Abstract
Safe alternative anticoccidial drug to chemical feed additives are herbal extracts, because they don’t results to tissue residue and drug resistance. In order to evaluate the effects of herbal extracts to control avian coccidiosis, 180 one-day-old broiler chickens were randomly divided into nine equal groups, as follows: (1) Biarum bovei (2) Nectaroscordum tripedale( 3) Dorema aucheri (4) Cichorium intybus (5) Prangos ferulaceae (6) diclazuril (7) Artemisia absinthium (8) infected control (9) uninfected control (each contains two groups). Administration of herbal extracts and supplementation of diclazuril was began 2 days before challenge and lasted for the duration of the experiment. The chicks of all the groups except uninfected control group were inoculated orally with sporulated oocysts (3 × 103 oocysts of Eimeria tenella) on the day 22 of age. The criteria employed were: body weight, feed conversion ratio, blood in feces, survival rate, lesion scoring, number of oocyst output per gram feces and histopathological changes. For histopathological evaluation, on day 12 post inoculation three birds from each group were randomly selected and humanly sacrificed. N. tripedale and diclazuril revealed better results in terms of growth performance, lesion score, extent of bloody diarrhea and oocyst count as compared to other herbal extracts. The increase in the severity of lesions was observed in groups of D. aucheri, A. absinthium, B. bovei, P. ferulaceae, C. intybus, diclazuril and N. tripedale, respectively. In conclusion, the current study showed that herbal extracts were effective in control of coccidiosis caused by the E. tenella infection.
Keywords: Herbal extract, Anticoccidial, Coccidiosis, Broiler chicken
Introduction
Coccidiosis is one of the most important diseases of poultry worldwide that characterized by enteritis. It is caused by genus Eimeria in chicken, which undergo a direct life cycle with transmission between hosts by way of a resistant oocyst. The most common conditions that facilitate the development of disease are as follows: Eimeria species and virulence, high oocyst challenge, poor ventilation, high stocking density, low immune status of the host, bacterial enteritis, high humidity in litter and anticoccidial drugs lack of effectivity (Shivaramaiah et al. 2014). Since 1939 it has been discovered that sulfonamide would cure coccidiosis in chickens (Levine 1939), many ionophorous and chemical anticoccidial feed additives have been used. Several poultry scientists all over the world are also actively engaged in research into the use of plants and plant derived products to fight and reduce the heavy economic losses in poultry industry caused by coccidiosis (Abbas et al. 2012). Because of the development of anticoccidial resistance to these products and the potential harmful effects on human health, there is need to find out the safe alternatives for the control of avian coccidiosis and the use of herbal remedies in poultry diets has been proposed because of their natural stimulation of the immune system, enhanced growth performance and/or anticoccidial effects. In the last decade plant extracts were widely investigated, used for controlling avian coccidiosis and improving poultry performance worldwide (Mathis et al. 1995; Allen et al. 2000; Silbergeld et al. 2008). D.aucheri is a plant that grows in Iran. It is the first umbelliferous plant found to produce exudate flavonoids (WollenWeber et al. 1995). P. ferulaceae is a plant that has been used in Iran as a medicinal plant under the common name of Djashir. The extracts of the roots and fruits of the plant have been used for the treatment of digestive disorders, healing scars and to stop bleeding in Iran, Turkey, India and different parts of Caucasia and central Asia (Shikishima et al. 2001; Tada et al. 2002). A.absinthium is a species of wormwood, native to temperate regions of Eurasia and northern Africa. Its active substances include silica, two bitter substances (absinthin and anabsinthine), thujone, tannic, resinous substances, malic acid, and succinic acid. It is used medicinally as indigestion and gastric painstrong antimicrobial activity, especially against Gram-infected pathogenic bacteria (Fiamegos et al. 2011). C.intybus is well known for its toxicity to internal parasites. Studies indicated that ingestion of C. intybus by farm animal results in reduction of worm burdens (Heckendorn et al. 2007; Athanasiadou et al. 2007; Tzamaloukas et al. 2006). It is variously used as a tonic and treatment for gallstones, gastro-enteritis, sinus problems, cuts, and bruises (Howard 1987). N. tripedale(also known as Allium tripedale) is indigenous to Armenia, Iran, and Iraq. S-butyl cysteine sulfoxide, S-methyl and S-1-propenyl cysteine sulfoxides have been found in A. tripedale (Kusterer and Keusgen 2010) that were observed to exhibit modest antimicrobial activity. The purpose of the present study was to evaluate and to compare the efficacy of these herbal extracts on body weight, feed conversion ratio (FCR), blood in feces, survival rate, lesion scoring, output per gram feces (OPG), and histopathological lesions in broiler chickens experimentally infected with E.tenella.
Materials and methods
Birds and management
After purchasing 180 one-day-old broiler chickens (Cobb 500) of mixed sex from local hatchery, they were reared as a single group from one-day-old till day 20 on wire mesh. At the day 20, the chicks were randomly divided into nine groups (20 birds/group) and housed in pens of identical size with a single tray per group to catch faecal material. Each group had two replicates (ten birds/pen). The groups were herbal extracts, diclazuril, infected control and uninfected control. Administration of herbal extracts and supplementation of diclazuril was began 2 days before challenge and lasted for the duration of the experiment. The herbal extracts were prepared from fresh leaves of plants and mixed with water at 5 % level which was administered with the assigned extracts at a dose rate of 300 mg/kg of body weight/day. Diclazuril–Clinacox® (Jamedat Afagh Pharmaceutical Company, Iran)—were used in dose recommended by the manufacturers (1 ppm). During the study, water and feed were provided ad libitum. All birds were fed a standard commercial diet based on corn and soybean meal and were formulated without any anticoccidial medication. Strict sanitation practices were maintained in the house before and during the course of experiment.
Extraction
The whole leaves of B. bovei,N. tripedale, D. aucheri, C. intybus and P. ferulaceae were collected from Yasuj, Iran. The leaves were washed then dried under air conditioning and reduced to powder with electric mill (THOMAS-Wiley). The powder was cold extracted in water/ethanol 95o mixture (1:1) for 72 h. The mixing obtained was homogenized, filtered and evaporated at 50oC in rotary evaporator. The solvents were evaporated to obtain dark hydro-alcohol extractions (Chikoto and Eloff 2005). Birds in each treatment group were fed the diets contained 30 mg/kg of extract.
Parasite and dose
Coccidial oocysts of E.tenella were obtained from the ceca of naturally infected chicks. Identification of the species was based on the morphological characteristics described by Thienpont et al. (1979) and the site of lesions. After collecting and sporulating the oocysts in 2.5 % potassium dichromate at 28 °C for 24 h, they were inoculated in three healthy chickens orally to propagate the oocysts. Eight days post inoculation, birds were euthanized and ceca contents were obtained. After concentration, the oocysts were sporulated as described above and kept in a refrigerator (2–5 °C) until use (Davies et al. 1963). Each bird was orally challenged with 3 × 103 oocysts of E.tenella at 22 days of age.
Observations procedures
Anticoccidial efficacy of herbal extracts was evaluated on the basis of body weight, FCR, blood in feces, survival rate, lesion scoring and OPG. The live body weight of all birds of each group on 4, 7, and 11 days post-inoculation (DPI) were taken. Feed intake (hen-day) and their FCR were calculated. The extent of blood in feces was assigned from 0 to 4 according to the method suggested by (Youn et al. 1993). To determine ceca lesion score, five chickens from each group were randomly chosen at day 8 DPI. Lesion scoring was performed according to the method was suggested by Conway and McKenzie (1991). For the OPG of feces, we counted the oocysts by McMaster technique from the description by Long et al. (1976). After medication, calculating OPG was performed on days 5, 6, 7, 8, and 9 DPI and their average was calculated.
For histopathological evaluation, at the end of the experiment (12 DPI) three birds from each group were euthanized (humanely killing the birds by cervical dislocation) and 2 cm2 tissue pieces from caeca were collected and fixed in 10 % buffered formalin solution. Multiple transverse slices were embedded in paraffin wax. Sections were cut at 5 µm, and all were stained with hematoxylin-eosin (Gridley 1960).
In vitro anticoccidial tests
Fresh fecal samples from clinically infected birds were collected and mixed in 50 ml water. After vigorously shaking tube, it was filtrated through a single thickness of muslin. The procedure to harvest oocysts was by floatation technique using saturated salt (NaCl) solution (Conway and McKenzie 1991). To determine the sporulation time of the oocysts, the salt solution was removed by washing the oocysts with phosphate buffered saline and centrifuged 3–4 times at 1,500 M for 2 min in graduated tubes. Finally, 10 ml of tap water was added to the salt-free oocyst suspension and oocyst per 1 ml was calculated. A number of 100 oocysts were added to dishes that contained the extracts and the set up was left at ambient temperature and oxygen. The suspension was examined by hemocytometer chamber at hours 12, 24, 48, and 72 to determine the sporulation time of oocysts. Then, the percentages of sporulated oocysts were recorded. Similar methods were made for oocysts sporulated in diclazuril and potassium dichromate (2 %) solutions which served as control.
Statistical analysis
The data were analyzed using computerized statistical program (SPSS version 16.0). Differences in mean body weight were tested by ONE-WAY ANOVA. Mean separation was accomplished using Tukey’s studentized range test. P < 0.05 was considered as significant level.
Results
Performance
The results of herbal extracts on body weight and FCR are shown in Table 1. The body weight was significantly higher in all treatment groups except D. aucheri in comparison with infected control one. At the end of the first week DPI birds which were received diclazuril and N. tripedale had the highest body weight and it was continued until 11 DPI (P < 0.05). At the end of the experiment among treated groups, the lowest (1.60) and the highest (1.75) FCR were observed for diclazuril and D. aucheri groups, respectively.
Table 1.
Body weight (BW) and feed conversion ratio (FCR) of chicken treated with herb extracts and challenged with E. tenella
| Groups | DPI | |||||
|---|---|---|---|---|---|---|
| 4 | 7 | 11 | ||||
| BW | FCR | BW | FCR | BW | FCR | |
| B. bovei | 1,062a | 1.40 | 1,205 a | 1.47 | 1,470 ad | 1.66 |
| N. tripedale | 1,037 a | 1.39 | 1,312 bd | 1.43 | 1,625 b | 1.63 |
| D. aucheri | 988 b | 1.41 | 1,057 c | 1.53 | 1,260 c | 1.75 |
| C. intybus | 1,082 a | 1.38 | 1,215 a | 1.46 | 1,466 ad | 1.62 |
| P. ferulaceae | 1,092 a | 1.37 | 1,242 a | 1.44 | 1,530 a | 1.63 |
| Diclazuril | 1,087 a | 1.39 | 1,277 ab | 1.44 | 1,610 b | 1.60 |
| A. absinthium | 1,072 a | 1.38 | 1,185 a | 1.49 | 1,396 d | 1.63 |
| Infected control | 962 b | 1.43 | 1,042 c | 1.54 | 1,210 c | 1.77 |
| Uninfected control | 1,112 a | 1.34 | 1,390 d | 1.42 | 1,680 b | 1.56 |
Means within a column with different superscript letters denote significant differences (P < 0.05)
Lesion and feces scoring
A complete survival rate was observed in diclazuril group. N. tripedale had the lowest mortality (10 %) among groups treated with herbal extracts. The lesion score of birds treated with N. tripedale was lower (2.8) among groups treated with herbal extracts. This parameter in the group treated with diclazuril was lower among the all treated groups. Bloody diarrhea was observed in almost all of the groups (except uninfected control one) from 5 to 9 DPI. The extent of bloody diarrhea in the A. absinthium and D. aucheri groups were more severe (3.6) than that of other treated groups (Table 2).
Table 2.
Blood in the feces, survival rate and lesion scoring of broilers treated with herb extracts and challenged with E. tenella
| Groups | Blood in feces (DPI) | Survival rate | Lesion scoring | |||||
|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | |||
| B. bovei | − | + | ++ | ++ | + | + | 80 | 3.4 |
| N. tripedale | – | + | + | ++ | + | + | 90 | 2.8 |
| D. aucheri | – | + | ++ | ++ | + | + | 80 | 3.6 |
| C. intybus | – | + | ++ | ++ | + | + | 80 | 3.4 |
| P. ferulaceae | – | + | ++ | ++ | + | + | 80 | 3.4 |
| Diclazuril | – | + | + | ++ | + | – | 100 | 2.4 |
| A. absinthium | – | + | ++ | +++ | ++ | + | 80 | 3.6 |
| Infected control | – | + | +++ | ++++ | ++ | + | 70 | 4.0 |
| Uninfected control | – | – | – | – | – | – | 100 | 0 |
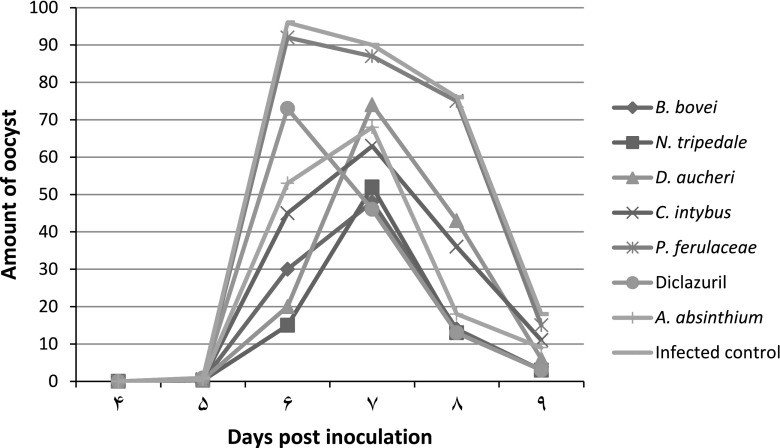
Amount of oocyst output per gram feces
Excretion of oocysts in the groups is presented in Table 3 and Fig. 1. The shedding of oocysts was recorded as early as 5th DPI. Oocyst outputs of treatment groups except P.ferulaceae were lower than that of the infected control group. Among the treated groups the birds treated with N. tripedale revealed better results in terms of oocyst count per gram of feces (83.3).
Table 3.
OPG from chickens treated with herb extracts and challenged with E. tenella (× 1,000)
| Groups | Counting days (PI) | Total | |||||
|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | 9 | ||
| B. bovei | 0 | 0.3 | 30 | 48 | 14 | 3 | 95.3 |
| N. tripedale | 0 | 0.3 | 15 | 52 | 13 | 3 | 83.3 |
| D. aucheri | 0 | 0.5 | 20 | 74 | 43 | 6 | 143.5 |
| C. intybus | 0 | 0.5 | 45 | 63 | 36 | 11 | 155.5 |
| P. ferulaceae | 0 | 0.5 | 92 | 87 | 75 | 15 | 269.5 |
| Diclazuril | 0 | 0.9 | 73 | 46 | 13 | 3 | 135.9 |
| A. absinthium | 0 | 0.5 | 53 | 68 | 18 | 9 | 148.5 |
| Infected control | 0 | 0.9 | 96 | 90 | 76 | 18 | 280.9 |
| Uninfected control | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Fig. 1.
OPG from chickens treated with herb extracts and challenged with E. tenella (×1,000)
Microscopic findings
On 12 DPI intracellular stages of E. tenella in mucosa and submucosa were observed in all treated groups. In addition, in group B. bovei coagulative necrosis and degeneration, crypt hyperplasia with oocysts was also evident in the epithelium. In group N. tripedale, D. aucheri and A. absinthium hemorrhagic fibrinopurulent enteritis was evident. In group P. ferulaceae fibrinopurulent enteritis with villus atrophy was seen. In general, the increase in the severity of lesions was observed in groups of D. aucheri, A. absinthium, B. bovei, P. ferulaceae, C. intybus, diclazuril and N. tripedale, respectively.
In vitro anticoccidial effect
P. ferulaceae and C. intybus extracts at the end of 72 h showed the highest (29.69 %) and lowest (8.51 %) percentage of sporulation inhibition, respectively (Table 4).
Table 4.
In vitro anti-coccidial effect of the plant extracts on the sporulation percentage of Eimeria oocysts
| Groups | Percentage of sporulation (%) | |||
|---|---|---|---|---|
| Hours | ||||
| 12 | 24 | 48 | 72 | |
| B. bovei | 51.22 | 61.11 | 63.83 | 78.75 |
| N. tripedale | 73.33 | 74.70 | 76.33 | 80.22 |
| D. aucheri | 34.57 | 68.96 | 70.43 | 72.55 |
| C. intybus | 71.54 | 78.10 | 86.95 | 91.49 |
| P. ferulaceae | 56.35 | 64.51 | 65.81 | 70.31 |
| Diclazuril | 48.07 | 63.13 | 70.00 | 75.20 |
| A. absinthium | 56.12 | 69.1 | 74.76 | 77.50 |
| Control | 74.8 | 86.4 | 94.6 | 95.1 |
Discussion
After challenge of chickens with E. tenella and their treatment with different herbal extracts the survival rates, bloody diarrhea, lesion scores, body weight, feed conversion rates, and excreted oocysts of feces were investigated. In different treated groups all parameters were improved in comparison with the infected control group. Herbal extracts in comparison with chemical anticoccidial drug (diclazuril) except in oocysts excretion had lower performance after infection with E. tenella. Lesion scores and survival rate in the group treated with diclazuril was more improved than other groups. B. bovei and N. tripedale produced the highest effect among herbal extracts (85,300 and 83,300 OPG in total, respectively).
The body weight in the group treated with diclazuril and N. tripedale at 11 DPI were more than other groups. The findings of the present study are similar to the findings of DU and HU (2004), who observed significantly higher body mass gains and mild lesion score in birds medicated with herbal complex for E. tenella infection in chicken in comparison with infected control group. Similarly Chandrakesan et al. (2009) observed that the herbal complex can cause better body mass gain between the 4th and 5th weeks and superior FCR after infection with E. tenella. The results of Arczewska–Wlosek and Swiatkiewicz (Arczewska-Wlosek and Swiatkiewicz 2012) were analogous to the current study that showed significantly lower body weight gain in groups were infected with Eimeria oocysts due to adverse effect on the digestion, absorption and assimilation of nutrients. Our study was in agreement with Christaki et al. (2004) that showed the mean body weight gain in the group were challenged with E. tenella was lower than in all other groups while in the Apacox groups (contains extracts from the plants Agrimonia eupatoria, Echinacea angustifolia, Ribes nigrum and Cinchonasuccirubra) was higher than in the challenged control group. Also the intensity of bloody diarrhoea and the numbers of oocysts per bird were lower in the Apacox groups than in the challenged control group. As the same Abbas et al. (2010) stated that the weight gain in the groups treated with turmeric were significantly higher than that of infected with E. tenella. In another study by Lillehoj et al. (Lillehoj et al. 2011) the body weight gain significantly enhanced in broiler birds fed with XT6930 (including carvacrol, cinnamaldehyde and Capsicum oleoresin) in comparison with E. acervulina-infected chickens. Significantly increased body weight and also decreased oocyst excretion in Schinopsis lorentzii extract group in broiler chicks as compared to that of were challenged with mixed suspension of sporulated oocysts of E. tenella, Eimeria maxima and Eimeria acervulina were observed due to proanthocyanidins in the S. lorentzii extract that have potential effect against a mixed Eimeria challenge (Cejas et al. 2011). Broiler chickens were experimentaly infected with E. tenella, showed more cecal lesion score and lower weight gain in challenged group as compared to negative controls (Conway et al. 2002). The chicks were inoculated by E. tenella revealed higher weight gain and significant reduction in the oocyst counts in medicated group with diclazuril in comparision with non-medicated group (Abbas et al. 2009). In broilers Artemisia annua group showed the highest weight gain compared to the control group due to its high protein content and presence of essential minerals such as sodium, potassium, zinc and manganese, amino acids and vitamins (Gholamrezaie Sani et al. 2013).
Using the fecal oocysts output of chickens as the indicator of effect of herbal extracts, our findings showed significant reduction in oocysts output in comparison with infected control group with the exception of P. ferulaceae group. Youn and Noh (2001) investigated on the infected broiler chickens with E. tenella, which received herbal extracts. They observed improvement in almost 15 treated groups in survival rates, lesion scores, body weight gains, bloody diarrhea, and oocysts excretions in comparison to the control group. Jang et al. (2007) evaluated the anticoccidial effect of green tea-based diets against E. maxima, observed that the green tea-fed chickens produced significantly reduced fecal oocysts (P < 0.05) when compared to the E. maxima infected group fed standard diet. Arczewska–Wlosek and Swiatkiewicz (Arczewska-Wlosek and Swiatkiewicz 2012) reported that an herbal complex comprising Salvia officinalis, Allium sativum, Thymus vulgaris, Echinacea purpurea and Origanum vulgare in broiler chickens has been effective against many species of Eimeria in terms of reducing oocyst output. Kostadinovic et al. (2012) in coccidial infection of broiler chickens were induced by E. tenella showed that A. absinthium extract reduced the severity of coccidial infection and caused significant decrease in output number of oocysts per gram of faeces and also bloody diarrhea in the group treated with A. absinthium extract was milder than in the other groups. A. absinthium could be a potential source of protection agents against coccidiosis. In the present study, the extent of bloody diarrhea in the group treated with diclazuril was milder than that of other groups. In broilers were experimentally infected with E. tenella, sever and mild bloody diarrhea were observed in the infected control group and A. annua group, respectively (Hady and Zaki 2012). Dragan et al. (2014; 2010) to evaluate the anticoccidial activity of A. annua and Foeniculum vulgare in chickens were challenged with E. tenella, reported significant reduction in faecal oocysts, bloody diarrhea and lesion score in A. annua group when compared to the positive control group and also the best feed conversion among all experimental groups. Artemisia herba-alba could have an interesting anticoccidial activity in decreasing excretion of E. tenella oocyst and bloody diarrhea (Messai et al. 2014).
In conclusion, the results of the present study showed that herbal extracts were effective in control of coccidiosis due to the E. tenella infection. In particular, N. tripedale was found to be more potent on the basis of OPG and live body weight gain. N. tripedale has promising efficacy as an effective alternative drug against coccidiosis. The use of herbal extracts could be beneficial in the control of coccidiosis however this needs to undergo more experimental studies to determine their effectiveness. But this result needs to be further investigated in the other species of coccidia that infect chickens.
References
- Abbas R, Iqbal Z, Khan M, Hashmi N, Hussain A. Prophylactic efficacy of diclazuril in broilers experimentally infected with three field isolates of Eimeria tenella. Int J Agric Biol. 2009;11:606–610. [Google Scholar]
- Abbas R, Iqbal Z, Khan M, Zafar M, Zia M. Anticoccidial activity of Curcuma longa L. in broilers. Braz Arch Biol Technol. 2010;53:63–67. doi: 10.1590/S1516-89132010000100008. [DOI] [Google Scholar]
- Abbas R, Iqbal Z, Khan A, Sindhu Z, Khan J, Khan M, Raza A. Options for integrated strategies for the control of avian coccidiosis. Int J Agric Biol. 2012;14:1014–1020. [Google Scholar]
- Allen P, Danforth H, Stitt P. Effects of nutritionally balanced and stabilized flaxmeal-based diets on Eimeria tenella infections in chickens. Poult Sci. 2000;79:489–492. doi: 10.1093/ps/79.4.489. [DOI] [PubMed] [Google Scholar]
- Arczewska-Wlosek A, Swiatkiewicz S. The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. J Animal and Feed Sci. 2012;21:133–142. [Google Scholar]
- Athanasiadou S, Gray D, Younie D, Tzamaloukas O, Jackson F, Kyriazakis I. The use of chicory for parasite control in organic ewes and their lambs. Parasitology. 2007;134:299–307. doi: 10.1017/S0031182006001363. [DOI] [PubMed] [Google Scholar]
- Cejas E, Pinto S, Prosdocimo F, Batalle M, Barrios H, Tellez G, De Franceschi M. Evaluation of quebracho red wood (Schinopsis lorentzii) polyphenolic vegetable extracts for the reduction of coccidiosis in broiler chicks. Int J Poultry Sci. 2011;10:344–349. doi: 10.3923/ijps.2011.344.349. [DOI] [Google Scholar]
- Chandrakesan P, Muralidharan K, Kumar V, Ponnudarai G, Harikrishnan T, Rani K. Efficacy of a herbal complex against caecal coccidiosis in broiler chickens. Vet arhiv. 2009;79:199–203. [Google Scholar]
- Chikoto, H, Eloff, JN (2005) Antioxidant. Patent NR 2005/09681
- Christaki E, Florou-Paneri P, Giannenas I, Papazahariadou M, Botsoglou N, Spais A. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Animal Res. 2004;53:137–144. doi: 10.1051/animres:2004006. [DOI] [Google Scholar]
- Conway D and McKenzie M (1991) Poultry coccidiosis,: Diagnostic and Testing Procedures, 3rd edn, Blackwell Publishing Professional, Ames, p 30
- Conway D, Mathis G, Lang M. The use of diclazuril in extended withdrawal anticoccidial programs: 1. Efficacy against Eimeria spp. in broiler chickens in floor pens. Poult Sci. 2002;81:349–352. doi: 10.1093/ps/81.3.349. [DOI] [PubMed] [Google Scholar]
- Davies S, Joyner L and Kendall S (1963) Coccidiosis
- Dragan L, Titilincu A, Dan I, Dunca I, Drăgan M, Mircean V. Effects of Artemisia annua and Pimpinella anisum on Eimeria tenella(Phylum Apicomplexa) low infection in chickens. Sci Parasitol. 2010;11:77–82. [Google Scholar]
- Dragan L, Gyorke A, Ferreira J, Pop I, Dunca I, Drăgan M, Mircean V, Dan I, Cozma V. Effects of Artemisia annua and Foeniculum vulgare on chickens highly infected with Eimeria tenella(Phylum Apicomplexa) Acta Vet Scand. 2014;56:1–7. doi: 10.1186/1751-0147-56-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Hu S. Effects of a herbal complex against Eimeria tenella infection in chickens. J Vet Med, Series B. 2004;51:194–197. doi: 10.1111/j.1439-0450.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- Fiamegos Y, Kastritis P, Exarchou V, Han H, Bonvin A, Vervoort J, Lewis K, Hamblin M, Tegos G. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE. 2011;6:e18127. doi: 10.1371/journal.pone.0018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamrezaie Sani L, Mohammadi M, Jalali Sendi J, Abolghasemi S, Roostaie Ali Mehr M. Extract and leaf powder effect of Artemisia annua on performance, cellular and humoral immunity in broilers. Iranian J Vet Res. 2013;14:15–20. [Google Scholar]
- Gridley M (1960) Manual of histologic and special staining technics, 3rd edn, Armed Forces Institute of Pathology, Washington, DC
- Hady M, Zaki M. Efficacy of some herbal feed additives on performance and control of cecal coccidiosis in broilers. APCBEE Procedia. 2012;4:163–168. doi: 10.1016/j.apcbee.2012.11.028. [DOI] [Google Scholar]
- Heckendorn F, Haring D, Maurer V, Senn M, Hertzberg H. Individual administration of three tanniferous forage plants to lambs artificially infected with Haemonchus contortus and Cooperia curticei. Vet parasito. 2007;146:123–134. doi: 10.1016/j.vetpar.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Howard M (1987) Traditional folk remedies: a comprehensive herbaledition, Century, p: 120
- Jang S, Jun M, Lillehoj H, Dalloul R, Kong I, Kim S, Min W. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet Parasitol. 2007;144:172–175. doi: 10.1016/j.vetpar.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kostadinovic L, Levic J, Galonja-Coghill T, Ruzicic L. Anticoccidian effects of the Artemisia absinthium L. extracts in broiler chickens. Arch Zootech. 2012;15:69–77. [Google Scholar]
- Kusterer J, Keusgen M. Cysteine sulfoxides and volatile sulfur compounds from Allium tripedale. J Agric Food Chem. 2010;58:1129–1137. doi: 10.1021/jf903581f. [DOI] [PubMed] [Google Scholar]
- Levine P. The effect of sulfanilamide on the course of experimental avian coccidiosis. Cornell Vet. 1939;29:309–320. [Google Scholar]
- Lillehoj H, Kim D, Bravo D, Lee S. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC proceedings. 2011;5:S34. doi: 10.1186/1753-6561-5-S4-S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P, Millard B, Joyner L, Norton C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976;6:201–217. [PubMed] [Google Scholar]
- Mathis G, Dale N, Fuller A. Effect of dietary raw soybeans on coccidiosis in chickens. Poult Sci. 1995;74:800–804. doi: 10.3382/ps.0740800. [DOI] [PubMed] [Google Scholar]
- Messai A, Bensegueni A, Abdeldjelil M, Agabou A, Redouane-Salah S. Effects of white wormwood (Artemisia herba-alba Asso), during an experimental coccidiosis in broilers. Ann Biol Res. 2014;5:61–66. [Google Scholar]
- Shikishima Y, Takaishi Y, Honda G, Ito M, Takeda Y, Kodzhimatov O, Ashurmetov O, LEE K. Chemical constituents of Prangos tschimganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem Pharm Bull. 2001;49:877–880. doi: 10.1248/cpb.49.877. [DOI] [PubMed] [Google Scholar]
- Shivaramaiah C, Barta JR, Hernandez-Velasco X, Téllez G, Hargis B. Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet Med: Res Repo. 2014;5:23–34. doi: 10.2147/VMRR.S57839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld E, Graham J, Price L. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Tada Y, Shikishima Y, Takaishi Y, Shibata H, Higuti T, Honda G, Ito M, Takeda Y, Kodzhimatov O, Ashurmetov O, Ohmoto Y. Coumarins and gamma-pyrone derivatives from Prangos pabularia: antibacterial activity and inhibition of cytokine release. Phytochem. 2002;59:649–654. doi: 10.1016/S0031-9422(02)00023-7. [DOI] [PubMed] [Google Scholar]
- Thienpont D, Rochette F and Vanparijs O (1979) Diagnosing helminthiasis through coprological examination. Janssen Research Foundation, Beerse, Belgium pp: 34-36
- Tzamaloukas O, Athanasiadou S, Kyriazakis I, Huntley J, Jackson F. The effect of chicory (Cichorium intybus) and sulla (Hedysarum coronarium) on larval development and mucosal cell responses of growing lambs challenged with Teladorsagia circumcincta. Parasitol. 2006;132:419–426. doi: 10.1017/S0031182005009194. [DOI] [PubMed] [Google Scholar]
- Wollenweber E, Dorr M, Rustiyan A. Dorema aucheri, the first umbelliferous plant found to produce exudate flavonoids. Phytochemistry. 1995;38:1417. doi: 10.1016/0031-9422(94)00840-P. [DOI] [Google Scholar]
- Youn H, Noh J. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet Parasitol. 2001;96:257–263. doi: 10.1016/S0304-4017(01)00385-5. [DOI] [PubMed] [Google Scholar]
- Youn H, Kang Y, Jang D. Effects of gamma-irradiation from cobalt-60 on pathogenicity of Eimeria tenella. Korean J Vet Res. 1993;33:649–655. [Google Scholar]