Abstract
Strongyloides species is a helminth of worldwide distribution primarily in tropical and subtropical regions. It is the only soil-transmitted helminth with the ability for autoinfection so; it may lead to severe systemic manifestations especially in immunosuppressed patients. Chemotherapy is currently considered the best therapeutic option for strongyloidiasis but some drugs are expensive and others have side effects as nausea, diarrhea and headache. Strongyloides larva is resistant to most chemical agents so, search for plant extracts may provide other effective but less expensive treatment. Lawsonia inermis Linn, popularly known as Henna, has been proven to have antihelminthic, antibacterial and antifungal properties. The current study was carried out to evaluate the efficacy of Lawsonia inermis on Strongyloides spp. In vitro using scanning electron microscopy. Fifty Strongyloides species. larvae and free living females were incubated with different concentrations of Lawsonia (1, 10, 100 mg/ml), for different incubation periods (24, 48, 72 and 96 h) in comparison to the same concentrations of flubendazole at the same different time points. The results showed that Lawsonia inermis in a concentration of 10 mg/ml incubated with Strongyloides spp. female for 24 h affected the parasite cuticular surface in the form of transverse and longitudinal fissures and transverse depression in comparison to no cuticular change with flubendazole (100 mg/ml). This suggests that Lawsonia inermis may be a promising phytotherapeutic agent for strongyloidiasis.
Keywords: Strongyloides, Lawsonia inermis, Flubendazole
Introduction
Strongyloidiasis is a major global health challenge that is underestimated in many countries. It is a chronic, soil-transmitted infection caused by Strongyloides spp., a helminth with a worldwide distribution, primarily in tropical and subtropical regions, where sanitary conditions are poor (Hall et al. 1994), such as Central and South America, sub-Saharan Africa, and South and Southeast Asia (Liu and Weller 1993; Concha et al. 2005). Foci of low endemicity are also reported in temperate climates, such as the Mediterranean Coast, mostly among elderly patients. Prevalence data indicate that 30–100 million people are infected worldwide, but the figure is presumably underestimated because infection is often subclinical (Olsen et al. 2009; Devi et al. 2011; Khieu et al. 2013).
In Egypt, recent infection rate is not known as little attention was directed towards this parasite, the prevalence rate ranges from 1.5 to 11 % (Salem et al. 1990; El-Shazly et al. 2006; El-Sherbini et al. 2008).
Due to its peculiar life cycle Strongyloides may remain indefinitely in the host, if not effectively cured (Concha et al. 2005). It is the only soil-transmitted helminth (STH) with the ability for auto-infection, and thus may lead to systemic infections with high parasite densities, particularly in immune-compromised hosts (Vadlamudi et al. 2006). Disseminated strongyloidiasis may lead to severe complications with substantial mortality. Therefore effective and timely use of antihelminthics is essential. The treatment must then reach the goal of the complete elimination of the parasite (Basile et al. 2010; Bisoffi et al. 2011).
Chemotherapy still the only effective tool to cure helminthic infection as effective vaccines against them have not been developed so far (Singh et al. 2002) Treatment options for strongyloidiasis include ivermectin, thiabendazole and albendazole. Ivermectin is currently considered the best therapeutic option (Gilbert et al. 2010), but its use is limited as it is relatively expensive. Also ivermectin can produce some side effects such as headache, dizziness, muscle pain, nausea, or diarrhea (Goodman and Gilman 2001; Singh et al. 2002; Bairagi et al. 2011).
Like the infective larvae of all nematodes, the Strongyloides larva is resistant to most chemical agents. Search for plant extracts may provide other effective but less expensive treatment and could be of value in preventing and treating helminthic multi-resistance (Nogueira et al. 2010; El-Sherbini and Osman 2013).
The identification of new antihelminthic drugs becomes a priority because of the availability of a handful of drugs, cost of treatments, and recent emergence of drug resistance. Medicinal plants are a good source of bioactive compounds for development of drugs. Most of the phytochemicals, secondary metabolites of plants, are physiologically active. Plants are known to provide high source of antihelminthic activity (Akhtar et al. 2000).
Lawsonia inermis Linn, popularly known as Henna or Mehandi in India belongs to family Lythraceae. Henna is naturalized and cultivated in the tropics of America, Egypt, India and parts of the Middle East (Bairagi et al. 2011). The leaf used for alleviating jaundice, skin diseases, venereal diseases and small pox. Seeds are effective against dysentery, liver disorders. Root is considered as a potent medicine for gonorrhea and herpes (Chaudhary et al. 2010).
The plant has been reported to have analgesic, hypoglycemic, astringent, antihemorrhagic, hepatoprotective, immunostimulant, anti-inflammatory, antibacterial, antimicrobial, antifungal, antiviral, antiparasitic, antitrypanosomal, antidermatophytic, antioxidant, tuberculostatic and anticancer properties. It is considered as a valuable source of unique natural products for development of medicines against various diseases (Sastri 1962; Gupta 2003). L. inermis L. was proved as antihelminthic by demonstrating the in vitro antihelminthic activity of petroleum ether (Bairagi et al. 2011), alcoholic (Sarojini et al. 2012) and aqueous and hydroalcoholic extracts (Eguale and Giday 2009).
In early Islamic culture henna usage is very evident in the book Prophetic Medicine where the medicinal practices of Prophet Mohammed (PBUH), as mentioned by his followers and others that were close to him in his household, were recorded (AL-Arnaoutt and AL-Arnaoutt 1987).
Lawsonia inermis L. constituents are made up of mannite, tannic acid, mucilage and gallic acid, but the main constituent is 2-hydroxynaphthoquinone, an important pigment called lawsone (Rahmoun et al. 2013).
Although naphthoquinones do appear to exhibit a wide spectrum of biological activities, the mechanisms of action remain somewhat unclear. However lawsone was shown to elicit in vivo lower toxic effects in mussel tissues than tissues in higher organisms. This may be due to the lower detectable levels of xanthine oxidase in the invertebrate mussels (Heinrich et al. 2004; Lall et al. 2003; Osman et al. 2004).
Based on these observations this study will be undertaken to determine the possible antihelminthic activities of L. inermis L. stems against free living female and filariform larvae of Strongyloides.
Materials and methods
Plant material and extraction procedure
Lawsonia inermis L. variety alba (Henna) plant was collected from National Center of Agriculture, Cairo, Egypt, 2009. It was kindly identified by Prof. El-Mewafy Abdou El-Mewafy, Head of Medicinal and aromatic plants Department, Horticulture Research Institute, Egypt. A voucher specimen no BUPD30 is deposited in Pharmacognosy Department, Faculty of Pharmacy, Beni-Suef University, Egypt. After removal of leaves, the stems were collected, left to dry in shade and powdered.
Preparation of the plant extract
The air-dried powdered stems of L. inermis L. (500 g) were extracted with 70 % methanol by cold maceration till exhaustion. The combined filtrates were evaporated to dryness using a rotary evaporator under reduced pressure. The residue was defatted with n-hexane. The semisolid residue left after defatting was transferred to a dark glass bottle and kept in a refrigerator until biological study.
Phytochemical screening
Phytochemical screening of the defatted methanol extract was carried out using standard procedures of different chemical tests and chromatographic techniques to identify the components (Harborne 1973; Trease and Evans 1989). Yield % of the defatted methanol was 18 % w/v. Results of phytochemical screening revealed the presence of phenolic compounds, flavonoids, quinine and tannins.
Strongyloides spp. isolation
Strongyloides spp. Larvae and free living females were isolated in Diagnostic and Research Unit of Parasitology Department, Faculty of Medicine, Ain Shams University, Egypt. Stool samples were collected from Stercoralis spp.-infected subjects and were inoculated directly onto the surface of 1.5 % non-nutrient agar (NNA) plates seeded with Escherichia coli bacterial suspension and incubated in a humidified chamber at 30 °C. The presence of Strongyloides spp. could be seen by examination of the agar plate growth was carried out daily for up to 7 days with a light and inverted microscopes using 40× objective. Subcultures were done after 2 weeks from positive cultures with confirmed growth. The plates were incubated in humidified chambers at 30 °C and examined after 24 h. Performing sub-culturing several times facilitated the isolation of Strongyloides spp.
Experimental design
In order to evaluate the in vitro strongyloidicidal activity of Lawsonia extract on Strongyloides spp. by scanning electron microscopy 50 Strongyloides spp. larvae and free living females were used for each control and experimental group. They were incubated with different concentrations of Lawsonia (100, 10, 1 mg/ml), for different incubation periods (24, 48, 72 and 96 h). The plates were sealed and incubated at 30 °C for different incubation periods. In addition, controls containing only the parasite in PBS as a non-treated control and parasite plus Flubendazole (100, 10, 1 mg/ml) as a reference drug control were submitted to the same procedure. Each concentration was performed in triplicate. After each incubation period, extraction from each test and control plates were processed for scanning electron microscopy.
Evaluation of the drug efficacy was carried out by scanning electron microscopy
The specimens were collected and rinsed in 2.5 % glutaraldhyde fixative solution buffered with 0.1 % phosphate buffer for 24 h. Specimens were then post fixed in osmium tetroxide for 15 min. The specimens were then dehydrated through ascending grades of ethanol and then were dried at critical point dryer using liquid carbon dioxide with Bal-Tec CPD030. The specimens were further mounted on brass studs with aluminum conducting tape and coated with gold in Bal-Tec SCD005 (Shoukry 2011). Specimens were examined with Philips XL30 scanning electron microscope operated at 10–30 kV, at the electron microscopy unit, Ain Shams University.
Results
Lawsoniainermis L at 10, 100 mg/ml after 24,48,72,96 h show ultrastructure changes while 1 mg/ml after 24,48,72,96 h show no ultrastructure changes. Flubendazole 1,10,100 mg/ml for 24,48,72,96 h show no ultrastructure change (Fig. 1).
Fig. 1.

Lawsonia inermis stem and leaves
Figures 2, 3, and 4 show an adult Strongyloides spp. female scanned by electron microscopy after incubation with flubendazole 100 mg/ml for 24 h as a reference drug control. They show no changes in the cuticle of the worm similar to untreated worms so used as a reference.
Fig. 2.
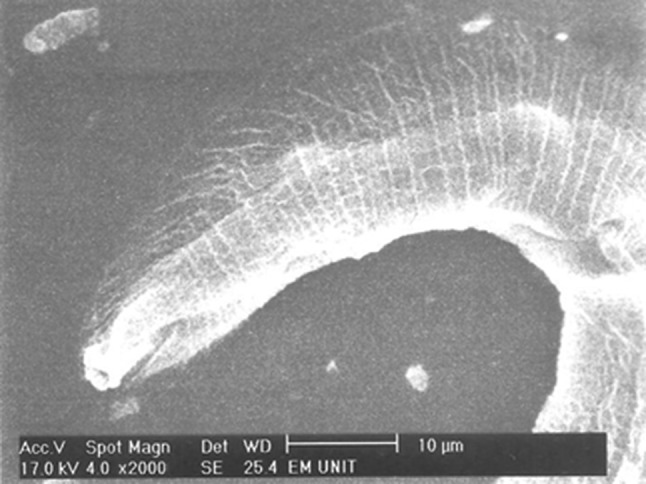
Adult Strongyloides stercoralis female after incubation with flubendazole 100 mg/ml for 24 h with no changes in the cuticle
Fig. 3.
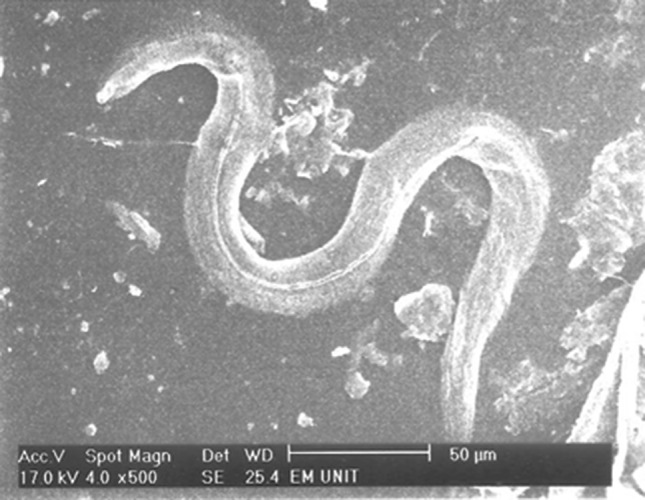
Adult Strongyloides stercoralis female after incubation with flubendazole 100 mg/ml for 24 h with no changes in the cuticle
Fig. 4.
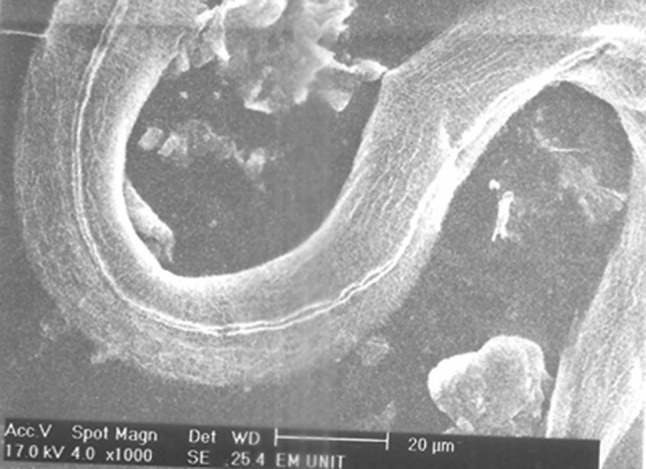
Adult Strongyloides stercoralis female after incubation with flubendazole 100 mg/ml for 24 h with no changes in the cuticle
Figures 5, 6, 7 and 8 show an adult Strongyloides spp. female scanned by electron microscopy after incubation with L. inermis L. 10 mg/ml for 24 h. In Fig. 5 there is a transverse fissure in the body of the worm. In Fig. 6 there is a longitudinal fissure and transverse depression in the body of the worm. Figure 7 shows a transverse fissure in the body of the worm and also in Fig. 8 there is a longitudinal fissure in the anterior part of the worm.
Fig. 5.
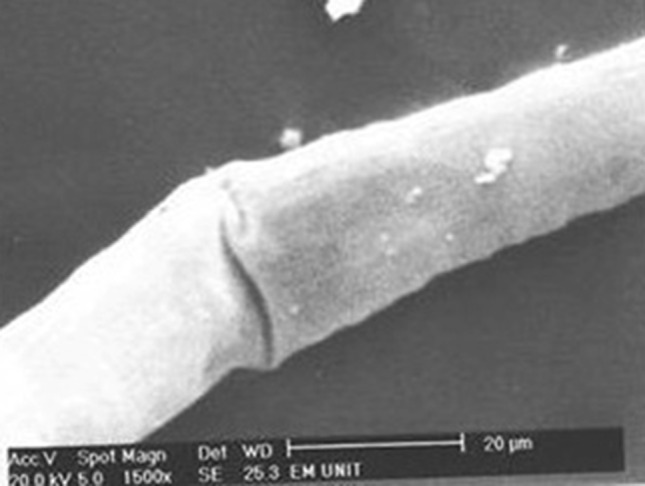
Adult Strongyloides stercoralis female after incubation with Lawsonia inermis L. 10 mg/ml for 24 h with a transverse fissure in the body of the worm
Fig. 6.

Adult Strongyloides stercoralis female after incubation with Lawsonia inermis L. 10 mg/ml for 24 h with a longitudinal fissure and transverse depression in the body of the worm
Fig. 7.

Adult Strongyloides stercoralis female after incubation with Lawsonia inermis L. 10 mg/ml for 24 h with a transverse fissure in the body of the worm
Fig. 8.

Adult Strongyloides stercoralis female after incubation with Lawsonia inermis L. 10 mg/ml for 24 h with a longitudinal fissure in the anterior part of the worm
We select the minimal dose which give ultrastructural effect, 10 mg/ml Lawsonia to demonstrate in figures.
The present study found that L. inermis L. exerts profound effects in parasite morphology in the form of transverse and longitudinal fissures and/or transverse depression in the body of the worm.
The morphological effects were described on females only but the larvae were disintegrated after incubation with both the herb and drug.
The present work did not investigate the effect of Lawsonia and flubendazole on motility and mortality of free living female or larvae because the main idea is to examine the effect of Lawsonia on ultrastructure of Strongyloides by scanning electron microscopy.
Discussion
The search for new anthelmintic agents is an important line of research because of the drug resistance acquired by several helminthes (Shaefler 1989; Shalit et al. 1989). The Strongyloides larva is resistant to most chemical agents. Albendazole is one of the principal drugs used to treat human strongyloidiasis (Horton 2000). However, the efficacy of the treatment of strongyloidiasis with albendazole, a benzimidazole derivative, has been inefficient due to benzimidazole resistance (Nontasut et al. 2005; Singthong et al. 2006).
Beta-tubulin represents the benzimidazole target. The benzimidazoles exert their anti-parasitic effect by binding directly to beta-tubulin and preventing its polymerization into microtubules, thereby affecting various mierotubule-based processes, which include cell division and intracellular transport (Lacey 1990). Mechanisms of benzimidazole resistance are well established, and are known to involve the target site mutations. It is associated with the deletion of a benzimidazole-susceptible beta-tubulin gene, decreasing the β-tubulin-benzimidazole interaction (Driscoll et al. 1989; Prichard 1994). Analysis of beta-tubulin isotype sequences from susceptible and resistant worms revealed the presence of a phenylalanine-to-tyrosine (Phe-Tyr) mutation at position 200 in all resistant worms examined (Kwa et al. 1994).
The search for natural antihelminthics is rewarding as it will lead to the development of a phytomedicine to act against parasite and have enormous therapeutic potential as they can serve the purpose without any side effects that are often associated with synthetic compounds (El-Sherbini and Osman 2013).
Medicinal plants have provided copious leads to combat diseases, from the dawn of civilization. The extensive survey of literature revealed that L. inermis L. is highly regarded as a universal panacea in the herbal medicine with diverse pharmacological activity spectrum. This versatile medicinal plant is the unique source of various types of accepted chemical compounds, which are responsible for various activities of the plant (Chaudhary et al. 2010).
It was reported that the phytochemical investigations of L. inermis L. leaves have shown the presence of steroids, saponins, tannins, flavonoids, β-sitosterolglucosides, quinonoids, naphthalene derivatives, luteolin, betulin, lupeol, gallic acid, coumarins, xanthones and phenolic glycosides (Dasgupta et al. 2003). These phytochemical constituents are good source of antimicrobial and antioxidant activity (Raja et al. 2013).
Sarojini et al. (2012) found that the ethanolic extract of L. inermis L. was potent as antihelminthic agent due to the presence of glycosides, terpenoids and flavonoids. On the other hand, the methanolic extract was effective probably due to the involvement of alkaloids, glycosides, terpenoids and flavonoids. Previously, also Sultana et al. (2009) demonstrated that ethanol extract and its components lawsone and gallic acid showed significant protein glycation inhibitory activity.
Few reports was undertaken on stems of Henna, so the present work aims the study testing the ability of L. inermis L. stems, as a natural antihelminthic agent against free living female and filariform larvae of Strongyloides spp.
The present study found that methanolic extract of L. inermis L. 10 mg/ml incubated with Strongyloides spp. female for 24 exerts profound effects on parasite morphology affecting the cuticular surface of female worms in the form of transverse fissure, longitudinal fissure and transverse depression in the body of the worm (Figs. 5, 6, 7, 8). Worms were distorted, and their cuticle did not appear to be intact, suggesting the possibility of direct cytotoxic damage affecting parasite morphology. In contrast, incubation with flubendazole 100 mg/ml for 24 h as a reference drug control resulted in no cuticular or surface alterations in female adult worms of Strongyloides. Flubendazole treated worms appeared transversally striated and the female posterior region was slightly dorsally bent (Figs. 2, 3, 4) similar to untreated worms.
It could be concluded that methanolic extract of L. inermis L. possesses dose dependant antihelminthic activity when compared to flubendazole. This effect of L. inermis L. could be due to the presence of phenolic compounds (e.g. gallic acids), flavonoids, quinine and tannins as proved by Sarojini et al. (2012).
The Lawsonia effective dose in this experiment was (10 mg/ml). Thus lower doses are not recommended, a fact which is supported by Eguale and Giday (2009) who found no statistically significant effect on the survival of adult Haemonchus contortus at a Lawsonia concentration ranging from 0.25 to 8.0 mg/ml.
The effect of Lawsonia and flubendazole on motility and mortality of free living female or larvae will not be discussed because the main concern of the present work was to examine the effect of Lawsonia on morphology of Strongyloides by scanning electron microscopy.
The possible mechanism of the antihelminthic activity of L. inermis L. could have been related to antioxidant activity, increasing the hepatic glutathione reductase (GR), superoxide dismutase (SOD) and catalase activities significantly as proved by (Dasgupta et al. 2003).
In addition, antihelminthic activity of Lawsonia may be due to its effect on inhibition of glucose uptake in the parasites and depletion of its glycogen synthesis (Bairagi et al. 2011), a mechanism which is similar to that of flubendazole which inhibits cytoplasmic microtubules in the intestinal or absorptive cells of the worms, thus inhibiting glucose uptake and glycogen storage depletion, leading to death of the worms within days (Jasra et al. 1990). However, Lawsonia has the additional advantage of being natural product with minimal side effects.
Lawsonia inermis L. may also have activated nicotinic cholinergic receptor in the worms resulting in either persistent depolarization or hyperpolarisation (Tripathi 2006).
The reported tuberculostatic and antimicrobial activities of L. inermis L. (Henna) would indicate the probable involvement of lawsone, i.e. 2-hydroxynaphthoquinone, which is known to be the main bioactive constituent of this herb, in the antimicrobial activity (Rahmoun et al. 2013). The binding ability of quinones in different oxidation states allows them to play an important role in biological systems properties (Valderrama et al. 2008). This antioxidant activity was also obtained by ethanol extract (Babili et al. 2013).
The antihelminthic potential of L. inermis petroleum and alcoholic extracts were previously studied against Indian adult earthworms due to its resemblance to intestinal roundworms of human beings. These effects were mainly on motility and viability not the morphology (Eguale and Giday 2009; Bairagi et al. 2011; Sarojini et al. 2012). However, morphological changes of the cuticle of adult worm reported in this study were also detected using L. inermis aqueous extract against the liver fluke Fasciola gigantica where detachment of spines noticed (Jeyathilakan et al. 2012).
No data retrieved from the literature so far report surface morphological changes caused by drugs on Strongyloides spp. adults. So the present work is the first to note these effects.
The surface alterations reported in this study, arise in distinct areas of the body of Lawsonia treated Strongyloides, leaving large regions apparently without alterations. These observations taken with absence of any alterations in flubendazole treated worms eliminate the hypothesis of the cuticular alterations due to technique artifacts and confirm that the cuticular alterations seen in treated worms are a drug induced surface effects.
However, movement and activity of the parasite were apparently normal. It is not possible, however, to understand whether or not these alterations have biological significance on the parasite itself or in the host-parasite interplay at longer time if would have an accumulative damage after yearly mass treatment as discussed by Ottesen et al. (1997) and Oliveira-Menezes et al. (2007) commenting on comparative analysis of a chemotherapy effect on the cuticular surface of Wuchereria bancrofti adult worms.
Morphological alterations as antihelminthic outcome was also observed by Oliveira-Menezes et al. (2007) who demonstrated cuticular changes of the W. bancrofti adult worm surface obtained from a patient treated with diethylcarbamazine (DEC) co-administered with albendazole (ALB). On the other hand, adult worms that were recovered from a patient treated with DEC alone after a single dose did not show such any abnormalities.
Other studies by scanning electron microscopy (SEM) with the third stage of subperiodic Brugia malayi showed damage on cuticular surface with ivermectin treatment, but not with DEC or ALB when used alone or in combination (Tippawangkosol et al. 2004). Moreover, tegumental changes were also reported by Keiser et al. (2006) who showed that tribendimidine acted similarly rapidly on the intestinal trematode E. caproni.
Most of the toxicological studies report that toxic effects due to the use of herbal medicine are associated with hepatotoxicity. Other toxic effects of the kidney, nervous system, blood and cardiovascular system, as well as mutagenecity and carcinogenicity have also been published in medical journals. Therefore, numerous advance biological experimental techniques have been used as standard safety test prior to the efficacy study. From the literature it has been noted that L. inermis L. exhibited significant hepatoprotective, antioxidant, antiinflammatory, antibacterial, analgesic and adaptogenic effects indicating that it is a safe substance to be used as a drug ordinarily (Chaudhary et al. 2010).
In our research we suggested that Strongyloides isolate is most probably flubendazole resistant isolate regarding structural changes. This work is the first research carried out on Strongyloides spp. to test the effect of Henna as an alternative for flubendazole. In vivo testing is a demand necessary to be carried out.
Contributor Information
Khadiga Ahmed Ismail, Email: khadigaahmed68@yahoo.com.
Ayman Nabil Ibrahim, Phone: +02 01005189049, Email: aymanibrahim91@yahoo.com.
Mona Abdel-Fattah Ahmed, Email: mona.fattah@hotmail.com.
Mona Hafez Hetta, Email: monahetta@gmail.com.
References
- Akhtar MS, Iqbal Z, Khan MN, Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in Indo-Pakistan subcontinent. Small Rumin Res. 2000;38:99–107. doi: 10.1016/S0921-4488(00)00163-2. [DOI] [Google Scholar]
- Al-Arnaoutt S, Al-Jozieh IK. Prophetic medicine. Beirut: Risala Publishing; 1987. [Google Scholar]
- Babili FE, Bouajila J, Valentin A, Chatelain C. Lawsonia inermis: its anatomy and its antimalarial, antioxidant and human breast cancer cells MCF7 activities. Pharm Anal Acta. 2013;4:203. doi: 10.4172/2153-2435.1000203. [DOI] [Google Scholar]
- Bairagi GB, Kabra AO, Mandade RJ. Anthelmintic activity of Lawsonia inermis L. Leaves in Indian Adult Earthworm. 2011;2:237–240. [Google Scholar]
- Basile A, Simzar S, Bentow J, Antelo F, Shitabata P, Peng SK, Craft N. Disseminated Strongyloides stercoralis: hyperinfection during medical immunosuppression. J Am Acad Dermatol. 2010;63:896–902. doi: 10.1016/j.jaad.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, Gobbo M, Bisoffi G, Gobbi F. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoSNegl Trop Dis. 2011;5(7):e1254. doi: 10.1371/journal.pntd.0001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: a phytopharmacological review. Int J Pharm Sci Drug Res. 2010;2:91–98. [Google Scholar]
- Concha R, Harrington W, Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39:203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- Dasgupta T, Rao AR, Yadava PK. Modulatory effect of Henna leaf (Lawsonia inermis) on drug metabolising phase I and phase II enzymes, antioxidant enzymes, lipid peroxidation and chemically induced skin and forestomach papillomagenesis in mice. Mol Cell Biochem. 2003;245:11–22. doi: 10.1023/A:1022853007710. [DOI] [PubMed] [Google Scholar]
- Devi U, Borkakoty B, Mahanta J. Strongyloidiasis in Assam, India: a community-based study. Trop Parasitol. 2011;1(1):30–32. doi: 10.4103/2229-5070.72110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, Dean E, Reilly E, Bergholz E, Chalfie M. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J CeU Biol. 1989;109:2993–3003. doi: 10.1083/jcb.109.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguale T, Giday M. In vitro anthelmintic activity of three medicinal plants against Haemonchus contortus. Inter J Green Pharm. 2009;1:29–34. doi: 10.4103/0973-8258.49371. [DOI] [Google Scholar]
- El-Shazly AM, Awad SE, Sultan DM, Sadek GS, Khalil HH, Morsy TA. Intestinal parasites in Dakahlia governorate, with different techniques in diagnosing protozoa. J Egypt Soc Parasitol. 2006;36(3):1023–1034. [PubMed] [Google Scholar]
- El-Sherbini G, Osman S (2013) Anthelmintic activity of unripe Mangifera indica L. (Mango) against Strongyloidesstercoralis. Int J Curr Microbiol App Sci 2(5):401–409
- El-Sherbini G, Abo El-Nooor M, Hegazy M. Parasitosis in handicapped children on Egyptian blind asylum. J Egypt Soc Parasitol. 2008;38(1):319–326. [PubMed] [Google Scholar]
- Gilbert DN, Moellering RCJr, Eliopoulos GM, Chambers HF, Saag MS (2010) The sanford guide to antimicrobial therapy, 40th edn. Antimicrobial Therapy, Inc, Sperryville, p. 220
- Goodman LS, Gilman A (2001) The pharmacological basis of therapeutics, 10th edn. Mcgraw Hill Medical Publishing Division, New York, p. 1121
- Gupta AK. Quality standards of Indian medicinal plants. Indian Counc Med Res. 2003;1:123–129. [Google Scholar]
- Hall A, Conway DJ, Anwar KS, Rahman ML. Strongyloides stercoralis in an urban slum community in Bangladesh: factors independently associated with infection. Trans R Soc Trop Med Hyg. 1994;88:527–530. doi: 10.1016/0035-9203(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Harborne JB (1973) Phytochemical methods. Chapman and Hall, Ltd, London, pp 49–188
- Heinrich M, Barnes J, Gibbons S, Williamson EM. In: Fundamentals of pharmacognosy and phytotherapy: important natural products and phytomedicines in pharmacy and medicine. London: Elsevier Health Science; 2004. [Google Scholar]
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121(suppl):S113–S132. doi: 10.1017/S0031182000007290. [DOI] [PubMed] [Google Scholar]
- Jasra N, Sanyal SN, Khera S. Effect of thiabendazole and fenbendazole on glucose uptake and carbohydrate metabolism in Trichuris globulosa. Vet Parasitol. 1990;35:201–209. doi: 10.1016/0304-4017(90)90055-G. [DOI] [PubMed] [Google Scholar]
- Jeyathilakan N, Murali K, Anandaraj A, Abdul Basith S. In vitro evaluation of anthelmintic property of ethno-veterinary plant extracts against the liver fluke Fasciola gigantica. J Parasit Dis. 2012;36:26–30. doi: 10.1007/s12639-011-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Shu-Hua X, Utzinger J. Effect of tribendimidine on adult Echinostoma caproni harbored in mice, including scanning electron microscopic observations. J Parasitol. 2006;92:858–862. doi: 10.1645/GE-793R.1. [DOI] [PubMed] [Google Scholar]
- Khieu V, Schär F, Marti H, Sayasone S, Duong S, Muth S, Odermatt P. Diagnosis, treatment and risk factors of Strongyloidesstercoralis in school children in Cambodia. PLoSNegl Trop Dis. 2013;7(2):e2035. doi: 10.1371/journal.pntd.0002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa MS, Veenstra JG and Roos MH (1994): Benzirnidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype l. Mol Biochem Parasito1 63:299–303 [DOI] [PubMed]
- Lacey E. Mode of Action of Benzirnidazoles. Parasit Today. 1990;6:112–115. doi: 10.1016/0169-4758(90)90227-U. [DOI] [PubMed] [Google Scholar]
- Lall N, Das Sarma M, Hazra B, Meyer JM. Antimycobacterial activity of diospyrin derivatives and structural analogue of diospyrin against Mycobcterium tuberculosis in vitro. J Antimicrobial Chemother. 2003;51:435–438. doi: 10.1093/jac/dkg068. [DOI] [PubMed] [Google Scholar]
- Liu LX, Weller PF. Strongyloidiasis and other intestinal nematode infections. Infect Dis Clin North Am. 1993;7:655–682. [PubMed] [Google Scholar]
- Nogueira PS, Martins FA, Duarte ER. Effect of Anacardium humile on the larval development of gastrointestinal nematodes of sheep. Vet Parasitol. 2010;171:361–364. doi: 10.1016/j.vetpar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Nontasut P, Muennoo C, Sa-nguankiat S, Fongsri S, Vichit A (2005) Prevalence of strongyloides in Northern Thailand and treatment with ivermectin vs albendazole. Southeast Asian J Troph Med Public Health 36(2):442–444 [PubMed]
- Oliveira-Menezes A, Lins R, Norões J, Dreyer G, Lanfredi RM. Comparative analysis of a chemotherapy effect on the cuticular surface of Wuchereria bancrofti adult worms in vivo. Parasitol Res. 2007;101:1311–1317. doi: 10.1007/s00436-007-0639-z. [DOI] [PubMed] [Google Scholar]
- Olsen A, Van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P. Strongyloidiasis—the most neglected of the neglected tropical diseases. Trans R Soc Trop Med Hyg. 2009;103:967–972. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Osman AM, Rotteveel S, Den Besten OJ, Van Noort PC. In vivo exposure of Dreissenapolymorphamussles to the quinines mendione and lawson. J Appl Toxicol. 2004;24(2):135–141. doi: 10.1002/jat.963. [DOI] [PubMed] [Google Scholar]
- Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- Prichard R. Anthelmintic resistance. Vet Parasitol. 1994;54:259–268. doi: 10.1016/0304-4017(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Rahmoun NM, Boucherit-Otmani Z, Benabdallah M, Boucherit K, Villemin D, Choukchou-Braham N. Antimicrobial activities of the henna extract and some synthetic naphthoquinones derivatives. Am J Med Biol Res. 2013;1:16–22. doi: 10.12691/ajmbr-1-1-3. [DOI] [Google Scholar]
- Raja W, Ovais M, Dubey A. Phytochemical screening and antibacterial activity of Lawsonia inermis leaf extract. Int J Microbiol Res. 2013;4:33–36. [Google Scholar]
- Salem SA, Mohamed NH, Azab ME, Soffar SA, El Kadery AA, Sabry NM. A survey for enteroparasites in Menoufia governorate, Egypt with special reference to Strongyloidesstercoralis. J Egypt SocParasitol. 1990;20(1):335–344. [PubMed] [Google Scholar]
- Sarojini N, Chandra Kanti C, Priyanka J, UshaKumari S, Das MSD. Correlation between the phytochemical constituents and anthelmintic activity of Lawsoniainermis leaf extracts. Int J Res Ayurveda Pharm. 2012;3:559. [Google Scholar]
- Sastri BN (1962) The wealth of India: raw materials, 6th edn, Vol. (L-M), CSIR, New Delhi, pp 47–50
- Shaefler S. Methicillin-resistant strains of Staphylococcusaureus resistant to quinolones. J Clin Microbiol. 1989;27(2):335–336. doi: 10.1128/jcm.27.2.335-336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I, Berger SA, Gorea A, Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcusaureus isolates in a general hospital. Antimicrob Agents Chemother. 1989;33(4):181–184. doi: 10.1128/AAC.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukry Y. scanning electron microscopy, a new approach of an old issue. EJH. 2011;34:179–181. [Google Scholar]
- Singh D, Swarnkar CP, Khan FA. Anthelminthic resistance in gastrointestinal nematodes in livestock in India. J Vet Parasit. 2002;16:115–130. [Google Scholar]
- Singthong S, Intapan PM, Wongsaroji T, Maleewong W (2006) Randomized comparative trial of two high-dose albendazole regimens for uncomplicated human strongyloidiasis. Southeast Asian J Trop Med Public Health 37 (Suppl 3):32–34 [PubMed]
- Sultana N, Choudhary MI, Khan AJ. Protein glycation inhibitory activities of Lawsonia inermis and its active principles. Enzyme Inhib Med Chem. 2009;24:257–261. doi: 10.1080/14756360802057500. [DOI] [PubMed] [Google Scholar]
- Tippawangkosol P, Choochote W, Na-Bangchang K, Jitpakdi A, Pitasawat B, Riyong D. The in vitro effect of albendazole, ivermectin, diethylcarbamazine, and their combinations against infective third-stage larvae of nocturnally subperiodic Brugia malayi (Narathiwat strain): scanning electron microscopy. J Vector Ecol. 2004;29:101–108. [PubMed] [Google Scholar]
- Trease GE, Evans WC. Pharmacognsy. 11. Brailliar Tiridel Can: Macmillian Publishers; 1989. [Google Scholar]
- Tripathi KD (2006) Essential of medical pharmacology, 4th edn. Jaypee Publication, New Delhi, pp 816–817
- Vadlamudi RS, Chi DS, Krishnaswamy G. Intestinal strongyloidiasis and hyperinfection syndrome. ClinMol Allergy. 2006;4:8. doi: 10.1186/1476-7961-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama JA, Leiva H, Rodriguez JA, Theoduloz C, Schmeda-Hirshmann G. Studies on quinones. Part 43: Synthesis and cytotoxic evaluation of polyoxyethylenecontaining 1,4-naphthoquinones. Bioorg Med Chem. 2008;16:3687–3693. doi: 10.1016/j.bmc.2008.02.018. [DOI] [PubMed] [Google Scholar]


