Abstract
Despite presence of anticoccidial drugs and vaccines in the market, coccidiosis continues to result in substantial economic losses to the poultry industry. There is wide-spread resistance to already known anticoccidial drugs. It is an infectious disease of poultry and rigorous management is required during vaccination. In addition there is possibility of drug residues in meat and other byproducts of such treated animals and consequently makes more imperative to explore and understand the role of natural products in livestock parasite management. Therefore a study was designed to evaluate the anticoccidial activity of aqueous extract of Ganoderma applanatum in broiler chicken. In-vivo anticoccidial activity of aqueous extract of G. applanatum was measured in comparison to the reference drug amprolium on the basis of oocysts per gram of faeces, weight gain and feed conversion ratio. Oocyst output was measured with the help of Mc-Masters counting technique. The results of this study established the virulence of coccidian oocysts and the effectiveness of both amprolium and aqueous extract of G. applanatum against coccidian oocysts, confirmed by the fact that treatment with aqueous extract of G. applanatum resulted in a noticeable reduction in coccidian oocysts output, leading to improved weight gain and better feed conversion ratio. The study highlighted the potential of G. applanatum as a natural source of bioactive components for controlling a protozoan parasite, which can be isolated and tested in a bioassay-guided manner and harnessed in the form of anticoccidial drugs.
Keywords: Coccidiosis, Poultry, Ganoderma applanatum
Introduction
Coccidiosis is a managemental disease of poultry and ranks among the most important diseases of poultry worldwide (Lee et al. 2009). It has been documented that coccidiosis is the most consistently reported health problem in poultry (Biggs 1982; Williams 1999). In all parts of the world where confinement rearing is practiced, coccidiosis represents a major disease problem demanding the attention of poultry producers, feed manufactures, and poultry disease experts (Reid 1978). Coccidiosis is alleged to be a universal depreciator or even a potential killer of our poultry. The control of these coccidian parasites is accomplished largely through the use of commercial synthetic anticoccidial drugs. However, these drugs can leave their residues in food (Nagata and Saeki 1986) and also pose threat to environment (Waller 2006). Besides this, widespread resistance to drugs is emerging in coccidian parasites (Long 1982; Ruff and Danforth 1996). The resulting letdown of treatment, pooled with side effects, make it crucial to seek out for new drugs by pharmacological screening.
Feed supplements with natural medicinal products have been widely used as growth and health promoters in farm animals in China for years (Li 1998). The mushroom is a macrofungus with a distinctive fruiting body large enough to be seen with the naked eye and with over 2,000 species that possess medicinal properties (Chang and Miles 2004). Medicinal mushrooms such as Pleurotus ostreatus and Ganoderma lucidum are used as food supplements and medicines in certain disease conditions (Chang and Buswell 1996; Chang and Mshigeni 2001; Anthony and Joyce 2007). Qualitative and quantitative analysis of these mushrooms have shown the presence of some biologically active compounds (Kadiri 1990; Ketiku and Ola 1999; Ogbe et al. 2008). These bioactive compounds belong to the group of polysaccharides and antioxidants, which protect the body against free radicals that damage body cells to induce diseases (Monro 2003; Oei 2003). The presence of polysaccharides in mushrooms suggests they can be useful as natural health promoters against parasites, bacteria and viruses (Oei 2003). Besides Ogbe et al. (2009) has reported effectiveness of aqueous of G. lucidum extract against Houghton strain of E. tenella. With this outlook a study was designed to confirm such studies and to evaluate anticoccidial activity of aqueous extract of a wild mushroom (G. applanatum) in vivo using broiler chicken.
Materials and methods
Ganoderma applanatum (Ganodermataceae)
Ganoderma applanatum known as conk is a general term used for a fungus that destroys wood and digest the brown lignin as food source and leave behind the white cellulose. G. applanatum, appropriately dubbed a shelf fungus due to its shape, a fan-shaped polypore ranges from 30 to 70 cm long, that makes it noticeable in the woods. It has a thick, hard, lumpy, brown top with several radiating zones. The spore surface is ochre in color and after scratching the spore surface becomes brown. The pores of the spore surface are tiny and regular in shape. G. applanatum creates a new pore surface each year, giving it a “stacked” appearance.
Identification and processing of mushrooms
Mushrooms collected were firstly identified at Kashmir University Herbarium (KASH), Centre of Plant Taxonomy, Department of Botany, University of Kashmir. A voucher specimen (voucher no. 1801) was also deposited in KASH. Mushrooms were then washed with distilled water. After washing they were processed for shade drying in a well ventilated room. To facilitate complete drying, the fruit bodies of mushroom were cut into small pieces and then dried in shade conditions. The dried mushrooms were milled to a fine powder using an electric blender. The mushroom powder was again dried for about 3 h in an oven at 40 °C before extraction.
Preparation of aqueous extract
The crude aqueous extracts of the selected mushroom was prepared according to the techniques described by Iqbal et al. (2004). The powdered mushroom parts (100 g) were extracted with distilled water (500 ml) at 90–100 °C in a Soxhlet extractor for 8 h. The aqueous extract was filtered, and stored at 4 °C until used.
Broilers and experimental design
Day-old broiler chicks were purchased from local market and screened for coccidial infection. The broiler chicks were reared under standard management practices in the animal house of the Department of zoology, University of Kashmir, for 5 weeks. The birds were maintained in a coccidian free atmosphere. The method of housing the broilers was an intensive deep-litter system. Before birds were placed, the houses were cleaned, washed, disinfected and provided with saw dust. The ambient temperature in experimental house was maintained at 29 °C during the first week and after than gradually decreased by 3 °C in the third week, and finally fixed at 22 °C thereafter. All birds were reared in cages, kept in strictly isolated room. To meet the nutrient requirements of the broiler chicken during the entire experimental period, a complete basal diet was formulated for each of the two stages of growth; starter and grower. The diets were formulated to meet the nutrients requirements of broilers as recommended by the National Research Council (N.R.C. 1994). The chicks were provided with standard coccidiostat free feed. The feed and water was provided ad libitum during the study period. Lighting of the environment was provided for 24 h. At 22nd day age, the birds were used for experimental purpose. All the birds were tagged to maintain their identity.
On day 22 the body weight of all chicks were taken and grouped into four experimental groups A, B, C and D each having ten chicks by random allocation. Underweight and weak chicks were excluded from the experiment. The birds in groups A, B and C were inoculated with mixed coccidial oocysts of Eimeria species at the rate 3,850–4,000 sporulated oocysts per bird (Williams 2001) introduced directly into the crop of each bird at 22nd day of age. They were finally treated with mushroom extracts and recommended medicine according to the following schedule: Group-A: Infected and treated with extract of mushroom (1) in water for 5 consecutive days. Group-B: Infected and treated with recommended medicine for 5 consecutive days. Group-C: Infected and un-medicated group. Group-D: Uninfected and un-medicated group.
Group D served as uninfected and un-medicated control, groups A–C were infected with sporulated oocysts of Eimeria on the 22nd day of age. Group C was infected and left untreated. Group B was infected, and treated with the allopathic drug amprolium. The Group A was infected and treated with aqueous extract of Ganoderma applanatum. Drinking water was provided ad libitum throughout the entire period of study.
An inventory of birds for procuring infection
An inventory of poultry birds in nature was made for getting the coccidian infection in nature. Coccidiosis suspected guts were collected from different poultry farms. All the intestines and caeca were opened and their contents (faeces) were collected in a beaker. The oocysts thus procured were kept in a medium for experimental infection.
Parasite inoculation
Feacal samples from all experimental groups were collected and examined for any contamination by coccidia parasites prior to the experimental infection. All groups were found negative for coccidial oocysts. On 22nd day of age each group was inoculated by coccidial oocysts of Eimeria species by direct gavaging with the help of a graduated syringe. The sporulated oocysts were given at the dose rate of 3,850–4,000 oocysts per bird (Williams 2001). One ml of oocyst suspension in distilled water was orally inoculated directly into the crop using a flexible plastic tube fitted to 5 ml syringe.
Determination of weight gain and feed conversion ratio
Performance of broilers was evaluated by recording body weight (BW), daily body weight gain (DWG), daily feed intake (DFI) and feed conversion ratio (FCR) during the entire experimental period. Mortality was recorded as it occurred.
| 1 |
Collection of faecal samples and laboratory examination
The birds started shedding oocysts 128 h post infection. The fecal droppings in each cage were collected on a polyethylene sheet placed on the fecal tray of the cages. The faecal samples were continuously observed after a time interval of 24, 48 and 72 h and severity of infection was confirmed. Diagnosis of Eimerian oocysts in faeces is an easy to get an impression of the infection level, direct smear method and both qualitative and quantitative techniques can be done to faecal sample. McMaster’s oocyst counting technique was used for counting the coccidian oocysts (Soulsby 1982).
Oocysts counting
To obtain accurate information with regard to severity of an infection, egg counting methods were carried out to determine number of eggs per gram (EPG) of faeces. For this purpose McMaster counting chamber was used. This method is generally used in litter oocyst counting procedures since the percentage of sporulation and oocyst dimensions are not required in this measurement. The McMaster chamber method is documented by Hodgson (1970), Long and Rowell (1958), and Long et al. (1976).
Statistical analysis
The whole data was fed into Microsoft Excel 2010, a computer programme (SPSS 11.5 for windows). The data was represented as mean of replicates followed by standard deviation i.e. mean ± standard deviation (SD). ANOVA was carried out to determine the variance of the data registered followed by Tukey’s test, to detect significant difference among groups. Differences between means were considered statistically significant at P ≤ 0.05.
Results and discussion
Oocyst per gram (OPG) counts
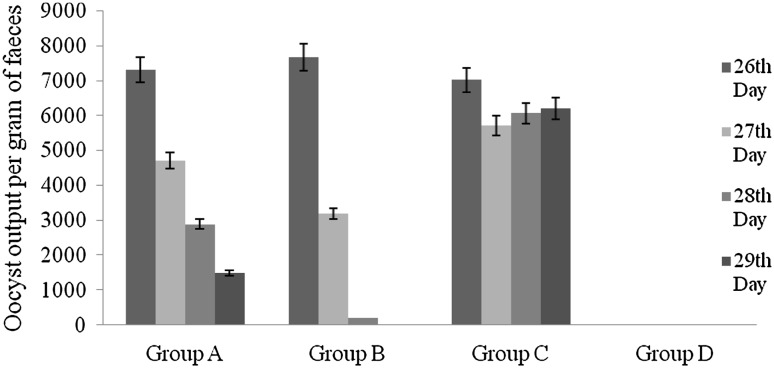
The OPG counts of different groups of chickens are represented in Table 1 (Fig.1). The highest oocyst count per gram of faeces (OPG) was recorded in group C as it was untreated group. Prior to treatment at 26th day the oocyst output of birds was 7,300.68 ± 32.792 oocysts/g faeces (group A), 7,658.12 ± 31.652 oocysts/g faeces (group B) and 7,013.89 ± 32.048 oocysts/g faeces (group C). The faeces of uninfected group D were free of coccidial oocysts. After treatment the oocysts detected in the G. applanatum treated group (A) on 27th day had reduced in number (4,700.92 ± 24.414 oocysts/g faeces) compared to un-treated group (C) which showed increase in oocysts released (5,709.61 ± 26.285 oocysts/g faeces). By 28th day, the oocysts released in A and B group had reduced to 2,886.67 ± 16.230 and 196.09 ± 3.864 and by day 29 the birds in group B were almost free of infection (16.44 ± 0.701) where as group A showed further reduction in number of coccidial oocysts (1,490.58 ± 13.652), while group C continued discharge high number of oocysts (6,200.45 ± 30.336 oocysts/g faeces).
Table 1.
Oocyst output of broilers infected with coccidian oocysts (Eimeria) and treated with aqueous extract of Ganoderma applanatum
| Age in days | Oocyst output per gram of faeces | ||||
|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | ||
| After infection | 26th day | 7,300.68 ± 32.792a | 7,658.12 ± 31.652a | 7,013.89 ± 32.048a | 0b |
| During treatment | 27th day | 4,700.92 ± 24.414d | 3,178.74 ± 23.259c | 5,709.61 ± 26.285a | 0b |
| 28th day | 2,886.67 ± 16.230d | 196.09 ± 3.864c | 6,057.32 ± 29.497a | 0b | |
| 29th day | 1,490.58 ± 13.652d | 16.44 ± 0.701c | 6,200.45 ± 30.336a | 0b | |
Group A Infected and treated with aqueous extract of Ganoderma applanatum at 1,000 mg/kg body weight, Group B Infected and treated with amprolium at 100 mg/kg body weight, Group C Infected but not treated, Group D Neither infected nor treated
Differences between means were considered significant at P ≤ 0.05 Superscripted letters a–d represent significant differences within one age group, where same letters represent no significant difference
Fig. 1.
Reduction in oocyst output of different treatment groups
Body weight gain records and feed conversion ratio
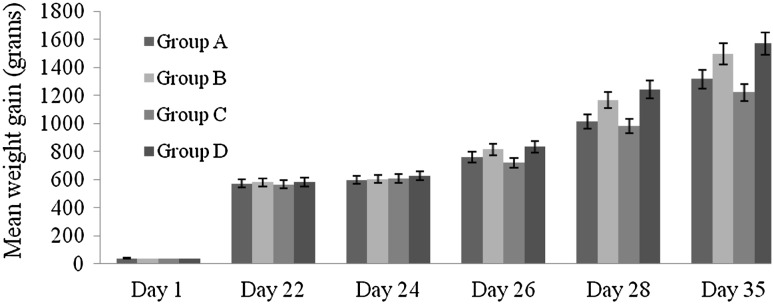
The impact of oral administration of sporulated coccidial oocysts on body weight gain of different groups of chickens followed by administration of G. applanatum extract are represented in Table 2 (Fig. 2). The mean initial weight of chicks for all groups was almost similar which was recorded on day 1–22nd day. Among the treated groups the significant improvement in body weight was recorded in group B. Chickens of group A gained the next highest body weight on the same day. The results further showed that infection with coccidial oocysts results in poor feed conversion ratio. Feed conversion ratio was higher in group C as compared to all the other groups. The mean weight gain of the birds in group C at day 35 was also significantly lower (1,220.77 ± 12.739 g) than other treated groups.
Table 2.
Group mean weight gain (in grams) of broilers infected with coccidia (Eimeria) and then treated with aqueous extract of Ganoderma applanatum and amprolium
| Age in days | Group mean weight gain (in grams) | ||||
|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | ||
| Initial weight | 1 | 39.33 ± 0.493a | 38.72 ± 0.562a | 37.88 ± 0.581a | 38.12 ± 0.620a |
| At pre-infection | 22 | 572.21 ± 2.72a | 580.95 ± 2.69a | 564.85 ± 2.27a | 583.36 ± 2.31a |
| At infection time | 24 | 597.87 ± 2.704a | 604.54 ± 3.171a | 607.64 ± 2.962a | 625.75 ± 3.105b |
| Before treatment | 26 | 761.54 ± 4.143d | 816.17 ± 4.864c | 720.29 ± 4.295a | 836.61 ± 5.169b |
| Three days after the treatments | 28 | 1,012.74 ± 8.672d | 1,165.4 ± 8.973c | 984.29 ± 8.380a | 1,244.52 ± 9.657b |
| Seven days after the treatments | 35 | 1,318.59 ± 12.004d | 1,496.63 ± 13.597c | 1,220.77 ± 12.739a | 1,570.36 ± 14.640b |
Group A Infected and treated with aqueous extract of Ganoderma applanatum at 1,000 mg/kg body weight, Group B Infected and treated with amprolium at 100 mg/kg body weight, Group C Infected but not treated, Group D Neither infected nor treated
Differences between means were considered significant at P ≤ 0.05 Superscripted letters a–d represent significant differences within one age group, where same letters represent no significant difference
Fig. 2.
Mean weight gain of chicks in different treatment groups
The experimental infection of the broiler chickens with coccidial oocysts showed clinical signs of weakness, reduced appetite, diarrhoea, and presence of oocysts in faeces. The experimental trials in all the infected birds showed a significant reduction in faecal oocyst output in birds that were treated with either aqueous extract of G. applanatum or amprolium. However the lowest OPG was recorded in amprolium treated group indicating the highest prophylactic efficacy among all groups. The reason for better efficacy of amprolium could be that it is already in the pure state and we can expect a bit low efficacy in the crude extracts of G. applanatum. In this study a gradual but significant differences in oocyst output of both infected-untreated and infected-treated groups were recorded. The results in terms of use of aqueous extract of G. applanatum to suppress oocysts of coccidia in broilers was in full agreement with Conway et al. (1993) who studied the effects of different levels of oocysts inocula of Eimeria acervulina, E. tenella and E. maxima on plasma constituents, packed cell volume, lesion scores and performance in chickens and Elmusharaf et al. (2006) who investigated the effect of a Manna-oligosaccharide (MOS) preparation on Eimeriatenella infection in broiler chickens. Besides reduction in oocyst output there was reduction in severity of clinical signs of bloody diarrhoea which was consistent with the studies of (Ogbe et al. 2008) and Ogbe et al. (2009). Moreover the results of this study are also in agreement with (Willis et al. 2010), they strongly suggest that a diet supplemented with 5 % FMG as an alternative control method in reducing Eimeria oocyst numbers during grow out.
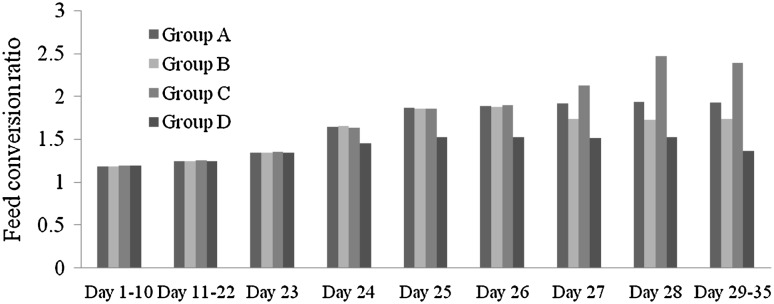
The highest feed conversion ratio observed in the infected, untreated birds (2.390) was observed provides an evidence of depression of feed intake due to infection with coccidian oocysts (Table 3) (Fig. 3). The highest feed conversion ratio reported in infected broilers resulted in significant reduction in the body weight. The study revealed that groups of birds not infected with coccidial oocysts consume more feed, while infected groups showed lower feed intake was due to coccidial stress. Hayat et al. (1991) supported the results of the present study and reported that coccidial infection decreased feed intake. Conway et al. (1993) also reported that a significant reduction in body weight occurred in broilers infected with a dose of 10,000 sporulated oocysts of E. tenella. The less effect of infection on growth performance may be related to the mildness of the infection. Under conditions of more severe infection with Eimeria, weight gain is generally reduced (Johnson and Reid 1970; Conway et al. 1993; McDougald 2003; Chapman et al. 2004).
Table 3.
Feed Conversion ratio of broilers infected with coccidia (Eimeria) and then treated with aqueous extract of Ganoderma applanatum and amprolium
| Age in days | Feed conversion ratio = Feed consumed/weight gained | ||||
|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | ||
| Not infected | 1–10 | 1.188 | 1.187 | 1.190 | 1.192 |
| 11–22 | 1.247 | 1.240 | 1.251 | 1.243 | |
| Infected | 23 | 1.344 | 1.349 | 1.352 | 1.340 |
| 24 | 1.645 | 1.656 | 1.639 | 1.452 | |
| 25 | 1.863 | 1.855 | 1.852 | 1.530 | |
| During treatment | 26 | 1.890 | 1.879 | 1.896 | 1.527 |
| 27 | 1.920 | 1.732 | 2.126 | 1.518 | |
| 28 | 1.937 | 1.725 | 2.464 | 1.522 | |
| After treatment | 29–35 | 1.925 | 1.738 | 2.390 | 1.361 |
Group A Infected and treated with aqueous extract of Ganoderma applanatum at 1,000 mg/kg body weight, Group B Infected and treated with amprolium at 100 mg/kg body weight, Group C Infected but not treated, Group D Neither infected nor treated
Fig. 3.
Feed conversion ratio of different treatment groups
The results of the present work showed in first experiment the birds of group A, infected and treated with aqueous extract of G. applanatum extract had higher mean weight gain (1,318.59 ± 12.004 g) and lower feed conversion ratio (FCR) (1.925), whereas birds of group C, infected but not treated gained lowest weight (1,220.77 ± 12.739 g) and highest FCR (2.390). Thus the poorest FCR was observed in birds which were infected but non-medicated. These results are supported by Voeten et al. (1988) who found that coccidiosis adversely affected growth and feed conversion.
Bioactive compounds or polysaccharides are known to block colonization of the intestine by pathogens, thereby improving their elimination from the body (Elmusharaf et al. 2006; Guo et al. 2004; Hughes et al. 1958). Many substances with immunomodulating effects have been found in mushrooms. Most medicinal mushrooms contain biologically active polysaccharides, glycoproteins and other valued substances. There is little doubt that mushroom-based products can serve as good dietary supplements. It is still up in the air exactly how these products work, but with the heightened interest around the world, the answers are forthcoming soon. Some biologically active compounds or organic acids, resins, and glycosides which include steroid and triterpenoid saponins are known to have therapeutic uses against microbes and parasites (Anon 2006; Die et al. 2004; Guo et al. 2004; Hobbs 1995). Some mushrooms contain polysaccharides that play a role in stimulating the activities of many interdependent cell types such as T and B-lymphocytes, macrophages, and natural killer (NK) cells, inducing production and secretion of cytokines and complement (Guo et al. 2004). Other mushrooms (e.g. Fraxinella, Boletus and Lactarius spp.) have also been reported to prevent intestinal coccidiosis in poultry (Guo et al. 2004; Harkonen 1998; Pang et al. 2000). Besides some mushrooms contain chemical substances that enhance the immune response and control certain parasitic and viral diseases (Anon 2006; Guo et al. 2004; Oei 2003; Wachtel et al. 2004; Wasser 2002; Zakhary et al. 1983). However, the active principles and the mechanisms of action of these mushrooms have not been fully elucidated, and should be the subject of future studies. This study showed that treatment with G. applanatum resulted in a marked reduction in the number of coccidian oocysts shed in the faeces, leading to improved weight gain and better feed conversion ratio. The results confirmed the virulence of coccidian oocysts and the effectiveness of both amprolium and G. applanatum extract against coccidian oocysts. Hence, the utilization of G. applanatum has potential as an alternative to other methods in coccidiosis intervention in elimination of clinical Eimerial infection in broiler chickens.
Acknowledgments
The authors are highly thankful to department of Zoology and Centre of Research for Development, University of Kashmir for providing the animal house and laboratory facilities for successful completion of this work. Thanks are due to Prof. M. Z. Chishti whose indispensable help and suggestions gifted during the study made it a success.
Conflict of interest
The authors declare that there is no conflict of interest.
Contributor Information
Shazia Ahad, Email: shaziaahad19@gmail.com.
Syed Tanveer, Email: syedtnvr@gmail.com.
Tauseef Ahmad Malik, Email: maliktsf@gmail.com.
References
- Anon (2006) Cultivation, utilization and medicinal effects of Ganoderma lucidum in Malaysia. Online at: http://www.canited.com/reishi97d-9.htm (Accessed 30 August 2006)
- Anthony MM, Joyce C. Proximate and nutrient composition of three types of indigenous edible wild mushrooms grown in Tanzania and their utilization prospects. Afr J Food Agric Nutr Dev. 2007;7(6):1–16. [Google Scholar]
- Biggs PM. The world of poultry disease. Avian Pathol. 1982;11:281–300. doi: 10.1080/03079458208436101. [DOI] [PubMed] [Google Scholar]
- Chang ST, Buswell JA. Mushroom nutriceuticals. World J Microbiol Biotechnol. 1996;12:473–476. doi: 10.1007/BF00419460. [DOI] [PubMed] [Google Scholar]
- Chang SI, Miles PE. Mushrooms cultivation, nutritional value, medicinial effects and environmental impact. Boca Raton: CRC Press; 2004. [Google Scholar]
- Chang ST, Mshigeni KE. Mushroom and their human health: their growing significance as potent dietary supplements. Windhoek: The University of Namibia; 2001. pp. 1–79. [Google Scholar]
- Chapman HD, Marsler P, LaVorgna MW. The effects of salinomycin and roxarsone on the performance of broilers when included in the feed for four, five or six weeks and infected with Eimeria species during the starter or grower phase of production. Poult Sci. 2004;83:761–764. doi: 10.1093/ps/83.5.761. [DOI] [PubMed] [Google Scholar]
- Conway DP, Sasai K, Gaafar SM, Smothers CD. Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella and E. maxima on plasma constituents, PVC, lesion scores and performance in chickens. Avian Dis. 1993;37:118–123. doi: 10.2307/1591464. [DOI] [PubMed] [Google Scholar]
- Elmusharaf MA, Bautista V, Nollet L, Beynen AC. Effect of a mannan oligosaccharide preparation on Eimeria tenella infection in broiler chickens. Int J Poult sci. 2006;5:583–588. doi: 10.3923/ijps.2006.583.588. [DOI] [Google Scholar]
- Guo FC, Kwakkel RP, Williams BA, Parmentier HK, Li WK, Yang ZQ, Verstegen MWA. Effects of mushroom and herb polysaccharides on cellular and humoral immune responses of Eimeria tenella-infected chickens. Poult Sci. 2004;83:1124–1132. doi: 10.1093/ps/83.7.1124. [DOI] [PubMed] [Google Scholar]
- Harkonen M. Uses of mushrooms by Finns and Karelians. Int J Circumpolar Health. 1998;57:40–55. [PubMed] [Google Scholar]
- Hayat CS, Nabi I, Hayat B, Iqbal Z, Khan MN. Comparative chemoprophylactic effect of different anticoccidials on performance of broilers. Pak Vet J. 1991;11:53–56. [Google Scholar]
- Hobbs C. Medicinal mushroom. Santa Cruz: Botanica Press; 1995. [Google Scholar]
- Hodgson JN. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Hughes DH, Lynch DL, Somers GF. Chromatographic identification of the amino acids and carbohydrates in cultivated mushroom. J Agric Food Chem. 1958;6:850–853. doi: 10.1021/jf60093a009. [DOI] [Google Scholar]
- Iqbal Z, Lateef M, Ashraf M, Jabbar A. Anthelmintic activity of Artemisia brevifolia in sheep. J Ethnopharmacol. 2004;93:265–268. doi: 10.1016/j.jep.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kadiri M (1990) The Physiological studies of some Nigerian mushrooms, Ph.D Thesis, University of Ibadan, Ibadan
- Ketiku AO, Ola L. Chemical composition and effects of mushroom (Lentinus subnudus, berk) preparations on the lipid profile of rats. Trop Anim Prod. 1999;2:169–174. [Google Scholar]
- Lee SH, Lillehoj HS, Park DW, Jang SI, Morales A, Garcia D, Lucio E, Larios R, Victoria G, Marrufo D, Lillehoj EP. Protective effect of hyperimmune egg yolk IGY antibodies against Eimeria maxima infections. Vet Parasitol. 2009;163:123–126. doi: 10.1016/j.vetpar.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Li CM. Chinese herb medicine feed additives (Chinese) 2. Beijing: Chinese Agricultural University Press; 1998. [Google Scholar]
- Long PL. The Biology of Coccidia. Baltimore: University Park Press; 1982. [Google Scholar]
- Long PL, Rowell JG. Counting oocysts of chicken coccidia. Lab Pract. 1958;7:515. [Google Scholar]
- Long PL, Joyner LP, Millard BJ, Norton CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Latina. 1976;6:201–217. [PubMed] [Google Scholar]
- McDougald LR. Coccidiosis. In: Saif YM, Barnes HJ, Fadly AM, Glisson JR, McDougald LR, Swayne DE, editors. Poultry diseases. Iowa: Iowa State Press; 2003. pp. 974–991. [Google Scholar]
- Monro JA. Treatment of cancer with mushroom products. Arch Environ Health. 2003;58:533–537. doi: 10.3200/AEOH.58.8.533-537. [DOI] [PubMed] [Google Scholar]
- Nagata T, Saeki M. Liquid chromatographic determination of amprolium in chicken tissues, using post-column reaction and fluorometric detection. J Assoc Off Anal Chem. 1986;69(6):941–943. [PubMed] [Google Scholar]
- N.R.C. Nutrient requirements of poultry. 8. Washington: National Academy Press; 1994. [Google Scholar]
- Oei P. Mushroom cultivation (3rd edn) Leiden: Technical Centre for Agricultural and Rural Cooperation (CTA), Backhuys; 2003. Benefits of mushrooms; pp. 1–7. [Google Scholar]
- Ogbe AO, Mgbojikwe LO, Owoade AA, Atawodi SE, Abdu PA. The effect of a wild mushroom (Ganoderma lucidum) supplementation of feed on the immune response of pullet chickens to infectious bursal disease vaccine. Electron J Environ Agric Food Chem. 2008;7:2844–2855. [Google Scholar]
- Ogbe AO, Atawodi SE, Abdu PA, Sannusi A, Itodo AE. Changes in weight, faecal oocyst count and packed cell volume of Eimeria tenella-infected broilers treated with a wild mushroom (Ganoderma lucidum) aqueous extract. J S Afr Vet Assoc. 2009;80:97–102. doi: 10.4102/jsava.v80i2.179. [DOI] [PubMed] [Google Scholar]
- Pang FH, Xie MQ, Ling HH. The investigation of Immunodulators tested for the results on the control of a coccidial infection. Chin J Vet Parasitol. 2000;8:1–3. [Google Scholar]
- Reid WM. Coccidiosis. In: Hofstad MS, Calnek BW, Helmboldt CF, Reid WM, Yoder HW Jr, editors. Diseases of poultry. 7. Ames: USA, Iowa State University Press; 1978. pp. 784–805. [Google Scholar]
- Ruff MD, Danforth HD (1996) Resistance of coccidian oocysts to medications. In: Proc. XX World’s Poultry Congress, II, 427–430
- Soulsby EJL. Helminths, Arthropods and Protozoan’s of domesticated animals. 7. London: Bailliere Tindall; 1982. [Google Scholar]
- Wachtel GS, Tomlinson B, Benzie IF. Ganoderma lucidum (Lingzhi), a Chinese medicinal mushroom: biomarker responses in a controlled human supplementation study. Br J Nutr. 2004;91:263–269. doi: 10.1079/BJN20041039. [DOI] [PubMed] [Google Scholar]
- Waller PJ. From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet Parasitol. 2006;139:1–14. doi: 10.1016/j.vetpar.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Williams RB. A compartmentalized model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol. 1999;29:1209–1229. doi: 10.1016/S0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Williams RB. Quantification of the crowding effect during infections with the seven eimeria species of the domesticated fowl: it’s importance for experimental designs and the production of oocyst stocks. Int J Parasitol. 2001;31(10):1056–1069. doi: 10.1016/S0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Willis WL, Isikhuemhen OS, Ibrahim S, King K, Minor R, Ohimain EI. Effect of dietary fungus myceliated grain on broiler performance and enteric colonization with bifidobacteria and salmonella. Int J of Poult Sci. 2010;9(1):48–52. doi: 10.3923/ijps.2010.48.52. [DOI] [Google Scholar]
- Zakhary JW, Taiseer M, Abo-Bakr A, El-Mahdy AR, El-Tabey SA. Chemical composition of wild mushrooms collected from Alexandria, Egypt. Food Chem. 1983;11:31–41. doi: 10.1016/0308-8146(83)90114-0. [DOI] [Google Scholar]