Abstract
Gastrointestinal trichostrongyles of small ruminants are one of the major causes of productivity loss. Epidemiological study was carried out to determine parasitic infection of sheep with abamossal nematodes at various abattoirs in Srinagar district of Kashmir Valley from August 2011 to July 2012. On the basis of necroscopy, out of representative 281 abamossa, 53.3 % were recorded to be infected with Haemonchus species and 41.2 % with Ostertagia spp. Thus, Haemonchus spp. were more prevalent than Ostertagia spp. in ovines (P > 0.05). Infection prevalence percentage of Haemonchus spp. was highest in late summer season and early rainy season (62.85 %) with peak value in the month of July (71.42 %) and lowest in winter (42.85 %) with minimum value in the month of February (40 %). Similar trend was seen with Ostertagia spp. having highest infection prevalence value during summer season (52.8 %) with peak values in the month of July (64.2 %) and lowest infection in winter (34.2 %) with minimum value in February (30 %). Moreover, non-local breeds were more prevalent than local ones (P = 0.05).
Keywords: Trichostrongyles, Sheep, Srinagar, Abamossa, Haemonchus and Ostertagia
Introduction
Animal husbandry plays a significant role in Jammu and Kashmir, as 0.13 per cent of gross domestic product (GDP) of the state is contributed by this sector (Jammu Kashmir development report). The unprecedented growth in demand for livestock products has recently acquired the label of the ‘livestock revolution’ (Delgado et al. 1999). Thus, it has become important to optimize agricultural production through improved management practices and the control of production limiting diseases such as helminth infections.
Infection by gastrointestinal trichostrongyles (Nematoda:Strongylida) is a major global threat for sheep production (Waller 1999). These parasites impair animal health and welfare and cause significant economic losses. The species of nematodes that affect sheep the most belong to superfamily Trichostrongyloidea (Bowman et al. 2003). Among these parasites, abamossal nematodes namely Haemonchus spp. and Ostertagia spp. cause great loss of small ruminants.
Haemonchus, the “barber pole worm” is the most deadly parasite in sheep and goats and is a highly pathogenic, blood sucking nematode causing clinical signs of anemia, hypoproteinia, edema, and death (Bennet 1983; Smith and Sherman 1994). Ostertagia, the “brown stomach worm” is recognized as the most economically damaging parasite for ruminants. The present study was designed to study the prevalence of Haemonchus spp. and Ostertagia spp. in relationship to breed and season.
Materials and methods
Epidemiological study was carried out to determine parasitic infection of sheep with abamossal nematodes at various abattoirs in Srinagar district of Kashmir Valley from August 2011 to July 2012. A sum of total 281complete abamossa from gastrointestinal tracts of sheep were obtained and brought to the Parasitology Lab, Department of Zoology, University of Kashmir, for examination. Nematodes were obtained from abamossa and identified under microscope. Each abomasum was isolated in situ with the use of a ligation between the small intestine and abomasum, after which the abomasum was removed and processed for recovery of adult nematodes. Abomasal contents and washes were combined and aliquoted, with 2–10 % duplicate aliquots being collected and preserved in ethanol (70 %) and examined with microscopy via temporary slide preparation(Cable 1958). Species identification was conducted as described previously (Solusby 1982). Data was properly synthesized and statistical analysis was done to see whether difference was significant using Mini Tab statistical programme. P value ≤ 0.05 was considered to be significant difference.
Results and discussion
Overall prevalence of Haemonchus and Ostertagia spp. in sheep
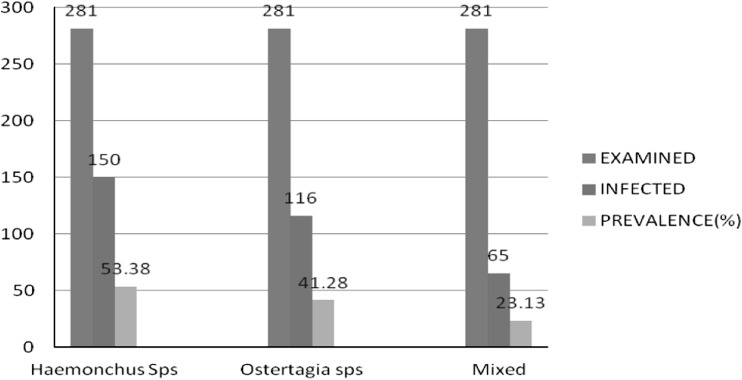
Out of representative 281 abamossae, 201 were found to be positive for two spp. of Trichostrongylides giving an overall prevalence of 71.5 %. Haemonchus spp showed higher prevalence of 53.4 % as compared to Ostertagia spp. of 41.3 %. The higher prevalence of Haemonchus spp. could be due to the fact that this nematode has a relatively short generation interval and ability to take the advantage of favorable environmental conditions (Grant 1981). Moreover; it was found that mixed infection of both spp. was 23.13 % (Fig. 1).
Fig. 1.
Species wise prevalence of abamossal nematodes
Month-wise prevalence rate of Haemonchus and Ostertagia spp. in sheep
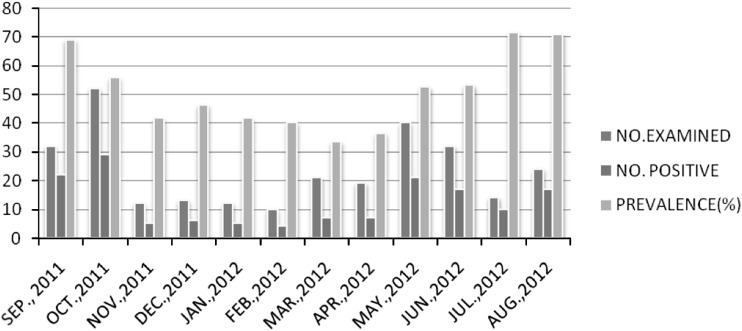
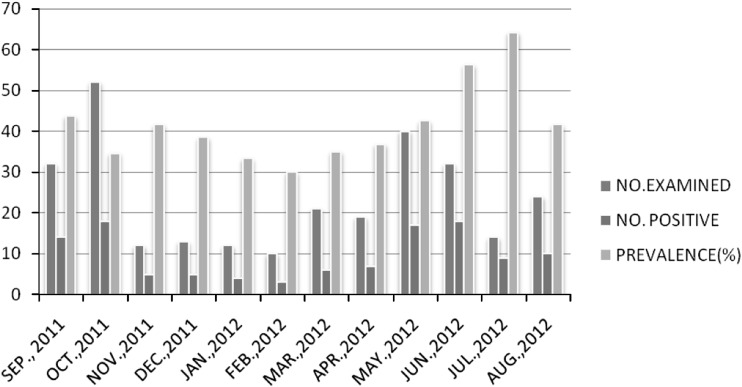
The findings revealed that the infected animals harbor Haemonchus and Ostertagia infection throughout the year with varied prevalence. The highest infection prevalence of Haemonchus was in summer season (62.85 %) with peak value in the month of July (71.42 %) and lowest in winter (42.85 %) with minimum value in the month of February (40 %). Similar trend was seen with Ostertagia sps having highest percentage infection value during summer season (52.8 %) with peak values in the month of July (64.2 %) and lowest infection in winter (34.2 %) with minimum value in February (30 %) (Figs. 2, 3).
Fig. 2.
Monthwise prevalence of Haemonchus spp
Fig. 3.
Monthwise prevalence of Ostertagia spp. during survey period
Season-wise prevalence rate of Haemonchus and Ostertagia spp. in sheep
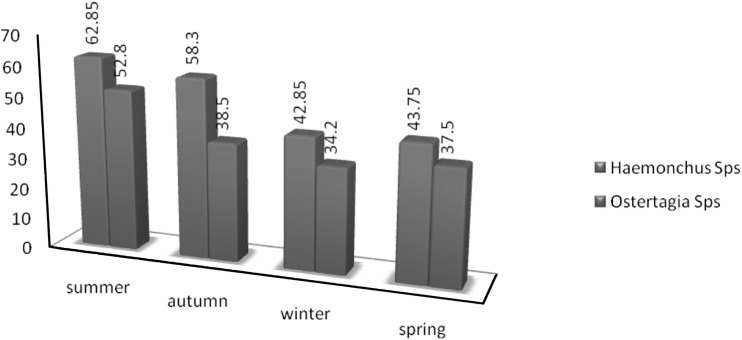
The seasonal dynamics is essential for parasite growth and development (Teel et al. 1996). The seasonal dynamics of nematode infection are the consequence of 20 complex inter-relationships between the sheep, their husbandry and the prevailing climate (Viassoff et al. 2001). The present study reported that the highest abamossal nematode infection was in summer season and declined onwards till spring with lowest prevalence in winter season. The low infection of Haemonchus spp. was reported in the winter season (42.85 %) which could be attributed to complete absence of grazing in winter and also important is low temperature which helps in hypobiosis in host (Ogunsuri and Eysker 1979). The presence of sufficient moisture and optimum temperature conditions during the rainy seasons (summer) favoured the survival of infective larvae of Haemonchus in the pasture and higher probability of uptake of the infective larvae leading to higher prevalence rate in the summer season (62.85 %). Our findings are consistent with those of Lateef et al. (2005); Nwosu et al. (2007). They reported that the high biotic potential of H. contortus results in rapidly assuming dominance at times when environmental conditions on pasture are favourable for the development and survival of the free living stages.The prevalence of Ostertagia species was also higher in summer (52.85 %) like that of Haemonchus species. The present findings, therefore, do not support the conclusions that the free–living stages of Ostertagia species thrive better in cool moist conditions (Gordon 1953) (Fig. 4).
Fig. 4.
Seasonal fluctuation of abamossal nemotodes
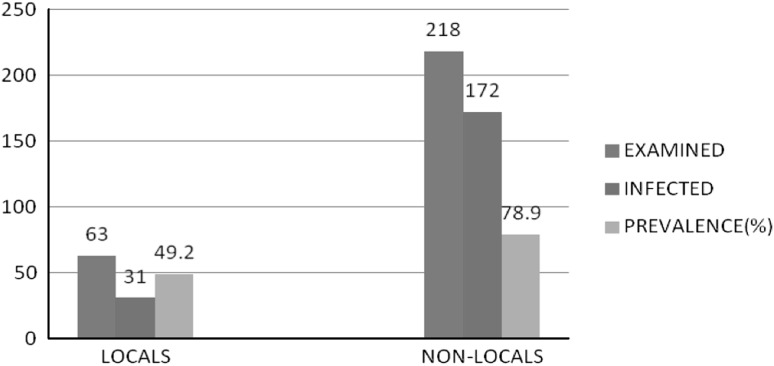
Prevalence rate of abamossal nematodes in sheep as affected by breed
The findings of present study reflected that the local breeds have lesser prevalence (49.2 %) than non-local breeds (78.9 %). The higher prevalence of infection in non-local sheep may be attributed to the environmental conditions i.e., higher temperature and humidity present in areas from where these sheep were bought into state (Pal and Qayyum 1992). Natural resistance based on genetic background and genetic variations might also be responsible for the differential prevalence of Haemonchus and Ostertagia spp. among different breeds of sheep (Baker et al. 1999) (Fig. 5).
Fig. 5.
Breedwise prevalence of Trichostrongylid parasite
Conclusion
Haemonchus was identified as the dominant Trichostrongylid sps. in sheep (58.4 %) suggesting that small ruminants are most susceptible and usual host of Haemonchus than Ostertagia. The prevalence of both Haemonchosis and Ostertagiosis seems high in ovines both in local and nonlocal breeds. The results of the present survey implies that infections of sheep with above mentioned helminths are responsible for condemnation of substantial quantities of affected organs and therefore of direct economic importance. It is thus suggested that the disease should be dealt with seriously by all concerned in order to provide a good support to the national economy.
Acknowledgments
The authors would like to extend their gratitude to Parasitology Laboratory, Department of Zoology, University of Kashmir, Jammu and Kashmir for its technical and material support in the realization of this study.
References
- Baker RL, Mwamachi DM, Audho JO, Aduda EO, Thorp W. Genetic resistance to gastrointestinal nematodes parasites in Red Massai x Dorper ewes in sub-tropics. Anim Sci. 1999;69:334–335. [Google Scholar]
- Bennet DG. Anemia and hypoproteinemia the veterinary clinics of north america. Large Anim Pract. 1983;5(3):514–521. doi: 10.1016/s0196-9846(17)30060-5. [DOI] [PubMed] [Google Scholar]
- Bowman DD, Lynn RC, Eberhard ML. Georgis’ Parasitiology for Veterinarians. 2003;8:66–69. [Google Scholar]
- Cable RM. An illustrated laboratory manual of parasitology. 4. Minneapolis15, Minnesota: Burges Pblishing Co; 1958. p. 156. [Google Scholar]
- Delgado C, Rosegrant M, Steinfeld H, Ehui S and Courbois C (1999). Livestock to 2020: the next food revolution. food,agric. envir. Discussion paper 28. Int,l Food Policy Res. Instt. (IFPRI), Food Agric. Org. of the United Nations (FAO), and the Int,l Livest. Res. Instt.,Washington, D.C.pp 72
- Gordon HM. The epidemiology of helminthosis in sheep in winter–rainfall regions of Australia. Aust Vet J. 1953;29:237–248. [Google Scholar]
- Grant JL. The epizootiology of nematode parasites of sheep in a high–rainfall area of Zimbabwe. J South African Vet Assoc. 1981;52:33–37. [PubMed] [Google Scholar]
- Lateef M, Iqbal Z, Jabbar A, Khan MN, Akhtar MS. Epidemiology of trichostrongylid nematode infections in sheep under traditional husbandry system in Pakistan. Int J Agric Biol. 2005;7:596–600. [Google Scholar]
- Nwosu CO, Madu PP, Richards W. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Vet Parasitol. 2007;144:118–124. doi: 10.1016/j.vetpar.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Ogunsuri RA, Eysker M. Inhibited development of Trichostrongylids of sheep in northern Nigeria. Res Vet Sci. 1979;26:108–110. [PubMed] [Google Scholar]
- Pal RL, Qayyum M. Breed, age and sex-wise distribution of gastrointestinal helminthes of sheep and goats in and around Rawalpindi region. Pak Vet J. 1992;12:30–60. [Google Scholar]
- Smith MC and Sherman DM (1994) Nematode gastroenteritis. In: Goat medicine. Lippincott Williams & Wilkins, Baltimore, pp 321–336
- Solusby EJL. Helminthes, arthropods and protozoa of domesticated animals. London: Bailliere Tindall; 1982. [Google Scholar]
- Teel PD, Matin SL, Grant WE. Simulation of host-parasite-land scape interactions: influence of season and habitat on cattle fever tick (Boophilus sp.) population dynamics. Ecol Modelling. 1996;84:19–30. doi: 10.1016/0304-3800(94)00142-1. [DOI] [Google Scholar]
- Viassoff A, Leathwick DM, Heath ACG. The epidemiology of nematode infections of sheep. N.Z. Vet J. 2001;49:213–221. doi: 10.1080/00480169.2001.36235. [DOI] [PubMed] [Google Scholar]
- Waller PJ. International approaches to the concept of integrated control of nematode parasites of livestock. Int, l. J Parasitol. 1999;27:155–164. doi: 10.1016/s0020-7519(98)00178-7. [DOI] [PubMed] [Google Scholar]