). Methods for detection of antihelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-U. [DOI] [PubMed] [Google Scholar]
- Coles GC, Jackson F, Pomroy WE, Prichard RK, Von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136(3–4):167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Debella A (2002) Manual of phytochemical screening of medicinal plants, Ethiopian health and nutrition research institute, p 84
- Eguale T, Tilahun G, Debella A, Feleke A, Makkonen E. Haemonchus contortus: in vitro and in vivo anthelmintic activity of aqueous and hydroalcoholic extracts of Hedera helix. Exp Parasitol. 2007;116:340–350. doi: 10.1016/j.exppara.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Gathuma JM, Mbaria JM, Wanyama J, Kaburia HFA, Mpoke L, Mwangi JN. Efficacy of Myrisine africana, Albizia anthelmintica and Hilctebrandtia sepalosa herbal remedies against natural sheep helminthosis in Samburu district, Kenya. J Ethnopharma. 2004;91:7–12. doi: 10.1016/j.jep.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Githiori JB, Hoglund J, Waller PJ, Baker RL. The anthelmintic efficacy of the plant, Albizia anthelmintica, against the nematode parasites Haemonchuscontortus of sheep and Heligmosomoides pofygyrus of mice. Vet Parasitol. 2003;116:23–34. doi: 10.1016/S0304-4017(03)00218-8. [DOI] [PubMed] [Google Scholar]
- Hammond JA, Fielding D, Bishop SC. Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Com. 1997;21:213–228. doi: 10.1023/A:1005884429253. [DOI] [PubMed] [Google Scholar]
- Hordegen P, Hertzberg H, Heilmann J, Langhans W, Maurer V. The anthelmintic efficacy of five plant products against gastrointestinal trichostrongylids in artificially infected lambs. Vet Parasitol. 2003;117:51–60. doi: 10.1016/j.vetpar.2003.07.027. [DOI] [PubMed] [Google Scholar]
- Hordegen P, Cabaret J, Hertzberg H, Langhans W, Maurer V. In vitro screening of six anthelmintic plant products against larval Haemonchuscontortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J Ethnopharm. 2006;108:85–89. doi: 10.1016/j.jep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Lateef M, Akhter MS, Gayur MN, Gilani AH. In vivo anthelmintic activity of ginger against gastrointestinal nematodes of sheep. J Ethnopharm. 2006;106:285–287. doi: 10.1016/j.jep.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Lateef M, Khan MN, Jabbar A, Akhter MS. Anthelmintic activity of Swertia chirata against gastrointestinal nematodes of sheep. Fitoterap. 2006;77(6):463–465. doi: 10.1016/j.fitote.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Jaliwala YA, Panda PK, Chourasia N, Kumar BN, Amit P, Mohanty PK. In vitro anthelmintic activity of aerial parts of Argemone mexicana Linn. J Phar Res. 2011;4(9):3173–3174. [Google Scholar]
- Ketzis JK, Taylor A, Bowmann DD, Brown DL, Warnick LD, Erb HN. Chenopodium ambrosioides and its essential oil as treatments for Haemonchus contortus and mixed adult nematode infections in goats. J Sm Rum Res. 2002;44:193–200. doi: 10.1016/S0921-4488(02)00047-0. [DOI] [Google Scholar]
- Khanu F, Anilakumar KR, Sudarshana Krishna KR, Viswanathan KR, Santhanam K. Anticarcinogenic effects of curry leaves in dimethylhydrazine-treated rats. Pl F Hum Nut. 2000;55:347–355. doi: 10.1023/A:1008148531495. [DOI] [PubMed] [Google Scholar]
- Lateef M, Iqbal Z, Khan MN, Akhtar MN, Akhtar MS, Jabbar A. Anthelmintic activity of Adhatoda vesica roots. Int J Agric Biol. 2003;5:86–90. [Google Scholar]
- Maciel MV, Morais SM, Bevilaqua CML, Camurca-Vasconcelos ALF, Costa CTC, Castro CMS. Ovicidal and larvicidal activity of Melia azedarach extracts on Haemonchus contortus. Vet Parasitol. 2006;140:98–104. doi: 10.1016/j.vetpar.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Maphosa V, Masika PJ, Bizimenyera ES, Eloff JN. In-vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotisleonurus and Elephantorrhiza elephantina against Haemonchus contortus. Trop Anim Heal Prod. 2010;42:301–307. doi: 10.1007/s11250-009-9421-9. [DOI] [PubMed] [Google Scholar]
- Min BR, Hart SP. Tannins for suppression of internal parasites. J Anim Sci. 2003;81:102–109. [Google Scholar]
- Molla SH, Bandyopadhyay PK (2013) In vitro anthelmintic activity of Psidium guajava against sheep gastrointestinal nematode, Haemonchus contortus. Env Ecol (In press)
- Ningappa MB, Dinesha R, Srinivas L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. F Chem. 2008;106:720–728. doi: 10.1016/j.foodchem.2007.06.057. [DOI] [Google Scholar]
- Rates SM. Plants as source of drugs. Toxic. 2001;39(5):603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Singh MP, Mahesh K, Ahmad AM. Efficacy of some ethnomedicinal plants against Haemonchus contortus. Ind J Vet Med. 2004;24:1–4. [Google Scholar]
- Soulsby EJL. Helminths, Arthropods and Protozoa of domesticated animals. 7. London: The English Language Book Society and Bailliere Tindall; 1982. pp. 763–773. [Google Scholar]
- Tandon V, Pal P, Roy B, Rao HSP, Reddy KS. In vitro anthelmintic activity of root-tuber extract of Flemengia vestita, an indigenous plant in Shillong, India. Parasitol Res. 1997;83:492–498. doi: 10.1007/s004360050286. [DOI] [PubMed] [Google Scholar]
- Waller PJ, Bernes G, Thamsborg SM, Sukura A, Richter SH, Ingebrigtsen K, Hoglund J. Plants as de-worming agents of livestock in the Nordic countries: historical perspective, popular beliefs and prospects for the future. Act Vet Scand. 2001;42:31–44. doi: 10.1186/1751-0147-42-31. [DOI] [PMC free article] [PubMed] [Google Scholar]